Abstract
Autoregulation of the ciliary beat frequency (CBF) has been proposed as the mechanism used by epithelial ciliated cells to maintain the CBF and prevent the collapse of mucociliary transport under conditions of varying mucus viscosity. Despite the relevance of this regulatory response to the pathophysiology of airways and reproductive tract, the underlying cellular and molecular aspects remain unknown. Hamster oviductal ciliated cells express the transient receptor potential vanilloid 4 (TRPV4) channel, which is activated by increased viscous load involving a phospholipase A2–dependent pathway. TRPV4-transfected HeLa cells also increased their cationic currents in response to high viscous load. This mechanical activation is prevented in native ciliated cells loaded with a TRPV4 antibody. Application of the TRPV4 synthetic ligand 4α-phorbol 12,13-didecanoate increased cationic currents, intracellular Ca2+, and the CBF in the absence of a viscous load. Therefore, TRPV4 emerges as a candidate to participate in the coupling of fluid viscosity changes to the generation of the Ca2+ signal required for the autoregulation of CBF.
Introduction
Epithelial ciliated cells are responsible for the mechanical clearance of mucus and trapped substances from the airways and the transport of gametes and embryos through the oviduct (Halbert et al., 1976; Afzelius, 1995; Knowles and Boucher, 2002). A primary determinant of mucociliary transport is the ciliary beat frequency (CBF), which is regulated by a variety of chemical and mechanical stimuli (Satir and Sleigh, 1990). Ciliated epithelia are exposed to physiological changes in mucus viscosity (Rutllant et al., 2002). Despite these variations in fluid viscosity, mucociliary transport efficiency is preserved. Johnson et al. (1991) have shown that ciliated cells are able to maintain relatively constant their CBF over a range of viscosities and proposed that this autoregulatory response of the CBF aimed to prevent the collapse of mucus transport under high viscous loads.
Several intracellular signals have been proposed to mediate the changes of CBF in response to different stimuli: cAMP, cGMP, nitric oxide, and Ca2+ (Jain et al., 1993; Geary et al., 1995; Wyatt et al., 1998; Evans and Sanderson, 1999). Among them, the role of Ca2+ in the control of CBF is particularly interesting as it has been associated with the ciliary response to mechanical stimuli. Mechanically stimulated ciliated cells increase intracellular Ca2+ and CBF (Lansley and Sanderson, 1999), a response that is lost in the absence of extracellular Ca2+ (Sanderson and Dirksen, 1986). The hypothesis that mechanical stimulation might be physiologically initiated by changes in mucus viscosity has been present for quite a while (Spungin and Silberberg, 1984), but the cellular mechanism linking the viscous load exerted by the presence of mucus to the control of CBF awaits to be resolved. In the present work, we aimed to elucidate the mechanism that couples mechanical stimulation (viscous load) to ciliary activity, a process that has been suggested to involve Ca2+ entry and subsequent activation of cilia (Spungin and Silberberg, 1984).
Over the past few years great advances have been made on the molecular characterization of the Ca2+ entry pathways activated in response to different stimuli, and a new class of calcium-permeable cationic channels, the transient receptor potential (TRP) superfamily, has emerged (Clapham, 2003). The vertebrate TRPV4 channel has been proposed as an osmo- and mechanosensitive channel (Liedtke et al., 2000, 2003; Strotmann et al., 2000; Wissenbach et al., 2000; Nilius et al., 2001; Arniges et al., 2004). Here, we report the role of TRPV4 and phospholipase A2 (PLA2) in the generation of the Ca2+ signal required to maintain CBF in hamster oviductal ciliated cells under conditions of mechanical stress induced by high viscous load, thereby preventing the collapse of the mucus transport.
Results and discussion
High viscosity–induced Ca2+-dependent autoregulation of the CBF in oviductal ciliated cells
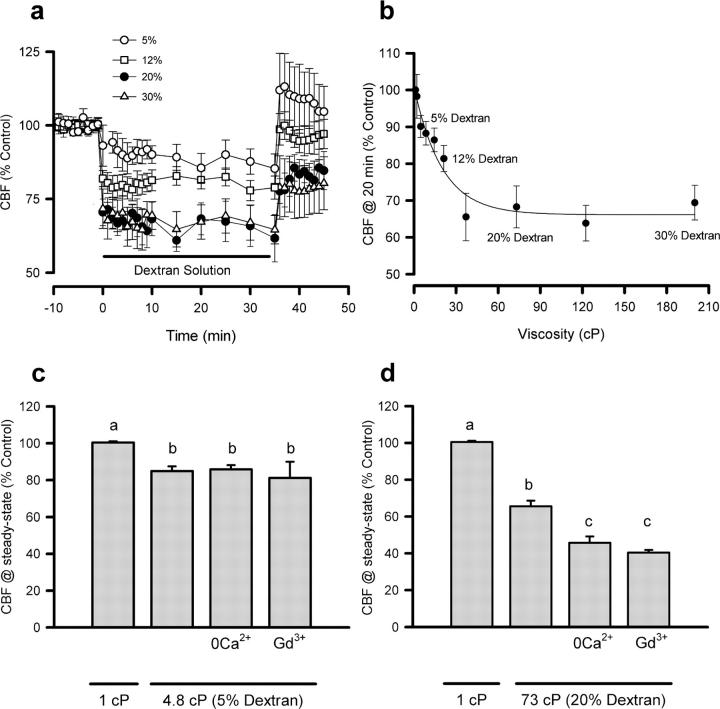
Exposure of primary cultures of hamster oviductal ciliated cells to increased viscous loading reduced the CBF, reaching a new stable value within the first 10 min (Fig. 1 a). The CBF dropped ∼35% within the range of 2–37 cP (2–15% dextran solutions), but no further decrease was observed at higher viscosities in the range of 37–200 cP (15–30% dextran solutions; Fig. 1 b). These results indicate that mucus transporting ciliated cells are capable of maintaining their CBF in high viscosity conditions and suggest the presence of an autoregulatory mechanism that allows ciliated epithelia to adjust their CBF to varying viscous loads without collapsing mucus transport. The signal coupling changes in mechanical load (fluid viscosity) to the autoregulation of CBF is still unknown, although early works pointed to the influx of Ca2+ into the cells as a probable candidate (Johnson et al., 1991). We tested the Ca2+ hypothesis by measuring CBF in ciliated cells exposed to either 5% (4.8 cP) or 20% dextran solutions (73 cP, viscosity value within the range where autoregulation of the CBF occurred) in the absence of extracellular Ca2+ or in the presence of 100 μM Gd3+, a blocker of mechanosensitive cation channels (Yang and Sachs, 1989). Fig. 1 c shows that neither the absence of extracellular Ca2+ or the presence of Gd3+ modified the CBF at low viscosity conditions but determined a marked reduction of the CBF at high viscous loads (73 cP; Fig. 1 d). These results suggested that the autoregulation of CBF at high viscous loads required the entry of Ca2+ into the ciliated cell, a process that apparently does not play a crucial role in the establishment of the steady-state CBF at lower viscosities.
Figure 1.
Effect of viscous loading on CBF. (a) Time course of CBF changes in hamster oviduct ciliated cells exposed to 5% (4.8 cP), 12% (21.5 cP), 20% (73 cP), and 30% (200 cP) dextran solutions. (b) Effect of viscous load on the CBF recorded after 20-min exposure to increased viscosity. CBF recorded at steady-state conditions (15–25 min) after exposure to 4.8 (c) or 73 cP (d) in the absence of extracellular Ca2+ or in the presence of 100 μM Gd3+. Results are the mean ± SEM of 5–10 separate cultures. Significant differences (P < 0.05) between groups are marked with different letters.
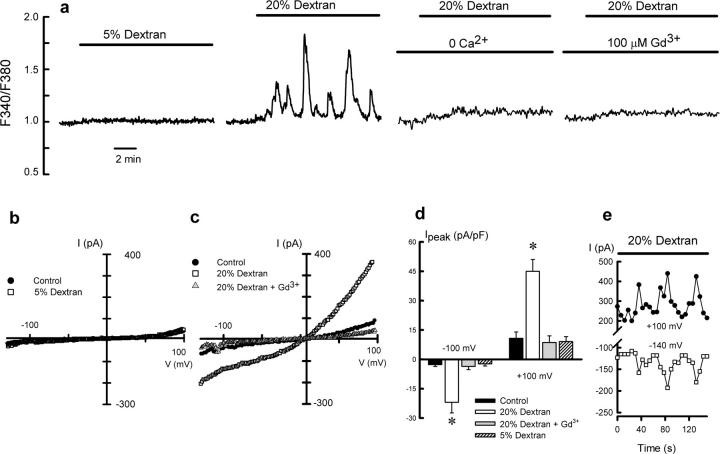
To further investigate the relationship between mechanical stimulation and intracellular Ca2+, we monitored changes in cytosolic Ca2+ in response to low (5% dextran) and high (20% dextran) viscous loads, the latter triggering the CBF autoregulatory response. Fig. 2 a shows the ratiometric fura-2 fluorescence obtained in primary cultures of single oviductal ciliated cells. Exposure to 5% dextran solution did not modify the intracellular Ca2+ levels (Fig. 2 a), whereas 20% dextran solution (73 cP) triggered an oscillatory Ca2+ response in ciliated cells, which was prevented in the absence of extracellular Ca2+ or in the presence of 100 μM Gd3+. Interestingly, the Ca2+ oscillatory pattern emerged at the viscosity values that trigger the autoregulatory process (>30 cP; unpublished data).
Figure 2.
Dextran-activated calcium entry pathway. (a) Cytosolic Ca2+ signal obtained in hamster oviductal ciliated cells exposed to 5 or 20% dextran and the effect of extracellular Ca2+ removal and 100 μM Gd3+ on the 20% dextran-induced Ca2+ signal. Traces are representative of five to six experiments under each condition. (b and c) Whole-cell cationic currents recorded in oviductal ciliated cells dialyzed with CsCl-containing pipette solution under control (1 cP) and 5% dextran (4.8 cP) (b) and 20% dextran solution alone (73 cP) or containing 100 μM Gd3+ (c). (d) Average current density measured at −100 mV and +100 mV under the following conditions: control (n = 27), 20% dextran (n = 15); 20% dextran + Gd3+ (n = 10); and 5% dextran (n = 11). *, P < 0.05, compared with control. (e) Oscillatory pattern of the cationic current obtained in a single cell exposed to 20% dextran.
Activation of a cationic current by high viscous load
The TRPV4 channel has been implicated in the cellular response to different mechanical stimuli (Liedtke et al., 2003; Suzuki et al., 2003); therefore, constituting a possible candidate to mediate Ca2+ entry in oviductal ciliated cells subjected to a high viscous load. Characterization of the putative TRPV4 cationic currents in response to increases in viscous load was evaluated in freshly dissociated single ciliated cells using the whole-cell patch-clamp technique. Exposure of a ciliated cell to a 5% dextran solution (4.8 cP) failed to activate a cationic current (Fig. 2 b), whereas exposure to a 20% dextran solution (73 cP) activated a large, outwardly rectifying current (Fig. 2 c), which was blocked by Gd3+ (100 μM). Mean normalized peak current responses to 5 and 20% dextran solutions are shown in Fig. 2 d. The TRPV4-like current generated in response to 20% dextran often presented an oscillatory pattern (Fig. 2 e) resembling the [Ca2+]i oscillations shown in Fig. 2 a.
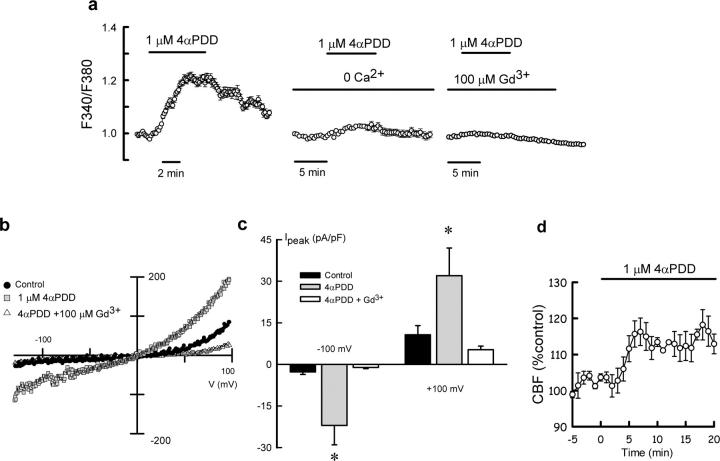
Functional TRPV4 channels in oviductal ciliated cells were also evaluated using a synthetic activator of TRPV4, 4α-phorbol 12,13-didecanoate (4αPDD; Watanabe et al., 2002), in the absence of viscous load (1 cP). Intracellular Ca2+ in ciliated cells increased in response to 4αPDD (Fig. 3 a), and, occasionally, showed an oscillatory pattern (not depicted). This response was prevented in the absence of extracellular Ca2+ (Fig. 3 a, middle) or in the presence of Gd3+ (Fig. 3 a, right), a blocker of nonselective cation channels. A whole-cell current with characteristics similar to that obtained with 20% dextran solutions was recorded when ciliated cells were exposed to 1 μM 4αPDD (Fig. 3, b and c). Analysis of the reversal potential of the currents activated by 20% dextran solutions (−4.7 ± 2.4 mV; n = 15) and 4αPDD (−5.5 ± 2.4 mV; n = 6) are not statistically different but showed a right shift compared with control currents (−12 ± 1.3 mV; n = 21) as previously described (Watanabe et al., 2002). Addition of 4αPDD also resulted in the increase of the CBF (Fig. 3 d) in the absence of mechanical stimuli (1 cP), thus adding support to the hypothesis linking the activity of TRPV4 channels to the control of CBF at high viscous loads. Changes in flow superfusion (1–15 ml/min) in the absence of dextran did not activate cationic currents, whereas exposure to 20% dextran solutions activated them either in the absence of fluid flow or under continuous perfusion at 1–3 ml/min. Therefore, it appears that high viscous load by itself is the main trigger of the response, although we cannot completely discard a shear stress component in the response elicited by high dextran solutions.
Figure 3.
Effect of the TRPV4 activator 4αPDD on cytosolic calcium, cationic currents, and CBF. (a, left) Cytosolic Ca2+ response of ciliated oviductal cells to 4αPDD (1 μM). Effect of removal of extracellular Ca2+ (middle) and 100 μM Gd3+ (right) on the 4αPDD response. Traces are means ± SEM obtained from 6–16 cells per culture (repeated on at least three cultures). (b) Whole-cell cationic currents recorded in an oviductal ciliated cell dialyzed with CsCl-containing pipette solution under control conditions, after application of 1 μM 4αPDD, and after application of 1 μM 4αPDD + 100 μM Gd3+. (c) Average current density measured at −100 mV and +100 mV under the following conditions: control (n = 15), 1 μM 4αPDD (n = 6), and 4αPDD + 100 μM Gd3+ (n = 3). *, P < 0.05, compared with control. (d) Time course of CBF response to 1 μM 4αPDD (n = 6).
Molecular identification of the dextran-induced cationic currents
All the functional data shown point to a TRPV4-like channel as the mediator in the increased [Ca2+]i required for the CBF autoregulatory response. Molecular identification of TRPV4 in hamster oviductal ciliated cells was investigated by single-cell RT-PCR and Western blot. Hamster TRPV4 has not yet been cloned, so we used primers directed against a region of the TRPV4 sequence highly conserved across species. Fig. 4 a shows single amplicons of ∼500 bp obtained from two hamster oviductal ciliated cells. Subsequent sequencing of the bands confirmed the expression of TRPV4 in the ciliated cells. Fig. 4 b shows a Western blot obtained with an antibody generated against a COOH-terminal epitope of the human TRPV4 protein. The antibody identified a double band of the expected size in human TRPV4-transfected HEK cells and a single band in hamster oviductal cells.
Figure 4.
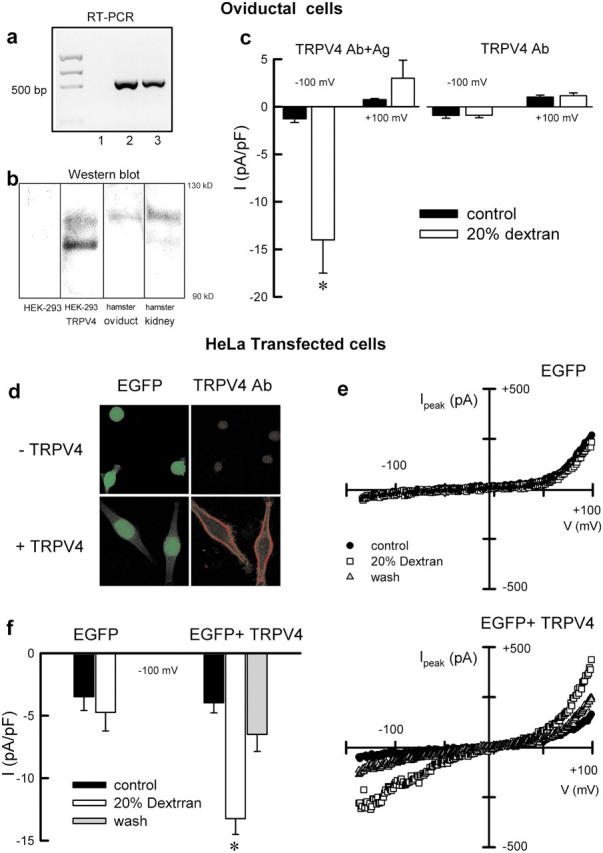
Molecular identification of dextran-activated cationic currents. (a) Detection of TRPV4 in two oviductal ciliated cells (lanes 2 and 3) by RT-PCR. (lane 1) Negative control where the cDNA was omitted. (b) Western blot showing bands at the predicted molecular mass for TRPV4 (∼100 kD) in hamster oviduct and kidney. Untreated HEK-293 and HEK-293 cells transfected with the human TRPV4 (isoform a) were used as negative and positive controls, respectively. (c) Mean current density recorded in oviduct ciliated cells dialyzed with NMDG-Cl solutions containing either 3.2 μg/ml (dilution 1:500) of TRPV4 antibody preabsorbed with a 10-fold excess of antigen (left; n = 5) or TRPV4 antibody alone (right; n = 6). Cells were superfused with 20% dextran solutions 10 min after the establishment of the whole-cell configuration. (d) Confocal images of HeLa cells transfected with EGFP (top) or EGFP+TRPV4 (bottom). EGFP fluorescence signal (green) and TRPV4 signal (red) are shown. (e) Whole-cell cationic currents recorded in HeLa cells with CsCl-containing pipette solution under control (1 cP), 20% dextran solution (73 cP), and washout. (f) Average current density measured at −100 mV in HeLa cells transfected with EGFP (n = 9) or EGFP+TRPV4 (n = 11). *, P < 0.05, compared with control.
Next, we functionally tested the blocking capability of the TRPV4 antibody on oviductal cells to confirm that a TRPV4-like channel is at the core of the ciliated cells' response to high viscous loads. Intracellular dialysis of hamster oviductal ciliated cells with the TRPV4 antibody prevented the activation of cationic currents under high viscous load conditions (Fig. 4 c, right). The specificity of the inhibitory effect of the TRPV4 antibody was demonstrated by repeating the experiments with antigen-preabsorbed TRPV4 antibody. Under these conditions, normal activation of TRPV4-like currents were observed in response to 20% dextran solutions (Fig. 4 c, left).
The activation of TRPV4 by dextran was also tested in a heterologous expression system. Immunolocalization of TRPV4 to the plasma membrane was confirmed in transiently cotransfected (EGFP+hTRPV4; Fig. 4 d, bottom) but not in EGFP-transfected Hela cells (Fig. 4 d, top). Exposure to 20% dextran solutions activated cationic currents only in EGFP+TRPV4–transfected cells (Fig. 4 e). Mean normalized currents obtained from EGFP- and EGFP+TRPV4–transfected HeLa cells are shown in Fig. 4 f.
Involvement of PLA2 in the autoregulation of the CBF
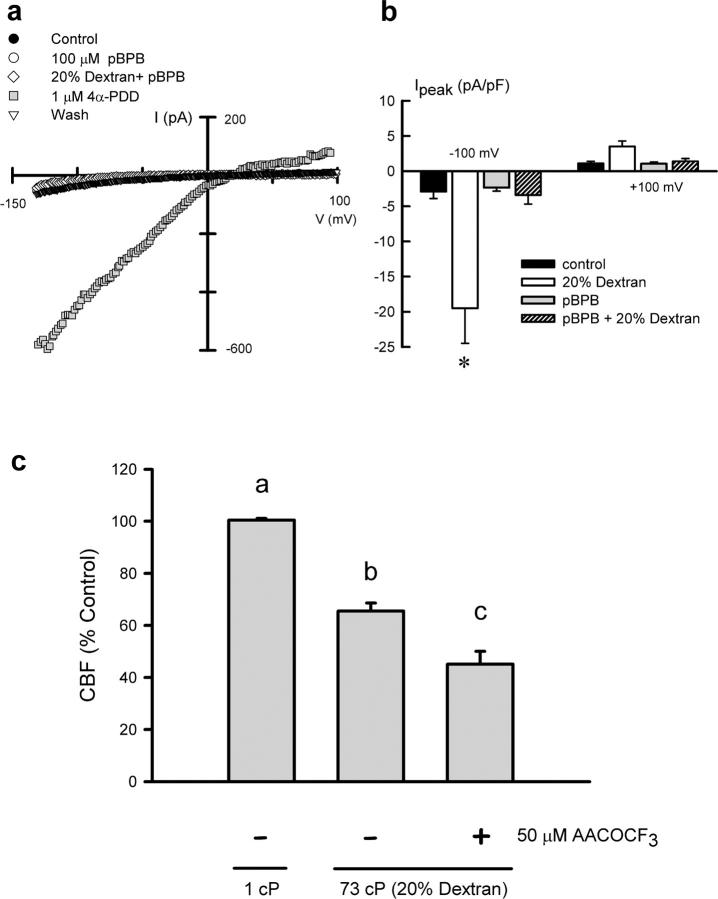
Cell swelling-dependent activation of TRPV4 requires the PLA2-mediated release of arachidonic acid (Vriens et al., 2004), suggesting that the TRPV4 channel is not an osmosensor per se, but a key element in the transduction of osmotic changes into Ca2+ signals. Both cell swelling and mechanical stress activate PLA2 and the formation of arachidonic acid (Lehtonen and Kinnunen, 1995; Pedersen et al., 2000). Therefore, we evaluated whether or not activation of the TRPV4-like current by 20% dextran solutions might also depend on the activity of PLA2 (Fig. 5). Currents were recorded with N-methyl-d-glucamine (NMDG) intracellular solutions, favoring the presence of inward currents. Incubation of single ciliated cells with the PLA2 inhibitor 4-bromophenacyl bromide (pBPB; 100 μM) prevented the activation of TRPV4-like currents under high viscosity conditions (Fig. 5, a and b) but did not affect current activation by 4αPDD (Fig. 5 a). These results are in accordance with previous observations reporting that swelling- and 4αPDD-induced activation of TRPV4 use distinct pathways (Arniges et al., 2004; Vriens et al., 2004). Moreover, inhibition of PLA2 activity with arachidonyl trifluoromethyl ketone (AACOCF3; 50 μM) also prevented the cilia autoregulatory response. In the presence of AACOCF3, the CBF in response to 20% dextran solutions (73 cP) dropped to 44% of the basal value (Fig. 5 c), similar to the CBF reduction observed in the absence of Ca2+ or in the presence of Gd3+ (Fig. 1 d).
Figure 5.
PLA 2 -dependent activation of TRPV4 under high viscous conditions. (a) Whole-cell currents recorded in a cell dialyzed with NMDG-Cl solutions and bathed consecutively in control solutions, 100 μM pBPB, 20% dextran + pBPB, and 1 μM 4αPDD. Basal current levels recovered after washout. (b) Average current density measured at −100 mV and +100 mV with NMDG-Cl–containing pipette solutions and Ca2+-free bathing solution under the following conditions: control (n = 16), 20% dextran (n = 16), pBPB (n = 12), and pBPB + 20% dextran (n = 12). *, P < 0.05, compared with control. (c) CBF recorded at steady-state conditions (15–25 min) under control (1 cP) and 20% dextran solutions (73 cP) in the absence or presence of 50 μM AACOCF3. Results are the mean ± SEM of 5–10 separate cultures. Significant differences (P < 0.05) between groups are marked with different letters.
In conclusion, our findings offer a first insight into the molecular basis of the mechanotransduction process required for the maintenance of CBF in high viscosity conditions. Mechanical stimulation of ciliated cells, physiologically achieved by changes in mucus viscosity, activates a TRPV4-like channel that elevates intracellular Ca2+. Channel opening requires the activity of PLA2 and occurs at high viscous loads; therefore, adapting cilia activity to a wide range of viscosities. Our results propose TRPV4 as a new target to consider in order to develop treatments for pathologies with altered mucociliary transport, including infertility and chronic respiratory diseases (Clarke, 1989; Afzelius, 1995).
Materials and methods
Chemicals and solutions
All chemicals were purchased from Sigma-Aldrich except dextran T-500 (500,000 D; Amersham Biosciences), fura-2AM (Molecular Probes), and AACOCF3 (Calbiochem). Bathing solutions used for CBF and intracellular Ca2+ measurements contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM glucose, 10 mM Hepes, pH 7.4, and 300 mosmol/Kg. Ca2+-free solutions were obtained replacing Ca2+ by Mg2+ and adding 5 mM EGTA. The viscosity of the bathing solution, a measure of the internal friction of a fluid (defined as the ratio = shear stress/shear rate), was increased by adding dextran without altering the solution's osmolality. 30% dextran solutions required a reduction of NaCl concentration (which did not affect CBF; Johnson et al., 1991) to maintain osmolality constant at 300 mosmol/Kg. Viscosity was measured using a cone-and-plate viscometer, and all the solutions used in this study behaved as Newtonian fluids (Johnson et al., 1991). After the exchange of the bathing solution by dextran solutions, the perfusion was stopped and the recordings were initiated. Alternatively, dextran solutions were superfused at a continuous flow rate of 1–3 ml/min.
Primary cultures of Golden Hamster oviductal ciliated cells
Primary cultures were obtained and maintained as described previously (Hermoso et al., 2001) and single cells were isolated by enzymatic treatment of oviductal samples using a method previously described (Lock and Valverde, 2000). Primary cultures were used when a monolayer of ciliated cells showed spontaneous ciliary activity (5–7 d) and single cell preparations were used within 48 h. Animals were maintained and experiments performed according to the guidelines issued by the Institutional Ethics Committees of the Institut Municipal d'Investigació Mèdica (Universitat Pompeu Fabra) and the Pontificia Universidad Católica de Chile.
Measurement of CBF and intracellular Ca2+
CBF was recorded using a microphotodensitometric technique (Hermoso et al., 2001). Hamster oviductal ciliated cells in culture beat spontaneously at 12.4 ± 0.9 Hz (mean ± SEM; n = 24). CBF data are expressed as a percentage of basal CBF at 1 cP (mean ± SEM). Cytosolic Ca2+ was determined at 30–37°C in cells loaded with 5 μM fura-2AM using an spectrofluorometric (Hermoso et al., 2001) or ratio-imaging technique (Fernández-Fernández et al., 2002). Cytosolic Ca2+ increases are presented as the ratio of emitted fluorescence (510 nm) after excitation at 340 and 380 nm relative to baseline.
Electrophysiology
Ionic currents were recorded in the whole-cell patch-clamp mode (Fernández-Fernández et al., 2002). Patch pipettes were filled with a solution containing 140 mM NMDG-Cl, 1 mM MgCl2, 1 mM EGTA, 10 mM Hepes, 4 mM ATP, and 0.1 mM GTP, or 20 mM CsCl, 100 mM CsAcetate, 1 mM MgCl2, 0.1 mM EGTA, 10 mM Hepes, 4 mM ATP, and 0.1 mM GTP (300 mosmol/l, pH 7.3). Cells were held at 0 mV and ramps from −140 mV to +100 mV (400 ms) were applied at a frequency of 0.2 Hz. Ramp data were acquired at 2 kHz and low-pass filtered at 1 kHz. Experiments were performed at RT (22–26°C).
Single-cell RT-PCR and transient expression of TRPV4
Single-cell RT-PCR experiments were conducted as described previously, with slight modifications (Roudbaraki et al., 1999). In brief, after whole-cell recordings in single oviductal ciliated cells, the cell contents were aspirated under visual control. The pipette content (∼8 μl) was ejected into an Eppendorf microtube with RNase inhibitor (20 U), and kept at −80°C until processed. Subsequently, one-step RT-PCR (QIAGEN) was performed using primers specific for a region of TRPV4 (GenBank/EMBL/DDBJ accession no. 263523) highly conserved among vertebrates (from exon 7 to 12, nucleotides 1382–1918). Cloning of TRPV4 from human tracheal epithelial cells and transient expression in HEK-293 and HeLa cells were performed as described by Arniges et al. (2004).
Immunodetection
Proteins from human TRPV4-transfected HEK-293 cells and hamster oviduct membranes were detected by Western blot technique using an affinity-purified polyclonal anti-human TRPV4 antibody (COOH-terminal residues CDGHQQGYPRKWRTDDAPL; dilution 1:500). Bands were visualized with HRP donkey anti–rabbit IgG (1:2,000) and chemiluminescence reagent (Supersignal West Fempto; Pierce Chemical Co.). Confocal immunofluorescence using a TCS SP microscope (oil 40×, 1.25 NA; Leica) and software (Leica) were performed in HeLa-transfected cells. Cells were fixed and permeabilized as described previously (Bahamonde et al., 2003). Cells were incubated with the TRPV4 antibody (1:3,000) and secondary Alexa 594 goat anti–rabbit (1:1,000).
Statistics
Data are expressed as the mean ± SEM. t test or ANOVA were performed with the SigmaPlot 5 or STATISTICA 6.0 programs. CBF data were analyzed after arcsin transformation. Tukey's test was used for post hoc comparison of means. The criterion for a significant difference was a final value of P < 0.05.
Acknowledgments
We thank A. Currid for proofreading the manuscript.
This work was supported by grants from the Spanish Ministerio de Ciencia y Tecnología (SAF2003-1240), Fondo de Investigación Sanitaria (Red HERACLES), and Generalitat de Catalunya (2002SGR-109) to M.A. Valverde and from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT-1040804) to M. Villalón. J.M. Fernández-Fernández is a Ramón y Cajal Fellow.
Abbreviations used in this paper: 4αPDD, 4α-phorbol 12,13-didecanoate; AACOCF3, arachidonyl trifluoromethyl ketone; CBF, ciliary beat frequency; NMDG, N-methyl-d-glucamine; pBPB, 4-bromophenacyl bromide; PLA2, phospholipase A2; TRPV4, transient receptor potential vanilloid 4.
References
- Afzelius, B.A. 1995. Role of cilia in human health. Cell Motil. Cytoskeleton. 32:95–97. [DOI] [PubMed] [Google Scholar]
- Arniges, M., E. Vazquez, J.M. Fernández-Fernández, and M.A. Valverde. 2004. Swelling-activated Ca2+-entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J. Biol. Chem. 279:54062–54068. [DOI] [PubMed] [Google Scholar]
- Bahamonde, M.I., J.M. Fernández-Fernández, F.X. Guix, E. Vazquez, and M.A. Valverde. 2003. Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated Maxi Cl− currents in C1300 neuroblastoma cells. J. Biol. Chem. 278:33284–33289. [DOI] [PubMed] [Google Scholar]
- Clapham, D.E. 2003. TRP channels as cellular sensors. Nature. 426:517–524. [DOI] [PubMed] [Google Scholar]
- Clarke, S.W. 1989. Rationale of airway clearance. Eur. Respir. J. Suppl. 7:599s–603s. [PubMed] [Google Scholar]
- Evans, J.H., and M.J. Sanderson. 1999. Intracellular calcium oscillations regulate ciliary beat frequency of airway epithelial cells. Cell Calcium. 26:103–110. [DOI] [PubMed] [Google Scholar]
- Fernández-Fernández, J.M., M. Nobles, A. Currid, E. Vazquez, and M.A. Valverde. 2002. Maxi K+ channel mediates regulatory volume decrease response in a human bronchial epithelial cell line. Am. J. Physiol. Cell Physiol. 283:C1705–C1714. [DOI] [PubMed] [Google Scholar]
- Geary, C.A., C.W. Davis, A.M. Paradiso, and R.C. Boucher. 1995. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am. J. Physiol. 268:L1021–L1028. [DOI] [PubMed] [Google Scholar]
- Halbert, S.A., P.Y. Tam, and R.J. Blandau. 1976. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 191:1052–1053. [DOI] [PubMed] [Google Scholar]
- Hermoso, M., N. Barrera, B. Morales, S. Perez, and M. Villalon. 2001. Platelet activating factor increases ciliary activity in the hamster oviduct through epithelial production of prostaglandin E2. Pflugers Arch. 442:336–345. [DOI] [PubMed] [Google Scholar]
- Jain, B., I. Rubinstein, R.A. Robbins, K.L. Leise, and J.H. Sisson. 1993. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem. Biophys. Res. Commun. 191:83–88. [DOI] [PubMed] [Google Scholar]
- Johnson, N.T., M. Villalon, F.H. Royce, R. Hard, and P. Verdugo. 1991. Autoregulation of beat frequency in respiratory ciliated cells. Demonstration by viscous loading. Am. Rev. Respir. Dis. 144:1091–1094. [DOI] [PubMed] [Google Scholar]
- Knowles, M.R., and R.C. Boucher. 2002. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 109:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley, A.B., and M.J. Sanderson. 1999. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+. Biophys. J. 77:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen, J.Y., and P.K. Kinnunen. 1995. Phospholipase A2 as a mechanosensor. Biophys. J. 68:1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke, W., Y. Choe, M.A. Marti-Renom, A.M. Bell, C.S. Denis, A. Sali, A.J. Hudspeth, J.M. Friedman, and S. Heller. 2000. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 103:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke, W., D.M. Tobin, C.I. Bargmann, and J.M. Friedman. 2003. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 100:14531–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock, H., and M.A. Valverde. 2000. Contribution of the IsK (MinK) potassium channel subunit to regulatory volume decrease in murine tracheal epithelial cells. J. Biol. Chem. 275:34849–34852. [DOI] [PubMed] [Google Scholar]
- Nilius, B., J. Prenen, U. Wissenbach, M. Bodding, and G. Droogmans. 2001. Differential activation of the volume-sensitive cation channel TRP12 (OTRPC4) and volume-regulated anion currents in HEK-293 cells. Pflugers Arch. 443:227–233. [DOI] [PubMed] [Google Scholar]
- Pedersen, S., I.H. Lambert, S.M. Thoroed, and E.K. Hoffmann. 2000. Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur. J. Biochem. 267:5531–5539. [DOI] [PubMed] [Google Scholar]
- Roudbaraki, M., A. Lorsignol, L. Langouche, G. Callewaert, H. Vankelecom, and C. Denef. 1999. Target cells of gamma3-melanocyte-stimulating hormone detected through intracellular Ca2+ responses in immature rat pituitary constitute a fraction of all main pituitary cell types, but mostly express multiple hormone phenotypes at the messenger ribonucleic acid level. Refractoriness to melanocortin-3 receptor blockade in the lacto-somatotroph lineage. Endocrinology. 140:4874–4885. [DOI] [PubMed] [Google Scholar]
- Rutllant, J., M. Lopez-Bejar, P. Santolaria, J. Yaniz, and F. Lopez-Gatius. 2002. Rheological and ultrastructural properties of bovine vaginal fluid obtained at oestrus. J. Anat. 201:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, M.J., and E.R. Dirksen. 1986. Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: implications for the regulation of mucociliary transport. Proc. Natl. Acad. Sci. USA. 83:7302–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir, P., and M.A. Sleigh. 1990. The physiology of cilia and mucociliary interactions. Annu. Rev. Physiol. 52:137–155. [DOI] [PubMed] [Google Scholar]
- Spungin, B., and A. Silberberg. 1984. Stimulation of mucus secretion, ciliary activity, and transport in frog palate epithelium. Am. J. Physiol. 247:C299–C308. [DOI] [PubMed] [Google Scholar]
- Strotmann, R., C. Harteneck, K. Nunnenmacher, G. Schultz, and T.D. Plant. 2000. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2:695–702. [DOI] [PubMed] [Google Scholar]
- Suzuki, M., A. Mizuno, K. Kodaira, and M. Imai. 2003. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278:22664–22668. [DOI] [PubMed] [Google Scholar]
- Vriens, J., H. Watanabe, A. Janssens, G. Droogmans, T. Voets, and B. Nilius. 2004. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA. 101:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, H., J.B. Davis, D. Smart, J.C. Jerman, G.D. Smith, P. Hayes, J. Vriens, W. Cairns, U. Wissenbach, J. Prenen, et al. 2002. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277:13569–13577. [DOI] [PubMed] [Google Scholar]
- Wissenbach, U., M. Bodding, M. Freichel, and V. Flockerzi. 2000. Trp12, a novel Trp related protein from kidney. FEBS Lett. 485:127–134. [DOI] [PubMed] [Google Scholar]
- Wyatt, T.A., J.R. Spurzem, K. May, and J.H. Sisson. 1998. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am. J. Physiol. 275:L827–L835. [DOI] [PubMed] [Google Scholar]
- Yang, X.C., and F. Sachs. 1989. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 243:1068–1071. [DOI] [PubMed] [Google Scholar]