Abstract
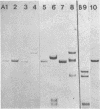
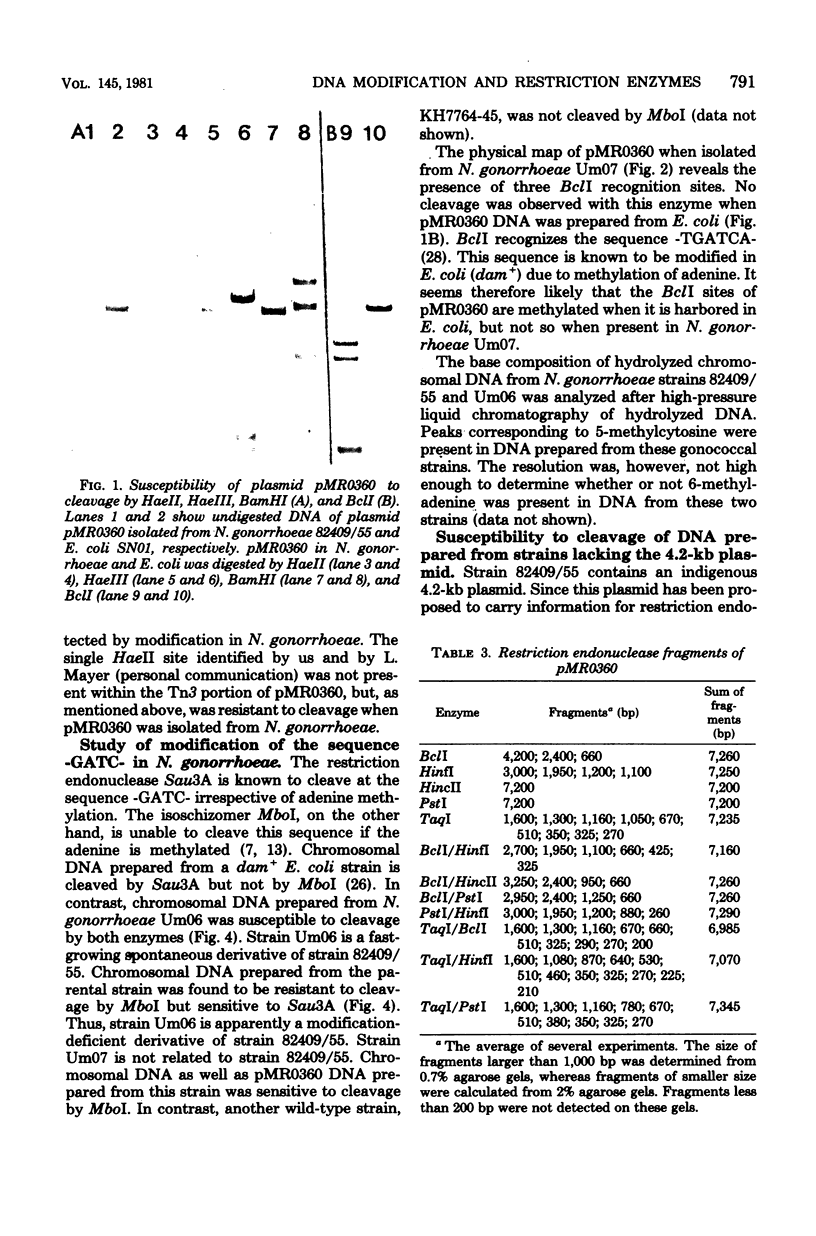
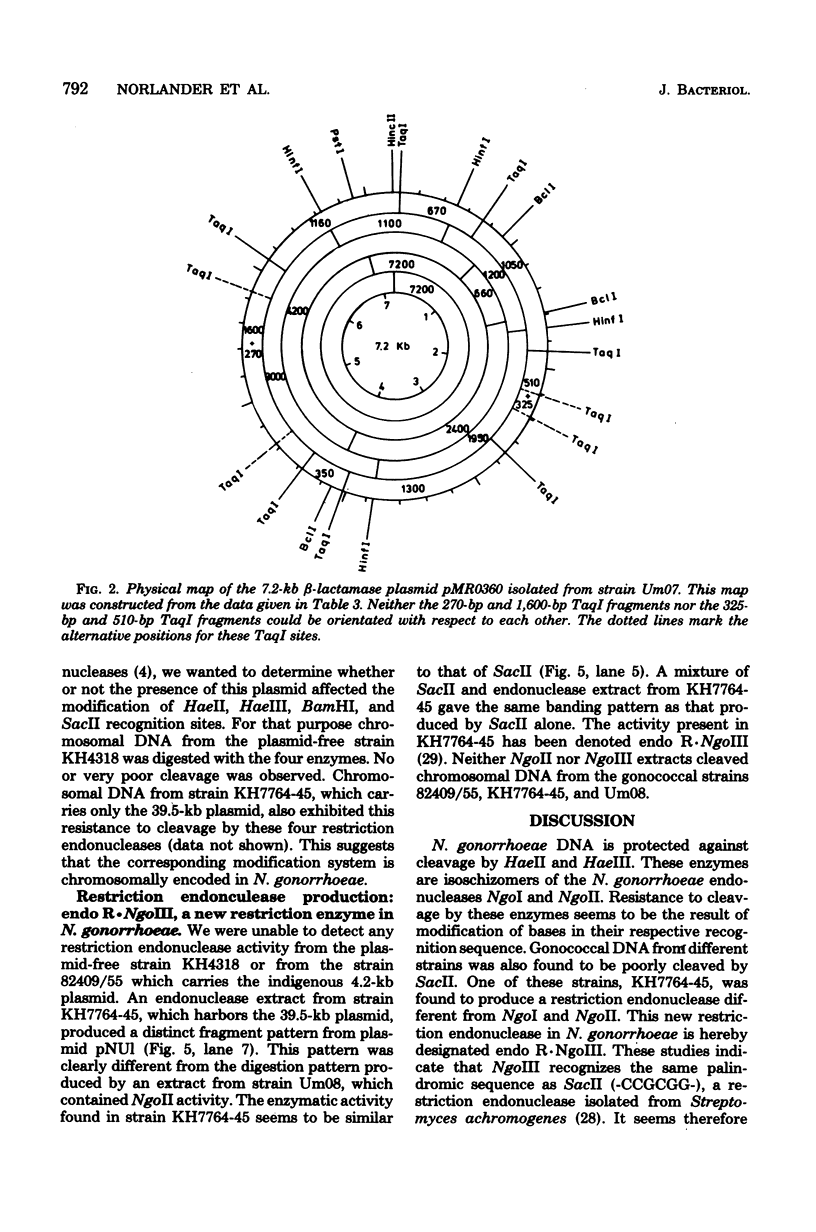
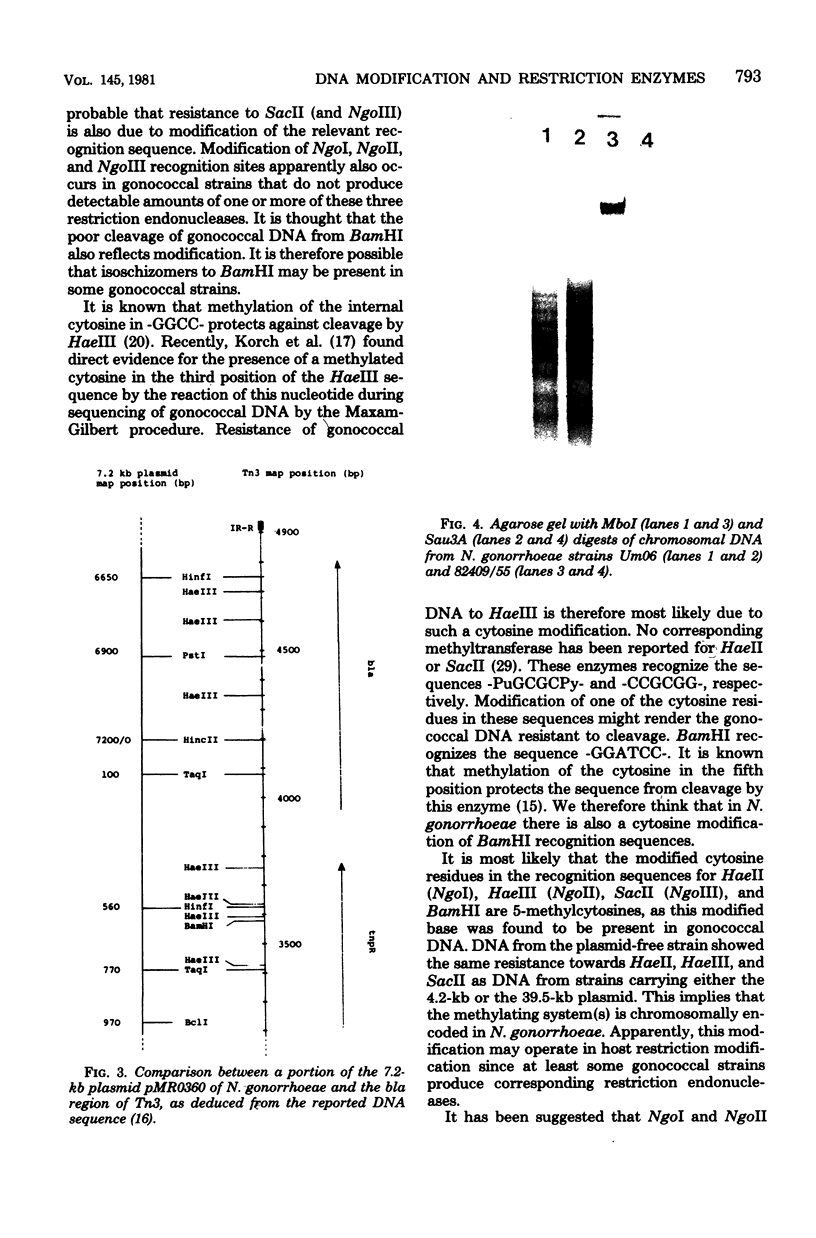
Modification of gonococcal deoxyribonucleic acid (DNA) was investigated, and the relationship with endonuclease production was explored. Both chromosomal and plasmid DNA from different gonococcal strains, irrespective of their plasmid content, was poorly cleaved by the restriction endonucleases HaeII, HaeIII, SacII, and BamHI. The fragment pattern of the Tn3 segment present on the 7.2-kilobase gonococcal resistance plasmid, when compared to its known DNA sequence, allowed us to conclude that the HaeIII and BamHI resistance was due to modification of these sites. A comparison of the fragment pattern of the resistance plasmid, when isolated from Escherichia coli or Neisseria gonorrhoeae, revealed that the resistance of HaeII must also be due to modification of its recognition sequence. Isoschizomers of HaeII and HaeIII can be found in isolates of N. gonorrhoeae (NgoI and NgoII, respectively). A new restriction endonuclease in gonococci, NgoIII, with a specificity similar to SacII, is reported here. High-pressure liquid chromatography of gonococcal DNA showed the presence of 5-methylcytosine. It is suggested that the methylation of cytosine residues in the HaeII (NgoI), HaeIII (NgoII), and SacII (NgoIII) recognition sites is the basis for the resistance of gonococcal DNA to cleavage by these enzymes. This methylation may be part of a host restriction modification system. In two out of five gonococcal strains the sequence -GATC- was modified. One strain unable to modify this sequence was a spontaneous mutant of a strain carrying such a modifying function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton D. J., Riggsby W. S., Miller R. V. NgoII, a restriction endonuclease from Neisseria gonorrhoeae. J Bacteriol. 1979 Mar;137(3):1299–1307. doi: 10.1128/jb.137.3.1299-1307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton D. J., Woodward J. M., Miller R. V. Identification of a new sequence-specific endonuclease, NgoII, from Neisseria gonorrhoeae. J Bacteriol. 1978 Jul;135(1):270–273. doi: 10.1128/jb.135.1.270-273.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Grundström T., Normark S. Isolation and characterization of DNA repetitions carrying the chromosomal beta-lactamase gene of Escherichia coli K-12. Mol Gen Genet. 1979 Jun 7;173(2):115–125. doi: 10.1007/BF00330301. [DOI] [PubMed] [Google Scholar]
- Edlund T., Grundström T., Normark S. Isolation and characterization of DNA repetitions carrying the chromosomal beta-lactamase gene of Escherichia coli K-12. Mol Gen Genet. 1979 Jun 7;173(2):115–125. doi: 10.1007/BF00330301. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Roberts M., Mayer L. W., Falkow S. Plasmid-mediated beta-lactamase production in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Mar;11(3):528–533. doi: 10.1128/aac.11.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C. W., Kuo K. C., Davis G. E., Suits R. D., Waalkes T. P., Borek E. Quantitative high-performance liquid chromatography of nucleosides in biological materials. J Chromatogr. 1978 Mar 21;150(2):455–476. doi: 10.1016/s0021-9673(00)88205-9. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Myers P. A., Roberts R. J. Two sequence-specific endonucleases from Moraxella bovis. J Mol Biol. 1977 Jul;114(1):169–179. doi: 10.1016/0022-2836(77)90290-x. [DOI] [PubMed] [Google Scholar]
- Gómez-Eichelmann M. C. Deoxyribonucleic acid adenine and cytosine methylation in Salmonella typhimurium and Salmonella typhi. J Bacteriol. 1979 Nov;140(2):574–579. doi: 10.1128/jb.140.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Keister T., Gottehrer A. Sequence specificity of DNA methylases from Bacillus amyloliquefaciens and Bacillus brevis. J Mol Biol. 1978 Oct 5;124(4):701–711. doi: 10.1016/0022-2836(78)90178-x. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Mamelak L., Boyer H. W. Genetic control of the secondary modification of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):57–62. doi: 10.1128/jb.104.1.57-62.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlander L., Davies J., Normark S. Genetic exchange mechanisms in Neisseria gonorrhoeae. J Bacteriol. 1979 Jun;138(3):756–761. doi: 10.1128/jb.138.3.756-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Edlund T., Grundström T., Bergström S., Wolf-Watz H. Escherichia coli K-12 mutants hyperproducing chromosomal beta-lactamase by gene repetitions. J Bacteriol. 1977 Dec;132(3):912–922. doi: 10.1128/jb.132.3.912-922.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M., Villani G., Boiteux S., Kinsella A. R., Glickman B. W., Spadari S. Replicational fidelity: mechanisms of mutation avoidance and mutation fixation. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):937–946. doi: 10.1101/sqb.1979.043.01.103. [DOI] [PubMed] [Google Scholar]
- Razin A., Urieli S., Pollack Y., Gruenbaum Y., Glaser G. Studies on the biological role of dna methylation; IV. Mode of methylation of DNA in E. coli cells. Nucleic Acids Res. 1980 Apr 25;8(8):1783–1792. doi: 10.1093/nar/8.8.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Falkow S. Conjugal transfer of R plasmids in Neisseria gonorrhoeae. Nature. 1977 Apr 14;266(5603):630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r63–r80. doi: 10.1093/nar/8.1.197-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Blackman E., Biswas G., Sparling P. F. Conjugative plasmids in Neisseria gonorrhoeae. J Bacteriol. 1978 Apr;134(1):278–286. doi: 10.1128/jb.134.1.278-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Sparling P. F. Transformation-derived Neisseria gonorrhoeae plasmids with altered structure and function. J Bacteriol. 1979 May;138(2):510–518. doi: 10.1128/jb.138.2.510-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vanyushin B. F., Belozersky A. N., Kokurina N. A., Kadirova D. X. 5-methylcytosine and 6-methylamino-purine in bacterial DNA. Nature. 1968 Jun 15;218(5146):1066–1067. doi: 10.1038/2181066a0. [DOI] [PubMed] [Google Scholar]