Abstract
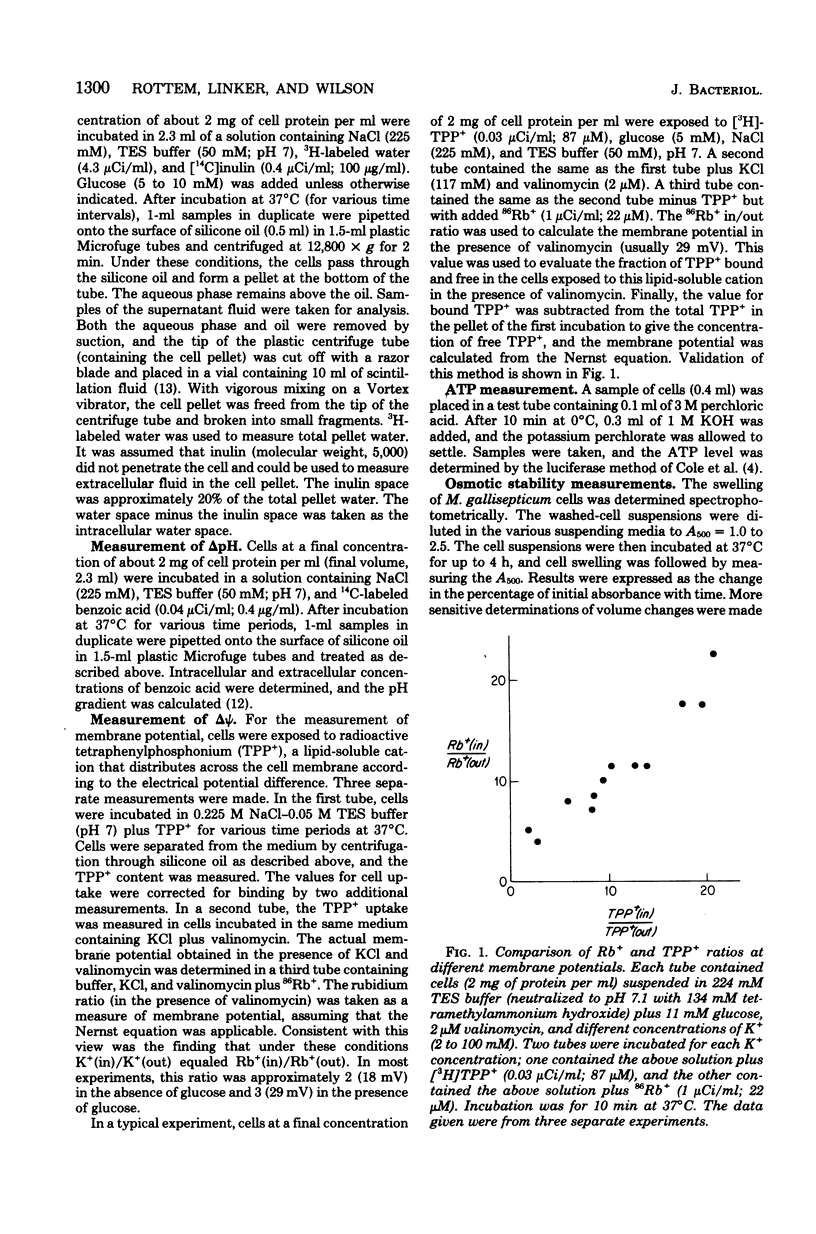
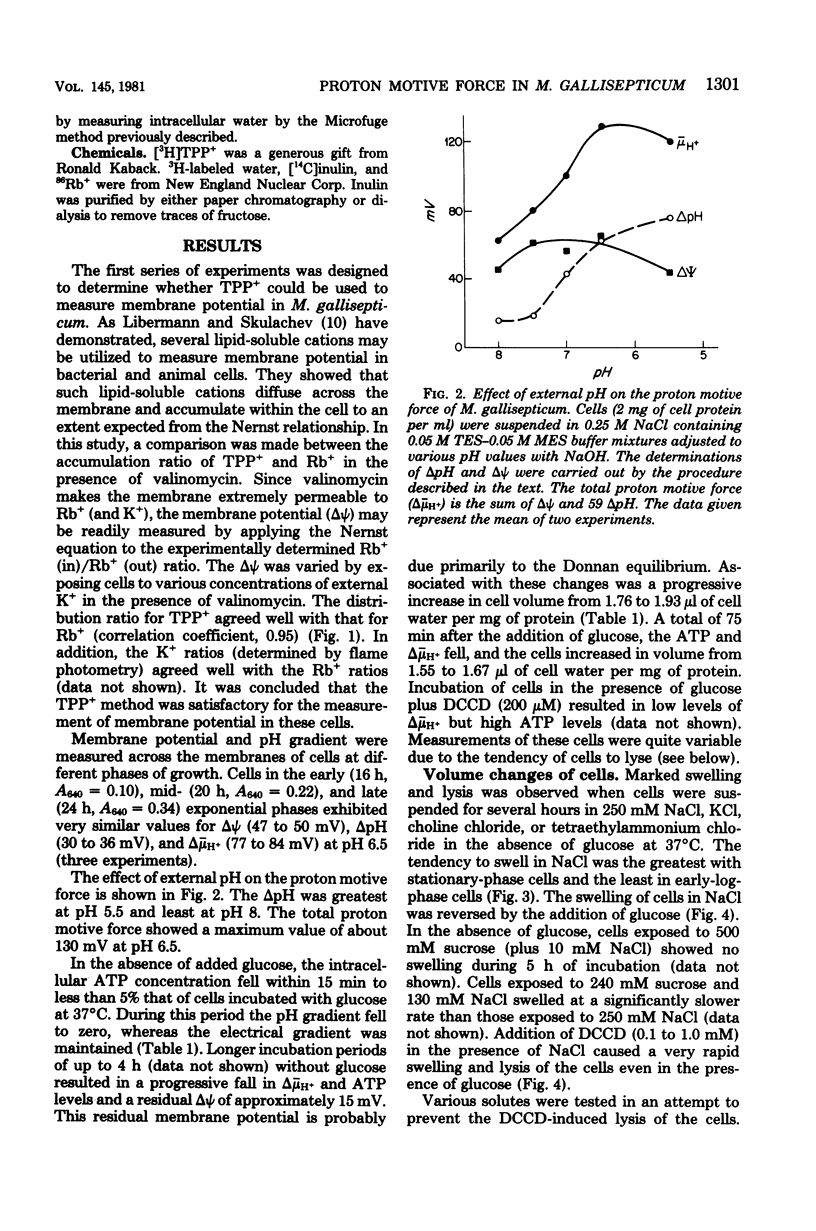
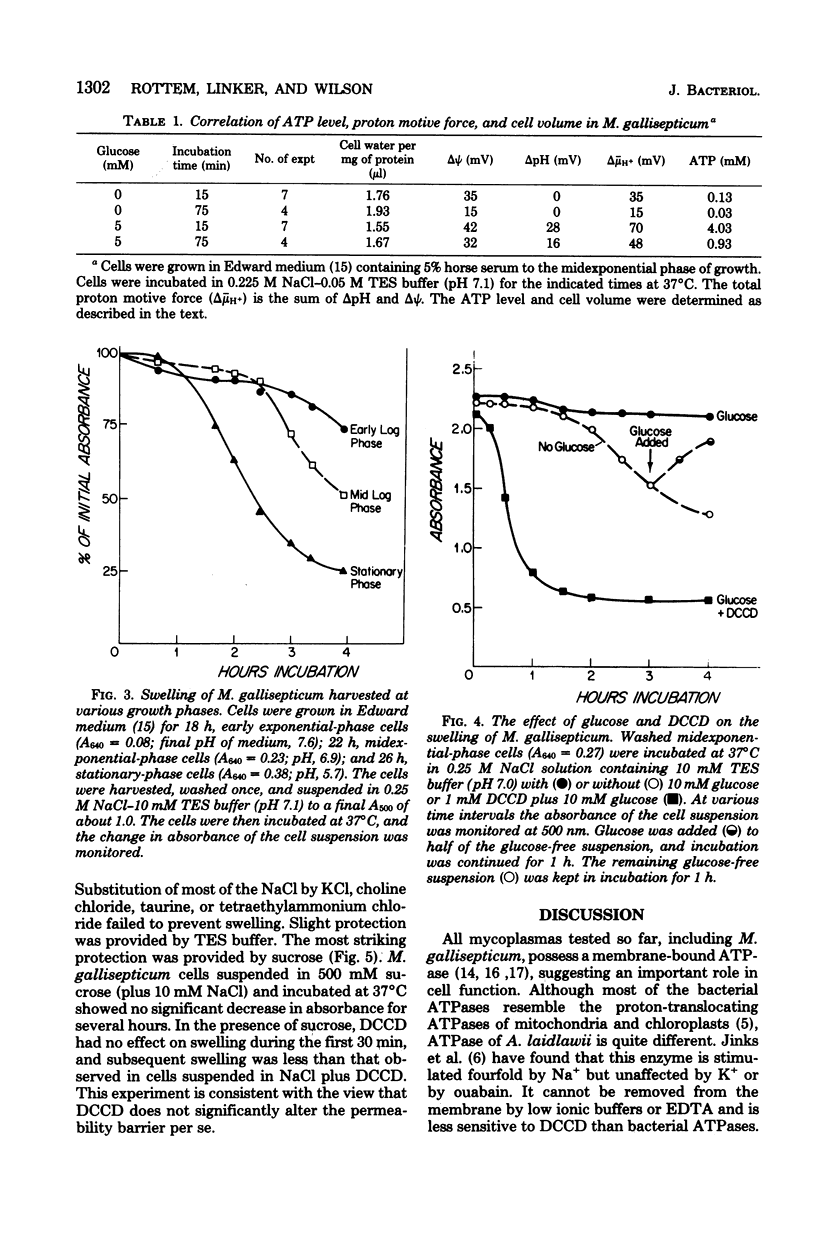
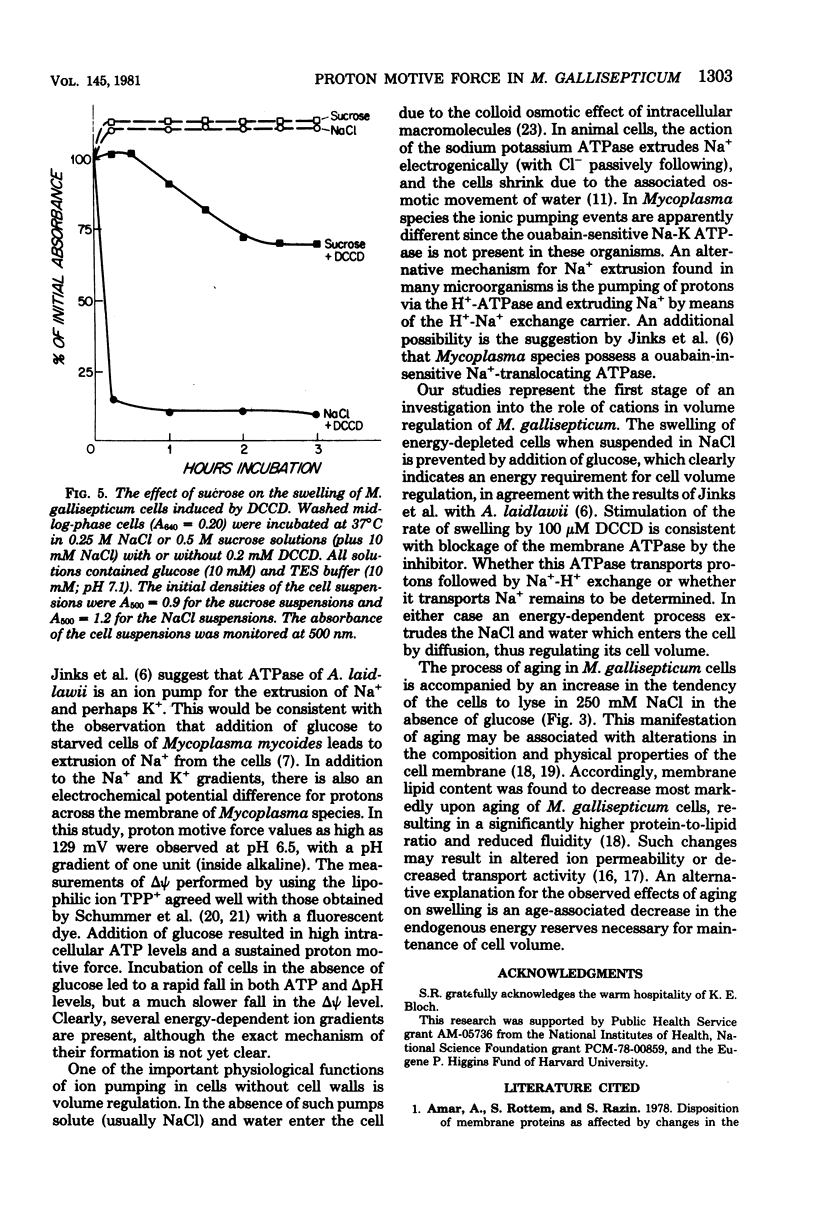
A proton motive force (delta (-) microH+) of 70 to 130 mV was measured across the membrane of Mycoplasma gallisepticum cells. The membrane potential was measured utilizing the lipid-soluble cation tetraphenylphosphonium. The method was validated by showing that in the presence of valinomycin the ratio of the concentrations (in/out) of tetraphenylphosphonium agreed well with those for K+ and Rb+. The pH gradient was calculated from the measured distribution ratio of benzoic acid. The proton motive force was approximately the same in cells harvested at early exponential, midexponential, and stationary phases of growth. The proportion of pH gradient to membrane potential varied with external pH. In the absence of glucose, cells incubated in an isosmotic NaCl solution showed low adenosine triphosphate and delta (-) microH+ levels and a tendency to swell and lyse compared with cells incubated with added glucose. It is concluded that energy is required for normal cell volume regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevers E. M., Leblanc G., Le Grimellec C., Op den Kamp J. A., van Deenen L. L. Disposition of phosphatidylglycerol in metabolizing cells of Acholeplasma laidlawii. FEBS Lett. 1978 Mar 1;87(1):49–51. doi: 10.1016/0014-5793(78)80130-6. [DOI] [PubMed] [Google Scholar]
- Clejan S., Bittman R., Rottem S. Uptake, transbilayer distribution, and movement of cholesterol in growing Mycoplasma capricolum cells. Biochemistry. 1978 Oct 31;17(22):4579–4583. doi: 10.1021/bi00615a001. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Jinks D. C., Silvius J. R., McElhaney R. N. Physiological role and membrane lipid modulation of the membrane-bound (Mg2+, na+)-adenosine triphosphatase activity in Acholeplasma laidlawii. J Bacteriol. 1978 Dec;136(3):1027–1036. doi: 10.1128/jb.136.3.1027-1036.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grimellec C., Leblanc G. Effect of membrane cholesterol on potassium transport in Mycoplasma mycoides var. Capri (PG3). Biochim Biophys Acta. 1978 Dec 4;514(1):152–163. doi: 10.1016/0005-2736(78)90085-8. [DOI] [PubMed] [Google Scholar]
- Leblanc G., Le Grimellec C. Active K+ transport in Mycoplasma mycoides var. Capri. Net and unidirectional K+ movements. Biochim Biophys Acta. 1979 Jun 13;554(1):156–167. doi: 10.1016/0005-2736(79)90015-4. [DOI] [PubMed] [Google Scholar]
- Leblanc G., Le Grimellec C. Active K+ transport in Mycoplasms mycoides var. Capri. Relationships between K+ distribution, electrical potential and ATPase activity. Biochim Biophys Acta. 1979 Jun 13;554(1):168–179. doi: 10.1016/0005-2736(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S. Membrane lipids of mycoplasmas. Biochim Biophys Acta. 1980 May 27;604(1):65–90. doi: 10.1016/0005-2736(80)90585-4. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Slutzky G. M., Bittman R. Cholesterol distribution and movement in the Mycoplasma gallisepticum cell membrane. Biochemistry. 1978 Jul 11;17(14):2723–2726. doi: 10.1021/bi00607a005. [DOI] [PubMed] [Google Scholar]
- Schummer U., Schiefer H. G., Gerhardt U. A novel method for the determination of electrical potentials across cellular membranes. II. Membrane potentials of Acholeplasmas, Mycoplasmas, Streptococci and erythrocytes. Biochim Biophys Acta. 1980 Aug 14;600(3):998–106. doi: 10.1016/0005-2736(80)90502-7. [DOI] [PubMed] [Google Scholar]
- Schummer U., Schiefer H. G., Gerhardt U. Mycoplasma membrane potential determined by a fluorescent probe. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):1023–1025. [PubMed] [Google Scholar]
- WILSON T. H. Ionic permeability and osmotic swelling of cells. Science. 1954 Jul 16;120(3107):104–105. doi: 10.1126/science.120.3107.104. [DOI] [PubMed] [Google Scholar]


