Abstract
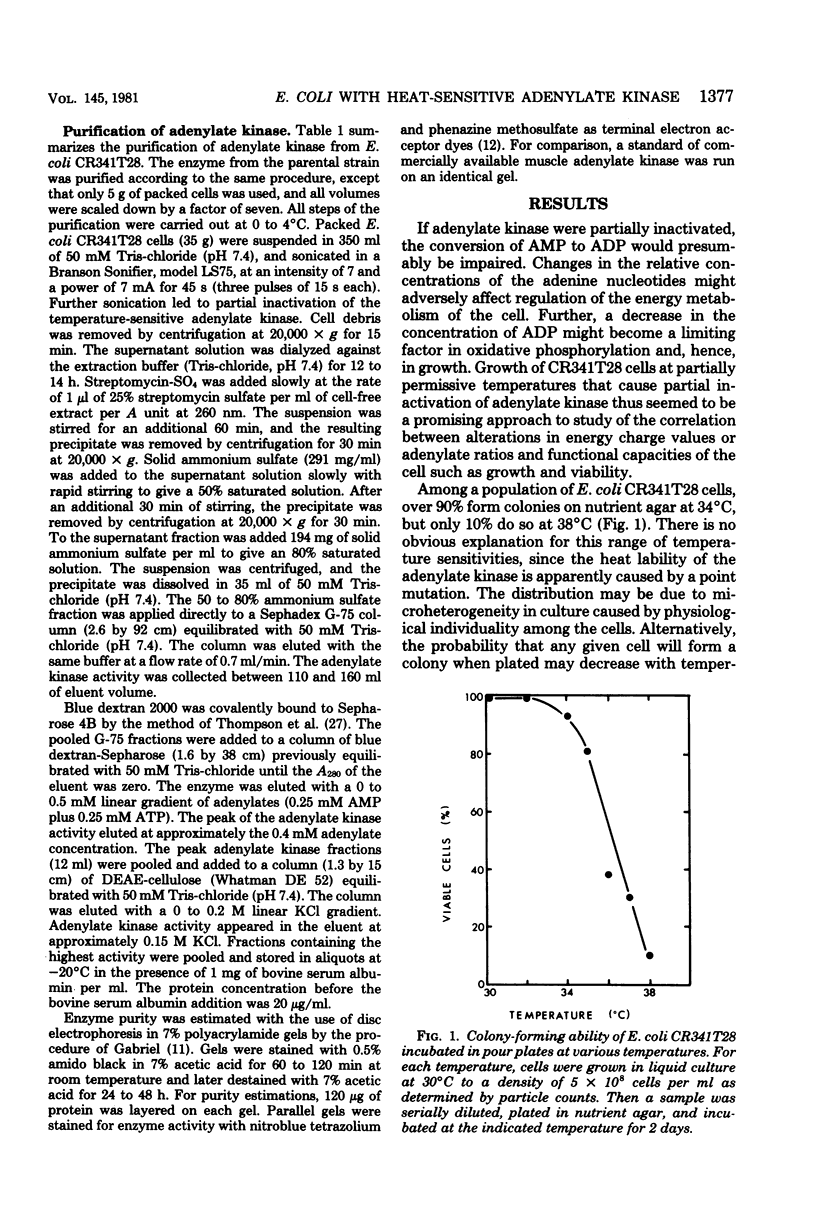
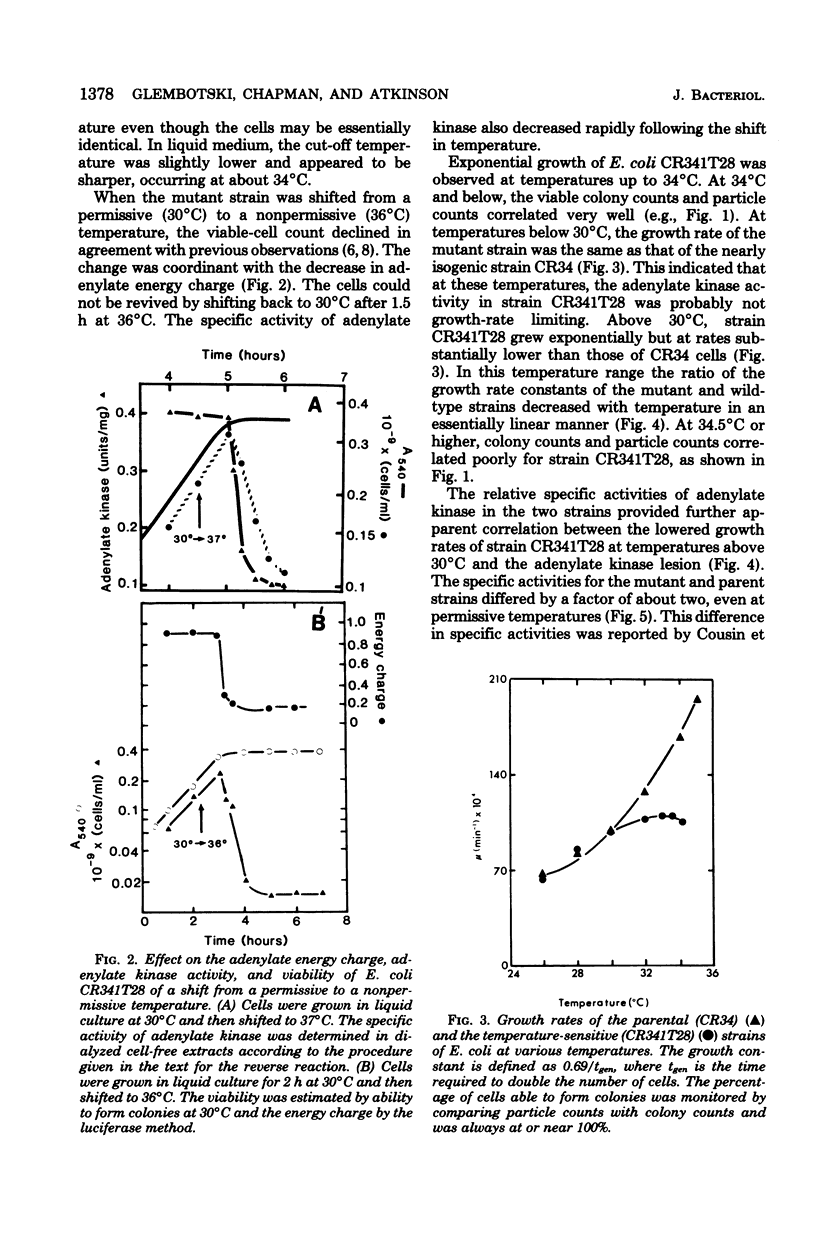
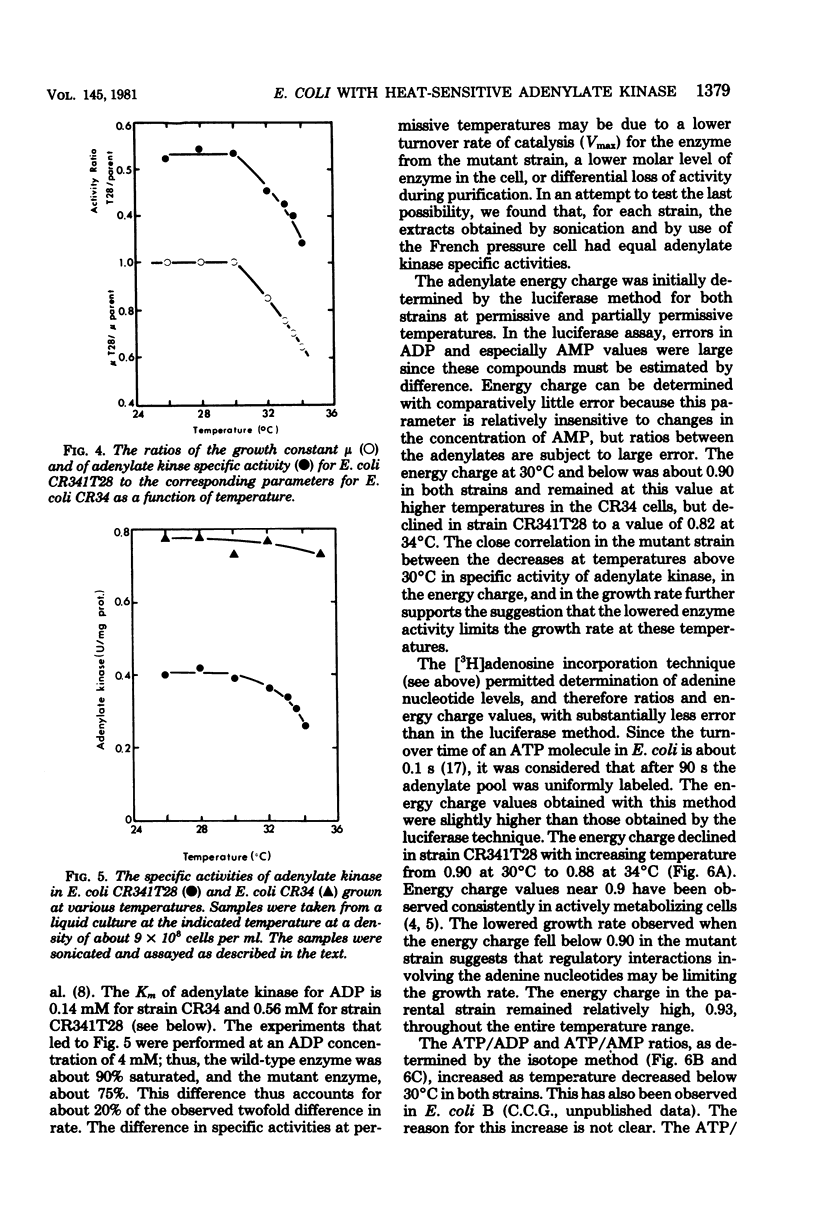
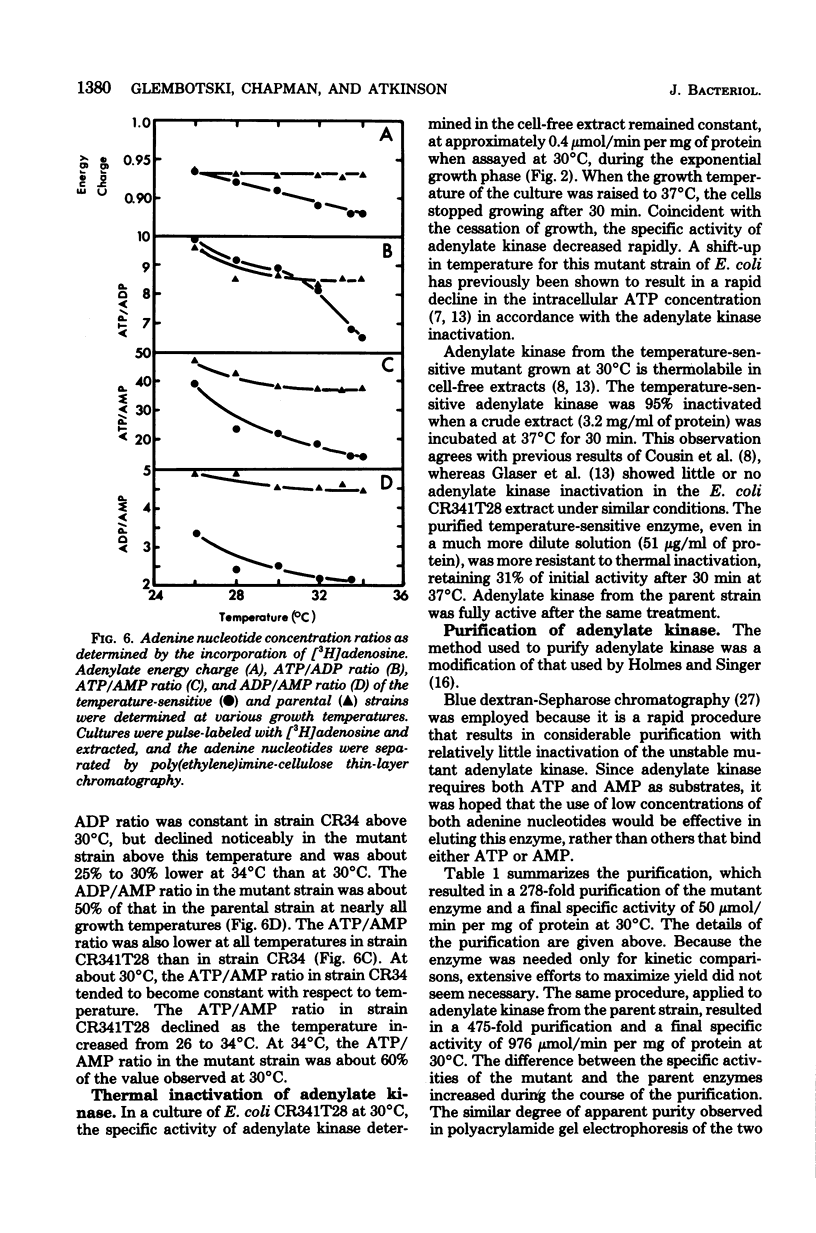
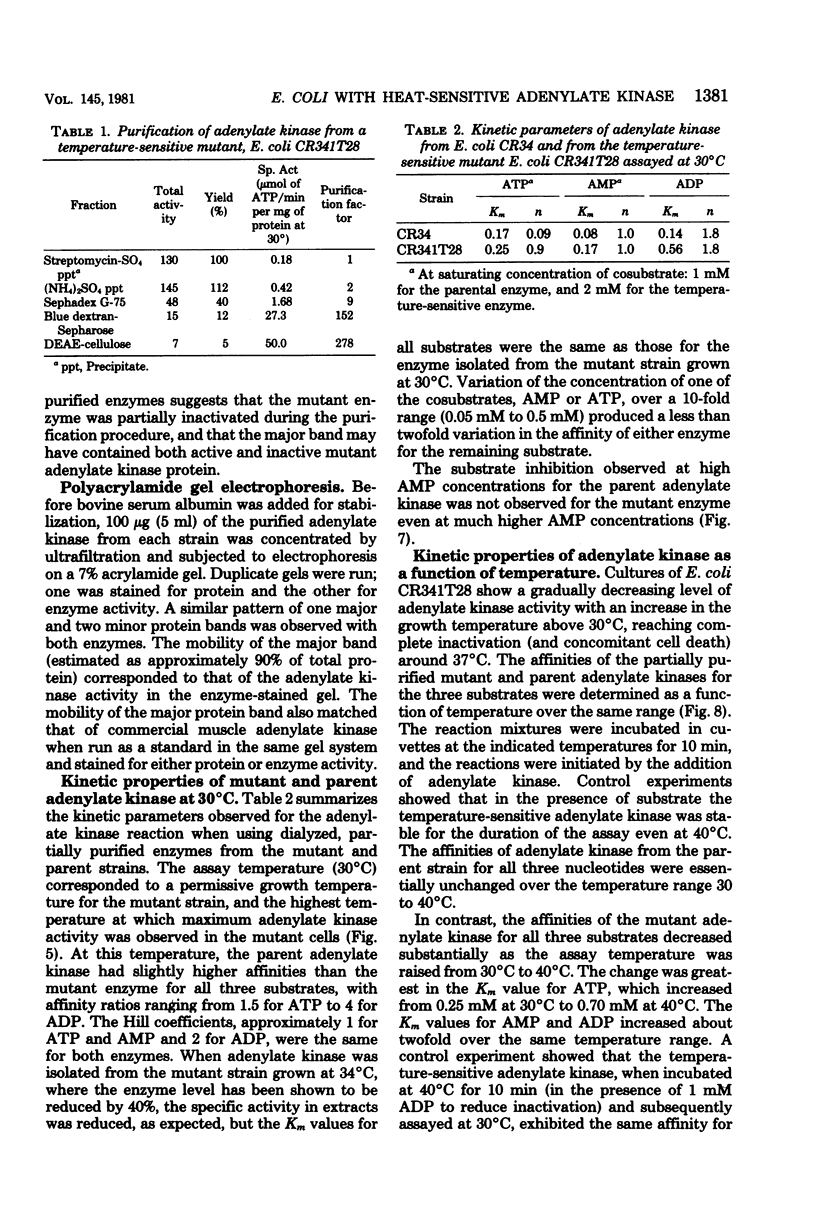
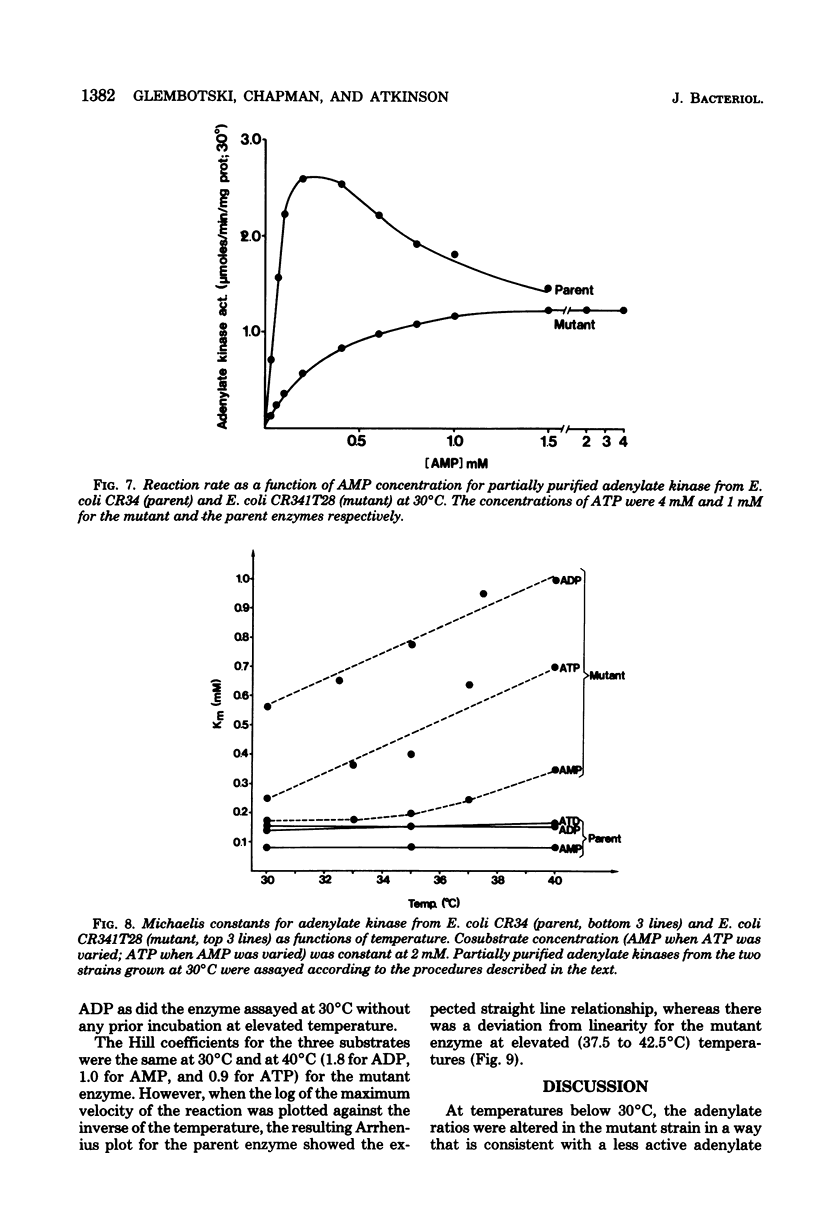
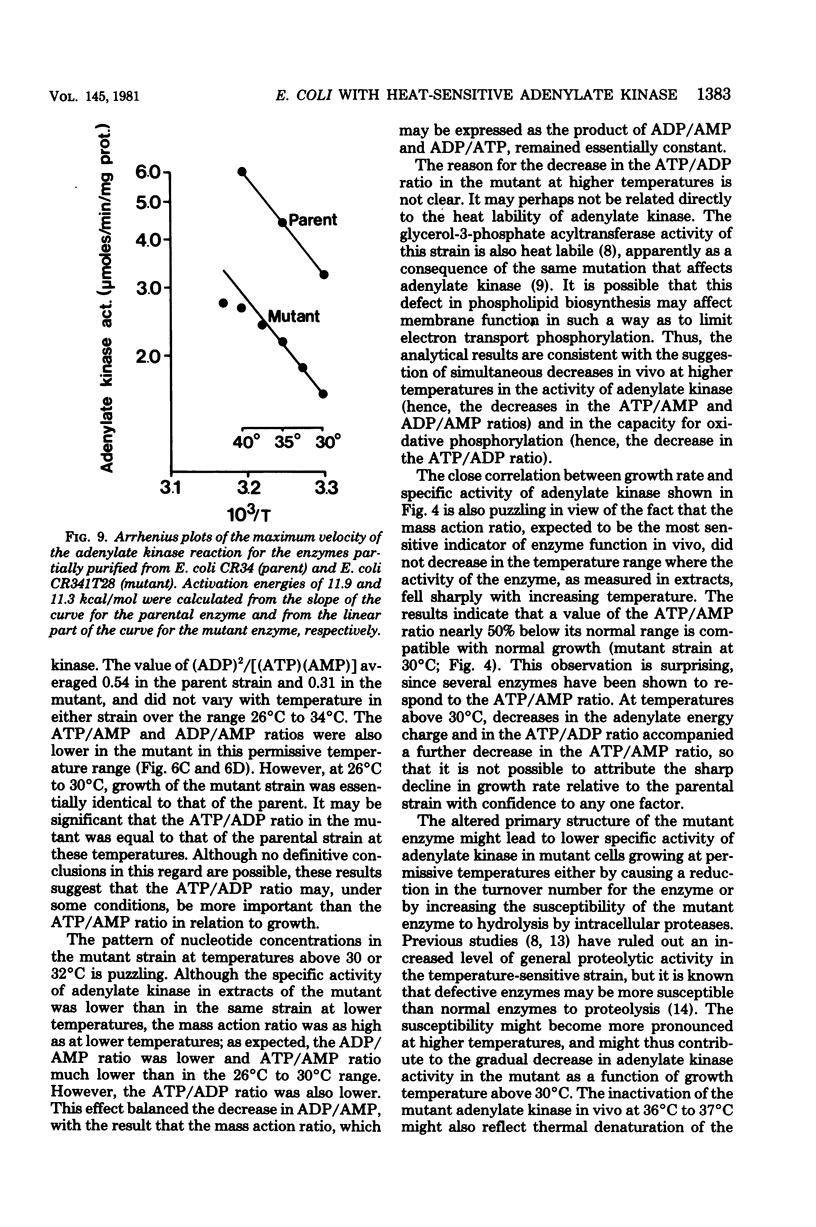
Escherichia coli strain CR341T28 will not grow at temperatures above 34 degrees C in liquid medium, and the adenylate kinase of this strain is heat sensitive. When a culture was shifted from a permissive (30 degrees C) to a nonpermissive (36 degrees C) temperature, the adenylate energy charge fell from 0.9 to 0.2, with a concurrent decrease in the number of viable cells and in the specific activity of adenylate kinase. When cultures of the temperature-sensitive strain were grown at temperatures above 30 degrees C, the adenylate energy charge, the specific activity of adenylate kinase, and the growth rate were lower than the corresponding parameters for the parental strain. By isotopic labeling of the adenine nucleotides in vivo, it was determined that increasing growth temperatures between 30 and 34 degrees C for the heat-sensitive strain resulted in a decrease in the adenosine triphosphate-to-adenosine monophosphate and adenosine triphosphate-to-adenosine diphosphate ratios. Between 26 and 30 degrees C the adenosine triphosphate-to-adenosine diphosphate ratio was essentially normal in the temperature-sensitive strain, but the adenosine triphosphate-to-adenosine diphosphate ratio was decreased. The adenylate ratios in the parental strain did not change between 30 and 34 degrees C. The adenylate kinase mass action ratio for each strain was essentially constant under all growth conditions. When assayed at 30 degrees C, the affinities of the enzyme from the mutant strain were somewhat lower than those of the parent adenylate kinase. The mutant enzyme also did not exhibit the substrate inhibition that was observed at high adenosine monophosphate concentrations with the parental enzyme. An increase in the assay temperature from 30 degrees to 40 degrees C had little or no effect on the Km values determined for the parental adenylate kinase, but caused the Km values determined for the mutant adenylate kinase to increase by a factor of two or more.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr, Atkinson D. E. Adenylate energy charge in Saccharomyces cerevisiae during starvation. J Bacteriol. 1975 Mar;121(3):975–982. doi: 10.1128/jb.121.3.975-982.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin D., Belaïch J. P. Sur une mutatio thermosensible d'Escherichia coli affectant une fonction énergétique. C R Acad Sci Hebd Seances Acad Sci D. 1966 Sep 19;263(12):886–888. [PubMed] [Google Scholar]
- Cousin D., Buttin G. Mutants thermosensibles d'Escherichia coli K12. 3. Une mutation létale d'E. coli affectant l'activité de l'adénylate-kinase. Ann Inst Pasteur (Paris) 1969 Nov;117(5):612–630. [PubMed] [Google Scholar]
- Cousin D. Mutants thermosensibles d'Escherichia coli K12. II. Etude d'une mutation létale affectant une réaction génératrice d'énercie. Ann Inst Pasteur (Paris) 1967 Sep;113(3):309–325. [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Nulty W., Vagelos P. R. Role of adenylate kinase in the regulation of macromolecular biosynthesis in a putative mutant of Escherichia coli defective in membrane phospholipid biosynthesis. J Bacteriol. 1975 Jul;123(1):128–136. doi: 10.1128/jb.123.1.128-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Cashel M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J Biol Chem. 1972 Nov 10;247(21):7067–7072. [PubMed] [Google Scholar]
- Holmes R. K., Singer M. F. Purification and characterization of adenylate kinase as an apparent adenosine triphosphate-dependent inhibitor of ribonuclease II in Escherichia coli. J Biol Chem. 1973 Mar 25;248(6):2014–2021. [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- Luria S. E., Suit J. L., Plate C. A. Initiation of transcription is temperature-dependent in an E. coli mutant with ts adenylate kinase. Biochem Biophys Res Commun. 1975 Nov 3;67(1):353–358. doi: 10.1016/0006-291x(75)90323-x. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T., PEEL J. L. Myokinase activity in microorganisms. Biochim Biophys Acta. 1956 May;20(2):390–392. doi: 10.1016/0006-3002(56)90302-x. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Swedes J. S., Dial M. E., McLaughlin C. S. Regulation of protein synthesis during early limitation of Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):162–170. doi: 10.1128/jb.138.1.162-170.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedes J. S., Sedo R. J., Atkinson D. E. Relation of growth and protein synthesis to the adenylate energy charge in an adenine-requiring mutant of Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6930–6938. [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]


