Abstract
Arf and Rab family GTPases regulate membrane traffic in cells, yet little is known about how they are targeted to distinct organelles. To identify sequences in Arf-1 necessary for Golgi targeting, we examined the localization of chimeras between Arf-1 and Arf-6. Here, we identify a 16–amino acid sequence in Arf-1 that specifies Golgi targeting and contains a motif (MXXE) that is important for Arf-1 binding to membrin, an ER-Golgi SNARE protein. The MXXE motif is conserved in all Arfs known to localize to the Golgi and enables Arf-1 to localize to the early Golgi. Arf-1 lacking these 16 aa can still localize to the late Golgi where it displays a more rapid Golgi-cytosol cycle than wild-type Arf-1. These studies suggest that membrin recruits Arf-1 to the early Golgi and reveal distinct kinetic cycles for Arf-1 at early and late Golgi determined by different sets of Arf regulators and effectors.
Introduction
A fundamental problem in membrane traffic in the biosynthetic and endocytic pathways is how organelles maintain their distinct composition of proteins and lipids in the face of continuous addition and removal of membrane. This is accomplished in part by the recruitment of cytosolic protein complexes, motors, and other components responsible for receiving and producing transport vesicles at the organelle. GTPases of the Rab and Arf families play key roles in regulating the recruitment of these protein complexes and influencing membrane lipid composition on distinct organelles (Munro, 2002), but how Rabs and Arfs target to specific organelles remains unclear.
Arfs are expressed in all eukaryotes and function in various membrane trafficking events and in the maintenance of organelle structure (Moss and Vaughan, 1995; Chavrier and Goud, 1999). There are six mammalian Arfs, and Arf-1 and Arf-6 are the least similar in amino acid sequence and the best-characterized members of the Arf family. Arf-1 is primarily localized to the Golgi complex where it regulates the binding of cytosolic coat proteins, including coat protein I (COPI), adaptor protein 1, and Golgi-localized, γ-ear–containing, Arf-binding proteins (GGA) to Golgi membranes (Bonifacino and Lippincott-Schwartz, 2003; Nie et al., 2003) and thus serves to regulate membrane traffic in the ER-Golgi system (Lippincott-Schwartz et al., 1998). Arf-6, by contrast, localizes to the plasma membrane and a membrane recycling system at the cell periphery (Donaldson, 2003).
An important characteristic of Arf-1 is that the inactive protein (Arf-1-GDP) is largely cytosolic, whereas the activated protein (Arf-1-GTP) is membrane bound. Arf proteins are all myristoylated and it has been postulated that Arf-1-GDP initially docks on the membrane through this myristoyl moiety before encountering the guanine nucleotide exchange factor (GEF) that catalyzes exchange of GTP for GDP (Antonny et al., 1997). Arf-1-GTP associates more tightly with the membrane and can then recruit coat proteins to the membrane until it encounters a GTPase-activating protein (GAP) that stimulates GTP hydrolysis and dissociation of Arf-1-GDP from the membrane.
In biochemical assays, all Arf proteins share common effector functions including: activation of phospholipase D (Liang et al., 1997) and phosphatidylinositol 4-phosphate 5-kinase (Honda et al., 1999), recruitment of COPI to membranes (Liang and Kornfeld, 1997), and binding to GGA (Takatsu et al., 2002). Yet in cells, Arfs have distinct functions and localizations. Arf-1 localizes to the Golgi complex, regulates the assembly of coat proteins onto membranes and mediates traffic in the ER-Golgi system (Bonifacino and Glick, 2004). Arf-6 localizes to the endosomal/plasma membrane system and not the Golgi and thus has no effect on regulating the assembly of COPI onto the Golgi complex in vivo (Peters et al., 1995). Thus, it is through organelle-specific targeting that Arf proteins attain their unique cellular functions.
In this study, we were interested in identifying amino acid residues in the Arf-1 sequence that determine its specificity for Golgi targeting and distinguish it from other Arf proteins, especially Arf-6. Because Arf proteins are highly conserved in structure (Goldberg, 1998; Pasqualato et al., 2001), we followed an approach that has been used by us and others, generating chimeric Arfs by exchanging amino- and carboxy-terminal portions of Arf-1 and Arf-6 and expressing them in cells (Liang et al., 1997; Jones et al., 1999; Al-Awar et al., 2000). As a result of this study, we identified a short sequence in Arf-1 containing a Golgi targeting signal, and in the sequence we identified a motif (MXXE) that is critical in vitro and in vivo for Arf-1 binding to membrin, an ER-Golgi SNARE protein. Because the MXXE motif enables Arf-1 to localize to the early Golgi, we suggest that membrin is an Arf-1 receptor at the early Golgi.
Results
Identification of the Golgi targeting sequence in Arf-1
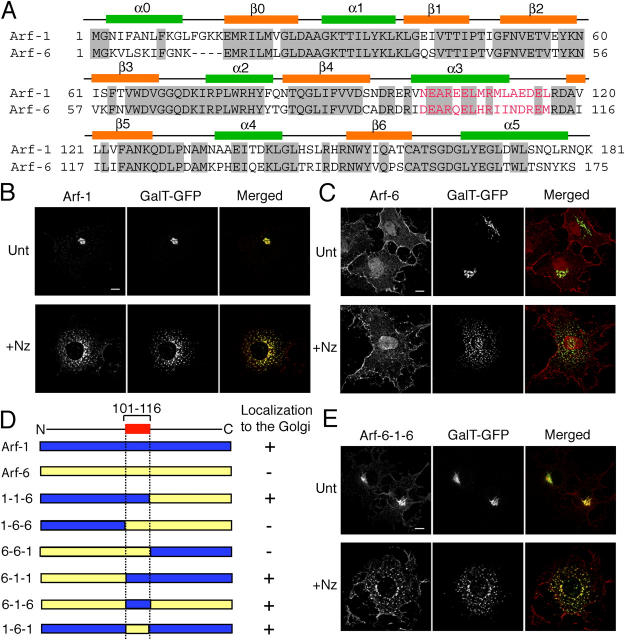
A comparison of human Arf-1 and Arf-6 is shown in Fig. 1 A where identical amino acid residues are shaded in gray and positions of the α-helices and β-sheets derived from structural studies (Amor et al., 1994; Menetrey et al., 2000) are indicated. To identify sequences in Arf-1 that are necessary for its Golgi localization, chimeric proteins of Arf-1 and Arf-6 were constructed and their localization was examined in COS-7 cells. Full-length Arf-1 localized to the juxtanuclear region in a pattern similar to that of galactosyltransferase (GalT)-GFP, a chimera of GFP and the transmembrane domain of a Golgi resident enzyme, GalT (Fig. 1 B, Unt). The Golgi localization of Arf-1 was also observed when cells were treated with nocodazole, a microtubule-disrupting drug that causes Golgi fragmentation (Fig. 1 B, +Nz). On the other hand, in cells overexpressing Arf-6, Arf-6 localized primarily to the plasma membrane, although some juxtanuclear labeling near the Golgi region was also observed (Fig. 1 C, Unt). This juxtanuclear staining was clearly not the Golgi because Arf-6 did not label the Golgi fragments after nocodazole treatment (Fig. 1 C, +Nz).
Figure 1.
Subcellular localization of Arf chimeras. (A) Numbering and secondary structural elements (α helices and β sheets) of human Arf-1 and Arf-6 are shown. Identical residues between Arf-1 and Arf-6 are shaded in gray. Residues marked in red indicate amino acids 101–116 and 97–112 of Arf-1 and Arf-6, respectively. (B, C, and E) COS-7 cells overexpressing GalT-GFP and HA-tagged Arf-1 (B), untagged Arf-6 (C), or untagged Arf-6-1-6 (E) were untreated (Unt) or incubated in the presence of 20 μg/ml nocodazole for 2 h (+Nz). Cells were fixed and immunolabeled with antibodies against HA (B) or Arf-6 (C and E) followed by Alexa 594 anti–mouse and anti–rabbit antibodies, respectively. Bars, 10 μm. (D) Diagrams of chimeras of Arf-1 and Arf-6 are shown. Blue and yellow regions represent Arf-1 and Arf-6 sequences, respectively. + and − indicate ability to localize to the Golgi.
As summarized in Fig. 1 D, all the chimeras that included amino acids 101–116 of Arf-1 localized to the Golgi (Fig. 1 D, 1-1-6, 6-1-1, and 6-1-6). On the other hand, 1-6-6 and 6-6-1, which lacked this sequence did not localize to the Golgi (Fig. 1 D). Most strikingly, Arf-6-1-6, the chimera containing amino acid residues 101–116 of Arf-1 inserted into the Arf-6 sequence (Fig. 1 D), clearly localized to the Golgi, even after nocodazole treatment (Fig. 1 E), although some plasma membrane labeling was also observed. These results show that residues 101–116 of Arf-1 are sufficient to target Arf-6 to the Golgi. However, we observed that Arf-1-6-1, which contains amino acids 97–112 of Arf-6, also localized to the Golgi (Fig. 1 D), indicating that additional sequences in the amino- and carboxyl-terminal halves of Arf-1 could together confer Golgi localization. We made similar observations with these Arf chimeras expressed in HeLa and NRK cells (unpublished data).
Arf-6-1-6 and Arf-1-6-1 are activated by a BFA-sensitive GEF and inactivated by a GAP on the Golgi membrane
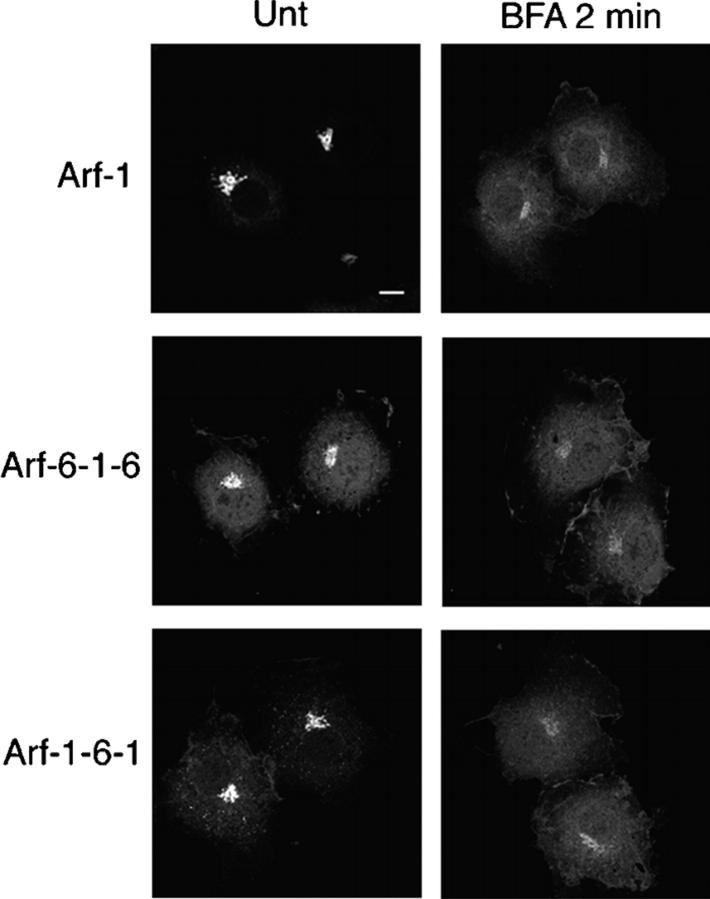
An important characteristic of Arf-1 is that the inactive, GDP-bound protein is in the cytosol whereas the active, GTP-bound protein is membrane bound, and cycling of Arf-1 between its activated and inactivated states is critical for Arf-1 function. When cells are treated with brefeldin A (BFA), a fungal toxin that inhibits Arf-GEFs that are associated with the Golgi complex (Jackson and Casanova, 2000), Arf-1 dissociates from Golgi membranes because activation of Arf-1 by GEF is inhibited, whereas GAP continues to inactivate Arf-1. To examine whether Arf-6-1-6 and Arf-1-6-1 can serve as substrates for GEFs and GAPs on the Golgi membrane like Arf-1, the dissociation of these Arf chimeras from the Golgi after addition of BFA were compared with that of Arf-1. In untreated COS-7 cells, clear Golgi localizations of Arf-6-1-6-GFP and Arf-1-6-1-GFP were observed (Fig. 2, Unt). After addition of BFA, both Arf-GFPs dissociated from the Golgi as rapidly as Arf-1-GFP (Fig. 2, BFA 2 min). These results show that both Arf-6-1-6 and Arf-1-6-1 can be inactivated through GTP hydrolysis by the GAP on the Golgi membrane. Furthermore, it suggests that activation of Arf-6-1-6 and Arf-1-6-1 is catalyzed by a BFA-sensitive Golgi GEF because neither Arf chimeras bound to the Golgi in the presence of BFA. Similar observations were made in HeLa cells (unpublished data).
Figure 2.
BFA-induced redistribution of Arf-6-1-6 and Arf-1-6-1. COS-7 cells overexpressing GFP-fused Arf-1, Arf-6-1-6, or Arf-1-6-1 were untreated (Unt) or incubated in the presence of 5 μg/ml BFA for 2 min and fixed. Bar, 10 μm.
110MXXE113 of Arf-1 is critical for Golgi targeting
To identify the amino acid residues in the Arf-1 sequence of Arf-6-1-6 that are necessary for Golgi targeting, we made amino acid substitutions converting the Arf-1 to the Arf-6 residues. There are 9 aa residues that are different between Arf-1 and Arf-6 in this region (Fig. 3 A). Substitutions of Arf-1 residues 101N, 105E, 108M, and 112A to Arf-6 residues D, Q, H, and N, respectively (6-1-6-a, Fig. 3 A), had no significant effect on Golgi localization (Fig. 3 B). In addition, single replacement of 110M, 111L, 113E, 114D, or 116L of 6-1-6-a with corresponding residues of Arf-6 revealed that the efficiency of Golgi localization of 6-1-6-a was significantly decreased if 110M or 113E were changed (Fig. 3, A and B, 6-1-6-b and 6-1-6-d). These results demonstrate that 110MXXE113 in Arf-1 is critical for the Golgi targeting information contained in helix α3. Examination of Arf sequences from yeast to human reveals that the MXXE motif is conserved in all class I Arfs (Fig. 3 C), with all that have been examined localizing to the Golgi membranes (Stearns et al., 1990; Takeuchi et al., 2002). It is also present in human Arf-like protein 1 (Arl1) that is also associated with the Golgi (Icard-Liepkalns et al., 1997; Lu et al., 2001), but is not present in other Arls. Importantly, this motif is absent in all class III Arfs (Fig. 3 C), which do not localize to the Golgi.
Figure 3.

Identification of critical amino acids in residues 101–116 of Arf-1 for Golgi targeting of the Arf-6-1-6 chimera. (A) Sequence comparison of residues 101–116 of Arf-1 and corresponding residues of Arf-6 and mutants of Arf-6-1-6 are shown. Bold characters indicate residues unique to Arf-6. (B) COS-7 cells overexpressing GalT-GFP and the Arf constructs indicated were fixed and immunolabeled with antibodies against Arf-6 followed by Alexa 594 anti–rabbit antibodies. 100 cells were counted and the fraction of transfected cells that show clear colocalization of overexpressed Arf proteins with GalT-GFP was noted. The result shown is the mean ± SD for three experiments. (C) Sequence comparison of residues 101–116 of Arf-1 and corresponding residues of related proteins are shown. Bold characters indicate residues identical to 110M and 113E of Arf-1.
To show more clearly that amino acids 101–116 of Arf-1 contain sufficient information for Golgi targeting, 101–116 of Arf-1 was fused to the carboxyl terminus of GFP (Fig. 4 A) and the subcellular localization of the fusion protein was examined in HeLa cells. Strikingly, in some cells expressing the GFP fusion protein at low to moderate expression levels, juxtanuclear localization of GFP-Arf-1(101–116) was observed that colocalized with β-COP, a subunit of COPI (Fig. 4 B). Although the efficiency of Golgi targeting of GFP-Arf-1(101–116) was low (observed in 5–10% of transfected cells), this result confirmed that 101–116 of Arf-1 is sufficient by itself to target to the Golgi, because GFP alone localized to the cytosol and never showed juxtanuclear localization (unpublished data). Moreover, in cells expressing GFP-Arf-1(101–116) at a higher level and showing juxtanuclear localization of the GFP fusion protein, the distribution of GM130, a Golgi matrix protein, was clearly different than in untransfected cells. In untransfected cells, GM130 localized to interconnected, ribbon-shaped elements (Fig. 4 C, inset I). This pattern was also observed in Arf-1-GFP–transfected cells (not depicted). In contrast, in cells showing juxtanuclear localization of GFP-Arf-1(101–116), the distribution of GM130 was changed into punctate structures (Fig. 4 C, inset II). Moreover, high level expression of GFP-Arf-1(101–116) caused COPI to dissociate from the Golgi complex (Fig. 4 D). These results suggest that overexpressed GFP-Arf-1(101–116) competes with endogenous Arf-1 for binding to the Golgi, and inhibits Arf-1 regulation of maintenance of Golgi structure and recruitment of COPI onto the Golgi complex.
Figure 4.

Amino acid residues 101–116 of Arf-1 are targeted to the Golgi. (A) Schematic representation of GFP-Arf-1(101–116). (B–D) HeLa cells expressing GFP-Arf-1(101–116) were fixed and immunolabeled with antibodies against β-COP (B and D) or GM130 (C) followed by Alexa 594 anti–rabbit and anti–mouse antibodies, respectively. Cells expressed GFP-Arf-1(101–116) at low to moderate level (B) or high level (C and D). The transfected cells in C and D are outlined. Images I and II in C show magnified views of boxed areas. Bars, 10 μm.
Arf-1, but not Arf-1-6-1, binds to membrin on Golgi membranes
In an attempt to identify the receptor on Golgi membranes that specifically recognizes 101–116 of Arf-1, we considered potential candidate receptors. Recently, Rein et al. (2002) showed that SNAREs may act as an Arf receptor on membranes in yeast. Using in vitro binding assays, they showed that Arf1p directly binds Bet1p, Sec22p, and Bos1p, SNAREs that function in transport pathways between the ER and the Golgi. The association with Bet1p is independent of the nucleotide bound to Arf1p (Rein et al., 2002), suggesting that the SNARE recruits Arf1p-GDP to membranes where the exchange of GDP to GTP, and thus the activation of Arf1p, takes place.
To test the possibility that those SNAREs act as Golgi membrane receptors for Arf-1 in mammalian cells, we coexpressed rbet1, sec22b, membrin, and syntaxin5, which are the mammalian homologues of Bet1p, Sec22p, Bos1p, and Sed5p, respectively, with Arf-1-GFP in HeLa cells. As reported (Hay et al., 1996, 1998), rbet1, membrin, and syntaxin5 mainly localized to the Golgi whereas sec22b localized to the ER. We noticed that, although Arf-1 generally colocalized with all of the Golgi-localized SNAREs (not depicted), Arf-1 localization was tightly coincident with membrin (Fig. 5 E), raising the possibility that Arf-1-GFP was directly binding to the overexpressed membrin and that membrin may be serving as an Arf-1 receptor in vivo.
Figure 5.
Arf-1, but not Arf-1-6-1, interacts and colocalizes with membrin. (A–D) His6-tagged Arf-1 and Arf-1-6-1, and Arf-1M110I, E113D were incubated with lysate from HeLa cells expressing myc-membrin in the presence of GDP. A cross-linking reagent DSP was added where indicated. Arfs were recovered and proteins were analyzed by immunoblotting. Shown is one experiment representative of three (A) or two (B) performed with similar results. In C and D, relative amount of membrin bound to His6-Arf for replicate experiments was determined using a densitometer. (E and F) HeLa (E) or NRK (F) cells overexpressing myc-membrin and Arf-1-GFP or Arf-1-6-1-GFP were fixed and immunolabeled with antibodies against myc followed by Alexa 594 anti–mouse antibodies. Bar, 10 μm. (F) Quantitative analysis of area overlap in the Golgi region for NRK cells as described in Materials and methods. Error bars are the mean ± SD.
To investigate whether membrin specifically interacts with Arf-1 and could be the basis for Golgi targeting of Arf-1, we used in vitro binding experiments. Arf-1 was expressed in E. coli as His6-tagged proteins where the amino-terminal 17 aa were replaced by the His6 affinity tag. We incubated affinity-purified Arf proteins with HeLa cell lysate expressing membrin in the presence of GDP. Assuming that the interaction between Arf-1 and its receptor would not be strong, we used a cross-linking reagent to cross-link the proteins bound to Arf. As shown in Fig. 5 (A and C), membrin efficiently bound to Arf-1 in the presence of the cross-linker. However, Arf-1-6-1, which lacks residues 101–116 of Arf-1 yet still localizes to the Golgi (Fig. 1 D), did not bind to membrin. Furthermore, when 110M and 113E (110MXXE113) were substituted with corresponding residues of Arf-6, the Arf-1 mutant failed to bind membrin (Fig. 5, B and D). We know that these mutations do not affect the general conformation of His6-Arf-1, because both His6-Arf-1-6-1 and His6-Arf-1M110I, E113D bound to the VHS-GAT domain of GGA as well as wild-type Arf-1 in the presence of GTPγS (Fig. S1, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200409138/DC1). Moreover, circular dichroism (CD) spectra of these Arf-1 mutants were essentially identical to that of Arf-1 (Fig. S1 C). Although we could detect binding of detergent-solubilized membrin to His6-Arf-1, we could not obtain binding using recombinant membrin (unpublished data). This might indicate that other proteins or membrane lipids are required for the interaction. Together, these results demonstrate that Arf-1-GDP binds membrin, and the association with membrin requires the MXXE motif of Arf-1.
Having demonstrated that Arf-1 can specifically bind to solubilized membrin, we examined more carefully the extent of colocalization of Arf-1 and membrin in intact cells. In HeLa cells, we observed a high degree of colocalization between Arf-1 and membrin (Fig. 5 E). However, colocalization of Arf-1-6-1 with membrin was partial and some membrin-positive structures were clearly devoid of Arf-1-6-1 (Fig. 5 E, inset, arrowheads). We observed similar immunolocalization results in NRK cells where we quantified the amount of colocalization (see Materials and methods), and found that 80% of Golgi-associated Arf-1 colocalized with membrin whereas only 41% of Golgi-associated Arf-1-6-1 colocalized with membrin (Fig. 5 F). Importantly, if we mutated Arf-1-6-1 such that it now contained the MXXE motif, 75% colocalization between Arf-1-6-1MXXE and membrin was now observed (Fig. 5 F). We also observed near complete colocalization of membrin with GFP-Arf-1(101–116) (unpublished data). These results suggest that Arf-1 can bind to membrin in vitro and in vivo but requires the MXXE motif. Arf-1-6-1, on the other hand, does not interact with membrin and thus must localize to the Golgi by an alternative mechanism.
The finding that Arf-1 could bind to membrin, both in cells and in in vitro binding assays, suggested that Golgi-associated membrin could act as the initial receptor for Arf-1-GDP before GEF-catalyzed nucleotide exchange. If this is true, it is possible that overexpression of membrin would partially protect Arf-1 from release from Golgi membranes during BFA treatment. When Arf-1 or Arf-1-6-1 was expressed alone in COS-7 cells, a significant amount of Arf dissociated from the Golgi within 2 min after BFA addition (Fig. 2, BFA 2 min). However, if membrin was coexpressed with Arf-1, Arf-1 labeling of the Golgi was still apparent after 2 min of BFA addition (Fig. 6 A). Even after 4 min, Golgi localization of Arf-1 was still prominent in membrin-transfected cells (Fig. 6 A, cell marked with arrowhead), whereas the bulk of the Arf-1 was dissociated in cells not coexpressing membrin (Fig. 6 A, cell marked with arrow). This association of Arf-1 with membrin was lost as Golgi membranes redistributed into the ER (see below). On the other hand, in Arf-1-6-1–transfected cells, overexpressed membrin did not protect the dissociation of Arf-1-6-1 from the Golgi by BFA (Fig. 6 A), suggesting that the 16 aa of Arf-1 including the MXXE motif were required for this effect. Overexpression of membrin did not affect COPI dissociation after BFA addition (Fig. 6 B), indicating that “protection” of Arf-1 dissociation did not lead to GTP loading onto Arf-1. Because rbet1 and syntaxin5 also showed Golgi localization in mammalian cells, we examined whether overexpression of rbet1 or syntaxin5 would protect Arf-1 from dissociation by BFA. As shown in Fig. 6 C, regardless of the level of expression, neither rbet1 nor syntaxin5 affected the distribution of Arf-1 after BFA addition, indicating that overexpression of these SNAREs did not retain Arf-1 at the Golgi in COS-7 cells. We made similar observations in HeLa cells and quantified the amount of Arf-1 and Arf-1-6-1 associated with the Golgi before and after BFA in cells coexpressing these ER-Golgi SNAREs (Fig. 6, D and E). In untreated cells, ∼17% of total cellular Arf-1 was associated with the Golgi and this dropped to 6% after 2 min BFA (Fig. 6 D, control). In cells expressing membrin however, Arf-1 associated with the Golgi drops only to 12% after BFA (Fig. 6 D, +membrin). On the other hand, neither rbet1 nor syntaxin5 expression affected the distribution of Arf-1 after BFA addition (Fig. 6 D, +rbet1 and +syntaxin5). In Arf-1-6-1–transfected cells, dissociation from the Golgi by BFA was similar in all cases and not affected by expression of any SNARE (Fig. 6 E). Together, these results indicate that membrin can specifically recruit Arf-1-GDP to Golgi membranes and transiently “protect” Arf-1-GDP from BFA-induced dissociation. Furthermore, that it is Arf-1-GDP that is binding to membrin is supported by the observation that Arf-1 Q71L did not colocalize with membrin in cells (unpublished data).
Figure 6.

Membrin “protects” Arf-1 from BFA-induced redistribution. (A–C) COS-7 cells overexpressing myc-membrin and Arf-1-GFP or Arf-1-6-1-GFP (A), myc-membrin and Arf-1-HA (B), or Arf-1-GFP and myc-rbet1 or HA-syntaxin5 (C) were untreated (Unt) or incubated in the presence of 5 μg/ml BFA for indicated times and fixed. Cells were immunolabeled with antibodies against myc (A), HA and β-COP (B), or myc or HA (C) followed by Alexa 594 anti–mouse (A and C), or Alexa 488 anti–mouse and Alexa 594 anti–rabbit (B) antibodies. Bars, 10 μm. (D and E) HeLa cells overexpressing Arf-1-GFP alone (D, control) or Arf-1-6-1-GFP alone (E, control), or with myc-membrin or myc-rbet1, or HA-syntaxin5 were untreated or incubated in the presence of 5 μg/ml BFA for 2 min and fixed and processed for immunolabeling with antibodies against myc or HA followed by Alexa 594 anti–mouse and anti–rabbit antibodies, respectively. To quantify Golgi and non-Golgi pools of Arf-1-GFP, one region of interest was drawn around Golgi and the other region of interest was drawn around the rest of the cell (representing total cellular fluorescence). The mean fluorescence intensity associated with the Golgi and total cell was measured for Arf-1-GFP. The total Arf-1-GFP fluorescence associated with the Golgi was expressed as a fraction of the total cellular fluorescence, and this procedure was repeated for 10 cells. Error bars are the mean ± SD.
Arf-1 and membrin colocalize on COPI-coated transport structures between the ER and Golgi
Having demonstrated that membrin appears to represent an initial Arf-1-GDP binding site on Golgi membranes, we wondered where the interaction occurs. Membrin is involved in the membrane transport pathway between the ER and the Golgi complex (Hay et al., 1997). Arf-1 also has been shown to function in this system (Dascher and Balch, 1994; Ward et al., 2001). These findings raise the possibility that both proteins might interact on transport structures from the ER to the Golgi. Discerning Arf-1 localization on peripheral, incoming ER to Golgi transport structures was difficult due to the Arf-1 presence in the cytosol. Therefore, we examined the distribution of membrin and Arf-1 during recovery from BFA in NRK cells, a process that involves rapid and massive anterograde membrane transport from the ER, resulting in reformation of the Golgi complex (Lippincott-Schwartz et al., 1990). After 30 min of BFA treatment, Arf-1 was cytosolic, whereas membrin localized to small, peripheral structures (Fig. 7 A, BFA) that colocalized with ERGIC53/p58 (unpublished data) and thus were ER exit sites (Tang et al., 1995; Ward et al., 2001). 10 min after BFA wash out, Arf-1 now colocalized with membrin on scattered peripheral structures (Fig. 7 A, WO 10 min). β-COP was also distributed on those structures, indicating that Arf-1 was activated and recruited COPI onto the structures (Fig. 7 B). After 15 min of recovery, most of the membrin and Arf-1 colocalized on larger juxtanuclear structures, although some small, peripheral structures were still observed (Fig. 7 A, WO 15 min). Arf-1-6-1 also showed cytosolic distribution in BFA-treated cells similar to Arf-1 (Fig. 7 A, BFA). However, 10 min after BFA wash out, the distribution of Arf-1-6-1 was still cytosolic, whereas membrin was observed on peripheral structures (Fig. 7 A, WO 10 min). Although some Arf-1-6-1 weakly colocalized with membrin on the juxtanuclear structures after 15 min of recovery, a major part of Arf-1-6-1 was still cytosolic and some membrin-positive transport structures were clearly devoid of Arf-1-6-1 (Fig. 7 A, WO 15 min, inset, arrowheads). After a 20-min recovery, Arf-1-6-1 could be observed to colocalize with membrin on the fully reassembled, juxtanuclear Golgi complex (Fig. 7 A, WO 20 min). Similar recruitment of Arf-1, but not Arf-1-6-1, to reforming Golgi structures was observed in COS-7 cells (unpublished data). The delay in recruitment of Arf-1-6-1 to the reforming Golgi did not slow Golgi recovery because endogenous Arf-1 could be recruited. Together, these results demonstrate that these 16 aa and the MXXE motif of Arf-1 allow Arf-1 recruitment to membrin-containing transport structures bound for the Golgi complex.
Figure 7.
Anterograde movement of membrin and Arf-1 into the Golgi in BFA-recovering cells. (A) NRK cells overexpressing myc-membrin and Arf-1-GFP or Arf-1-6-1-GFP were incubated in the presence of 5 μg/ml BFA for 30 min, washed free of BFA, and incubated in the absence of BFA for the indicated times of wash out (WO) and fixed. Cells were immunolabeled with antibodies against myc followed by Alexa 594 anti–mouse antibodies. (B) NRK cells overexpressing myc-membrin were treated as in A and fixed. Cells were immunolabeled with antibodies against myc and β-COP followed by Alexa 488 anti–mouse and Alexa 594 anti–rabbit antibodies. Bars, 10 μm.
Arf-1-6-1 localizes to the trans-Golgi, but not to the cis-Golgi, and is regulated differently from Arf-1
Because Arf-1-6-1 did not associate with reforming Golgi during BFA wash out (Fig. 7 A), we wondered how and where Arf-1-6-1 is recruited to the Golgi. The Golgi complex can be divided into cis- and trans-sides, shown to have distinct functions (Bonifacino and Glick, 2004). Golgi-specific BFA resistance factor 1 (GBF1) and BFA-inhibited GEF (BIG) 1/2 are Arf-GEFs known to localize mainly to cis-and trans-elements of the Golgi, respectively, and limited overlap between the distribution of GBF1 and BIG1 has been observed (Yamaji et al., 2000; Kawamoto et al., 2002; Shinotsuka et al., 2002a,b; Zhao et al., 2002). Indeed, double staining of NRK cells with antibodies for GBF1 and BIG1 confirmed the feasibility of distinguishing the distributions of these Arf-GEFs at the light microscopy level (unpublished data). To identify more specifically the distributions of Arf-1 and Arf-1-6-1 in the Golgi complex, we compared their distributions to the localizations of GBF1 or BIG1. The distribution of Arf-1 overlapped extensively with that of GBF1 with nearly superimposable images (Fig. 8 A). By contrast, Arf-1-6-1 overlapped less with GBF1 (Fig. 8 A). Quantifying the extent of colocalization revealed that 82% of the Golgi-associated Arf-1 colocalized with GBF1 whereas only 38% of the Golgi-associated Arf-1-6-1 colocalized with GBF1 (Fig. 8 B). On the other hand, Arf-1-6-1 seemed to colocalize more with BIG1 at the trans-Golgi than did Arf-1 (59% and 38% overlapping, respectively; Fig. 8, C and D). Importantly, mutagenesis of Arf-1-6-1 to include MXXE shifted the colocalization of Arf-1-6-1MXXE back to that observed with Arf-1, 72% colocalized with GBF1 (Fig. 8 B) and 38% colocalized with BIG1 (Fig. 8 D). These results demonstrate that Arf-1-6-1 localizes mainly to the trans-Golgi, and not to the cis-Golgi. Because the distribution of membrin was almost identical to that of GBF1, whereas membrin did not colocalize well with BIG1 (unpublished data), these results suggest that interaction with membrin may be required for Arf-1 to localize to the cis-Golgi.
Figure 8.
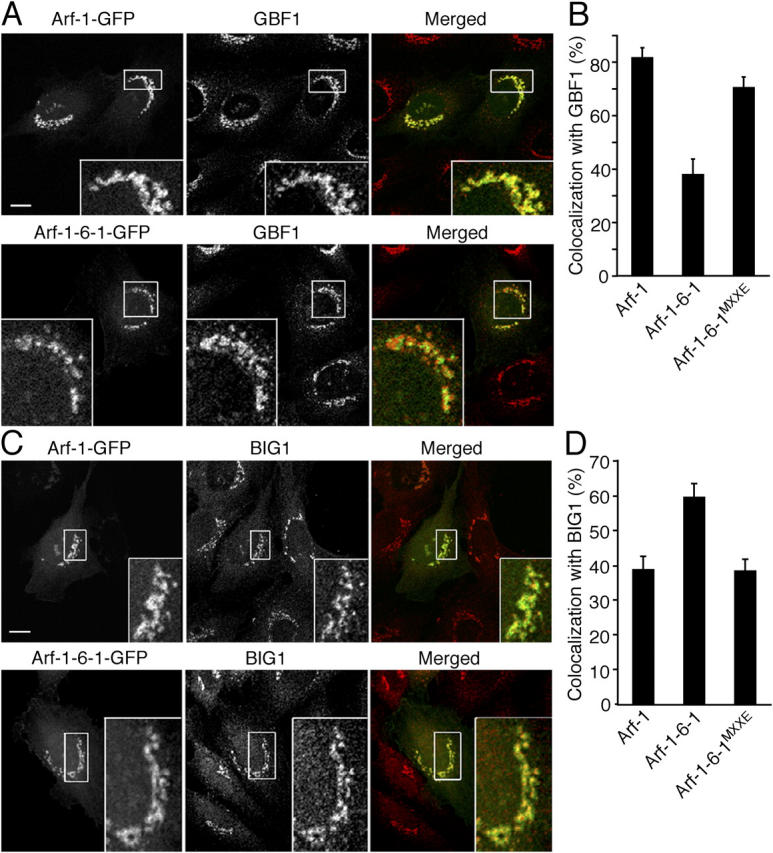
Comparison of the distribution of Arf-1 and Arf-1-6-1 to Arf-GEFs. NRK cells overexpressing GFP-fused Arf-1, Arf-1-6-1, and Arf-1-6-1MXXE were fixed and immunolabeled with antibodies against GBF1 (A) or BIG1 (C) followed by Alexa 594 anti–mouse and anti–rabbit antibodies, respectively. Bars, 10 μm. (B and D) Quantitative analysis of area overlap in the Golgi region as in A and C. Error bars are the mean ± SD.
Although our data suggest that Arf-1 binding to membrin is the mechanism for recruitment of Arf-1 to the early Golgi, it is possible that Arf-1 is recruited to the cis-Golgi through direct interaction and activation by GBF1. Arf-1-6-1 might not bind to the early Golgi because it cannot interact with GBF1 due to its Arf-6 sequence. Indeed, some Arf-GEFs may recognize subtle folding differences between Arf-1 and Arf-6 explaining their selectivity for different Arfs (Macia et al., 2001). To examine whether Arf-1-6-1 can be activated by GBF1, we measured the amount of GTP-bound Arf-1 and Arf-1-6-1 in cells overexpressing HA-tagged GBF1 (see Materials and methods). As shown in Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200409138/DC1), overexpressed GBF1 activated Arf-1-6-1, although not to the same extent as Arf-1, suggesting that Arf-1-6-1 can be activated by GBF1 in cells.
In addition to GEFs, distinct GAPs may regulate Arf-1 at the Golgi in mammalian cells (Cukierman et al., 1995; Miura et al., 2002). It is likely that distinct GEFs and GAPs regulate Arf-1 at cis- and trans-elements of the Golgi complex. Having demonstrated that Arf-1-6-1 localizes mainly to the trans-Golgi, and not to the cis-Golgi, we wondered if Arf-1-6-1 is regulated differently from Arf-1. FRAP is a powerful technique to examine the kinetics of dissociation and association of Arf-1 at the Golgi in live cells (Vasudevan et al., 1998; Presley et al., 2002). Because the kinetics reflect GAP and GEF activities on Arf-1, we examined the FRAP of the Golgi region in Arf-1-GFP- and Arf-1-6-1-GFP–transfected cells. Representative FRAP kinetic data are shown in Fig. 9 A. The recovery of Arf-1-GFP fluorescence showed kinetics (t 1/2 = 20 s) similar to previous studies (Presley et al., 2002). By contrast, the recovery kinetics of Arf-1-6-1-GFP (t 1/2 = 10 s) was much more rapid, indicating that Arf-1-6-1 spends less time on the Golgi per cycle than Arf-1. Because Arf-1-6-1 primarily localizes to the late Golgi (Fig. 8), the difference in the FRAP data for Arf-1 and Arf-1-6-1 suggests that Arf-1 cycling at the trans-Golgi might be faster than at the cis-Golgi. We hypothesized that failure to bind to membrin at the early Golgi leads to Arf-1-6-1 being activated and inactivated at the late Golgi with more rapid kinetics. To test this, we measured the FRAP kinetics of Arf-1-6-1MXXE-GFP and observed that Arf-1-6-1MXXE-GFP now exhibited FRAP kinetics (t 1/2 = 20 s) identical to that of wild-type Arf-1 (Fig. 9 A).
Figure 9.

Arf-1 and Arf-1-6-1 are regulated differently at Golgi membranes. (A) The kinetics of recovery of GFP-fused Arf-1, Arf-1-6-1, and Arf-1-6-1MXXE in the Golgi region after photobleaching are shown. The Golgi regions of cells expressing Arfs-GFP were photobleached at maximal laser intensity. Immediately after the bleach, cells were scanned every 5 s. The fluorescence intensity in the bleach area was measured. Shown is one experiment representative of three performed with similar results. (B) Models for membrane association/dissociation cycle of Arf-1 and Arf-1-6-1. See text for details.
Discussion
We have identified a 16–amino acid region in Arf-1 that is necessary and sufficient for Arf-1 targeting to the early Golgi. These 16 aa include helix α3 (Fig. 1 A) and contains a motif (MXXE) that is found in all Arfs known to associate with the Golgi complex, and in all eukaryotes examined. Membrin, an ER-Golgi SNARE, binds Arf-1-GDP via the helix α3 and is responsible for recruiting Arf-1 to the early Golgi. An Arf-1 mutant lacking this region (Arf-1-6-1) cannot bind membrin and does not localize to the early Golgi although it does associate with the late Golgi through other means that require both amino- and carboxyl-portions of the protein. Although SNAREs are believed to function in membrane fusion events (Hay, 2001; Bonifacino and Glick, 2004), our results show an additional role of membrin as a membrane receptor for Arf-1 at the Golgi. Because Bos1p, the yeast homologue of membrin, has also been shown to interact with Sar1p, a component of the coat protein II (COPII) coat (Springer and Schekman, 1998), membrin recruitment of Arf-1 might coordinate the exchange of Sar1/COPII to Arf-1/COPI coats on incoming, pre-Golgi structures.
The first suggestion that ER-Golgi SNAREs could recruit Arf-1 came from in vitro binding studies in yeast (Rein et al., 2002) showing that nonmyristoylated, Δ17Arf1p-GDP could bind directly to GST fusion proteins of Bet1p, Sec22p, and Bos1p. In that study, Arf1p binding to Bet1p was stronger than to Bos1p and, curiously, required the presence of an Arf-GAP protein, Glo3p, to prime the SNAREs (Rein et al., 2002). Our study shows that membrin, but not rbet1, sec22b or syntaxin5, can bind specifically to full-length, myristoylated Arf-1 through the MXXE motif in mammalian cells. Because our cells express mammalian ArfGAP1, it is possible that this endogenous GAP could function in vivo to prime membrin for Arf-1 binding. Interestingly, the proximal region of helix α3 makes contact with ArfGAP1 in a co-crystal of Δ17Arf-1-GDP and the GAP domain of ArfGAP1 (Goldberg, 1999), raising the possibility that the recruitment of Arf-1 to the early Golgi by membrin might also involve ArfGAP1. Because yeast Arf1p also contains MXXE, and can be recruited to Golgi when expressed in mammalian cells (unpublished data), this motif may be used for Golgi targeting in yeast cells. Indeed, evidence that the α3 region in yeast promotes Golgi recruitment of Arf-1 comes from a genetic study that identified amino acid residues in the distal portion of helix α3 as an intragenic suppressor of a dominant lethal mutation (Click et al., 2002).
The other class of Golgi membrane proteins that have been suggested to serve as Arf-1 receptors are the abundant p23 and p24 family proteins. The p23 protein was shown by chemical cross-linking to bind directly to Arf-1-GDP, but not to Arf-1-GTP, in vitro and the interaction site was mapped to the carboxyl-terminal 22 aa (Gommel et al., 2001). Although this interaction was also observed in an in vivo study examining fluorescence resonance energy transfer between p23-CFP and Arf-1-YFP (Majoul et al., 2001), it may not represent the interaction that targets Arf-1-GDP specifically to the Golgi. We did not detect a Golgi targeting motif in the last 22 aa of Arf-1 because Arf-6-6-1, which lacked the 16–amino acid sequence and the amino-terminal half of Arf-1 but contained Arf-1 sequence from 117–181, did not localize to the Golgi (Fig. 1 D). Our data suggest that membrin serves as a specific receptor for Arf-1-GDP. However, it does not preclude an interaction of Arf-1-GDP with p23 proteins either simultaneously or subsequently during the interaction cycle of Arf-1 with Golgi membranes.
Arf proteins are highly conserved both in sequence and in structure with nearly identical switch I and II regions (Goldberg, 1998; Pasqualato et al., 2001). Helix α3 is positioned on the “backside” of the molecule, on the opposite side of the GTP binding pocket. It alone appears to contain sufficient information for Golgi targeting (Fig. 4) and presumably membrin binding. In the context of the Arf-1 protein, the MXXE motif in helix α3 appears to be required for Golgi targeting. We do not know whether the M or E make direct contact with membrin, but they are necessary for Arf-1 binding to membrin.
We propose that in the ER-Golgi system, membrin recruits Arf-1-GDP to the membrane subsequent to the release of the COPII coat (Fig. 9 B, I). Arf-1-GDP is then activated by GBF1, the early Golgi GEF, and Arf-1-GTP can then recruit coatomer or other effectors and Arf-GAP, leading to GTP hydrolysis and release of Arf-1-GDP back into the cytosol. The Arf-1 construct that cannot bind to membrin (Arf-1-6-1) can still associate with the Golgi complex, possibly by direct interaction with an Arf-GEF such as BIG1 and 2, which are known to reside at the trans-Golgi (Fig. 9 B, II; Shinotsuka et al., 2002a,b; Zhao et al., 2002). Arf-1-6-1 colocalizes less with GBF1 (Fig. 8, A and B) and, during recovery from BFA, Arf-1-6-1 cannot associate with the peripheral, COPI-labeled, reforming Golgi structures (Fig. 7 A). Intriguingly, we observed a distinct difference between the FRAP kinetics of Arf-1 and Arf-1-6-1 (Fig. 9 A). Namely, that Arf-1-6-1-GFP had a much shorter recovery time after photobleaching (t 1/2 = 10 s) than did Arf-1-GFP (t 1/2 = 20 s). We speculate that the shorter residency time for Arf-1-6-1 could be due to interaction with trans-Golgi GEFs (BIG1 and 2) and GAPs. In contrast, the residency time of Arf-1 on the Golgi is longer and includes interaction of Arf-1 with membrin, GBF1 and ArfGAP1 at early Golgi. These differences in kinetic cycles may reflect the different GEFs and GAPs residing at the early and late Golgi (Fig. 9 B, I, II).
Functional relationship between Arf-1 and SNAREs
SNAREs have an essential role in regulating membrane fusion events throughout the secretory pathway (Hay, 2001; Bonifacino and Glick, 2004). SNAREs form cytoplasmic coiled-coil bundles that bridge two membranes about to undergo membrane fusion. Furthermore, as SNAREs are compartment specific and can engage in various SNARE pair conformations, they have the potential to serve as exquisite membrane receptors for cytosolic components such as COPI and COPII components whose regulated assembly controls vesicle formation.
Current models for transport in the early secretory pathway depict secretory cargo and the SNAREs syntaxin5, rbet1, and membrin being recruited into COPII vesicle budding from the ER (Fig. 9 B, I; Springer and Schekman, 1998). Our observations that Arf-1 cannot bind to membrin when it is in the ER or at ER exit sites, i.e., 30 min BFA (Fig. 7) suggest that the altered conformation of membrin in the ER or association with the COPII coat may prevent Arf-1 binding. The tethering protein p115 associates with these newly budded vesicles by binding directly to the SNAREs in a Rab1-dependent fashion (Allan et al., 2000). At the Golgi, p115 and these SNAREs are believed to facilitate fusion of pre-Golgi structures to Golgi membranes or to other incoming pre-Golgi structures. GBF1 associates with p115 (Garcia-Mata and Sztul, 2003) and has been shown to be involved in COPI recruitment onto membranes (Claude et al., 1999; Kawamoto et al., 2002). Furthermore, GBF1 is essential for COPI recruitment to the transport intermediates bound for the Golgi (Garcia-Mata et al., 2003). These observations support the idea that p115 and membrin might coordinately recruit GBF1 and Arf-1-GDP onto the post-ER, transport structure, leading to Arf-1 activation and the recruitment of COPI (Fig. 9 B, I).
Materials and methods
Cells, antibodies and reagents
Cells were grown in DME supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. Rabbit anti–Arf-6 antibody that recognizes a carboxyl-terminal peptide of the protein was used as described previously (Radhakrishna and Donaldson, 1997). Rabbit anti-BIG1 antibody was provided by C.L. Jackson (NIH; Yamaji et al., 2000). Monoclonal antibodies against the HA epitope (16B12) and the myc epitope (9E10) were purchased from Covance and Zymed Laboratories, respectively. Monoclonal anti-GM130 and anti-GBF1 antibodies were purchased from BD Biosciences. Rabbit anti–β-COP antibody was purchased from Affinity BioReagents, Inc. Secondary antibodies (Alexa 594, Alexa 488, anti–mouse, and anti–rabbit) were purchased from Molecular Probes. Dithiobis(succinimidyl propionate) (DSP) was purchased from Pierce Chemical Co. E. coli containing a vector for GST-fusion proteins comprising VHS-GAT domain of human GGA3 was provided by J.S. Bonifacino (NIH; Dell'Angelica et al., 2000). All other reagents were purchased from Sigma-Aldrich.
Plasmids and transient transfections
The chimeras of Arf-1 and Arf-6 were created by a two-step PCR procedure as described previously (Al-Awar et al., 2000). All other mutants of Arf-1 and Arf-6 were created using QuickChange site-directed mutagenesis kit (Stratagene). The cDNAs of untagged or carboxyl-terminally HA-tagged wild-type and mutant Arf were subcloned into the modified pCDL-SRα expression vector (Takebe et al., 1988) or pEGFP-N1 vector (CLONTECH Laboratories, Inc.). Amino acids 101–116 of Arf-1 were shuttled into pEGFP-C3 vector (CLONTECH Laboratories, Inc.). The plasmids for amino-terminally myc-tagged membrin, rbet1, sec22b, and HA-tagged syntaxin5 were created as described previously (Hay et al., 1996, 1997). The plasmids for GalT-GFP and HA-tagged GBF1 were provided by J. Lippincott-Schwartz (NIH; Cole et al., 1996) and C.L. Jackson, respectively.
Cells were grown on glass coverslips and transfected using FuGENE 6 (Roche) according to the manufacturer's instructions. For FRAP experiments, HeLa cells were plated in Lab-Tek chambered coverglass (Nalge Nunc International).
Confocal microscopy
20 h after transfection, cells were treated as indicated, fixed, and subjected to immunofluorescence staining in the presence of 0.2% saponin as described previously (Radhakrishna and Donaldson, 1997). All fluorescence images were obtained with a confocal microscope (model LSM 510; Carl Zeiss MicroImaging, Inc.). Most images of fixed cells were captured using 63× 1.4 NA objective with a pinhole corresponding to a focal depth of 0.7 μm. To quantify Golgi-associated Arf-1-GFP, a 40× 0.7 NA objective was used with a pinhole corresponding to a focal depth of 12.4 μm. For FRAP experiments, cells were incubated in CO2-independent media (Invitrogen), and imaged at 20°C on a confocal microscope (model LSM 510; Carl Zeiss MicroImaging, Inc.) using a 40× 0.7 NA objective with a pinhole corresponding to a focal depth of 2.5 μm. Image processing for figures was accomplished in Adobe Photoshop 5.5.
Analysis of colocalization
To quantify the level of colocalization for Golgi-associated Arfs with membrin, GBF1, and BIG1, 20 cells were randomly selected on the same coverslip. Levels for the laser power and detector amplification were optimized for each channel before starting the quantification. Images of the Golgi region were acquired at the same magnification and processed by Metamorph 4.6 (Universal Imaging Corp.).
In vitro binding experiments
To examine the interaction between His6-Arf-1 and membrin, HeLa cells overexpressing myc-membrin were lysed in binding buffer (25 mM Hepes, pH 7.5, 100 mM NaCl, 0.5 mM EDTA, 1 mM MgCl2, 1 mM DTT, 1 mM AEBSF, 3 μg/ml leupeptin, 3 μg/ml aprotinin, 3 μg/ml pepstatin A) containing 0.5% Triton X-100. Supernatants of the lysate were incubated with His6-tagged Δ17Arf-1 prebound to Ni-NTA beads (QIAGEN) in the presence of 1 mM GDP for 3 h at 4°C, and cross-linking reagent DSP was added at a final concentration of 1 mM. After incubation for 20 min at RT, DSP activity was quenched by addition of 50 mM glycine. The beads were washed and the proteins bound to beads were subjected to SDS-PAGE and immunoblot analysis with anti-myc antibody.
Online supplemental material
Fig. S1 shows that Arf-1-6-1 and Arf-1M110I, E113D when loaded with GTPγS could bind to GST-VHS-GAT as well as wild-type Arf-1. Also, measurement of CD spectra of these Arf-1 mutants demonstrated that the general conformations of Arf-1-6-1 and Arf-1M110I, E113D are essentially identical to that of Arf-1. Fig. S2 shows that overexpressed GBF1 can activate Arf-1-6-1 in cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200409138/DC1.
Acknowledgments
We thank J. Lippincott-Schwartz, C.L. Jackson, and J.S. Bonifacino for reagents; and J. Lippincott-Schwartz, C.L. Jackson, F. Brown, R. Weigert, and E. Korn for comments on the manuscript. We also thank R.S. Lipsitz and N. Tjandra for support in measuring CD spectra.
A. Honda was supported by the JSPS Research Fellowships for Japanese Biomedical and Behavioral Researchers at NIH.
Abbreviations used in this paper: BFA, Brefeldin A; BIG, BFA-inhibited GEF; CD, circular dichroism; COPI, coat protein I; COPII, coat protein II; DSP, dithiobis(succinimidyl propionate); GalT, galactosyltransferase; GAP, GTPase-activating protein; GBF1, Golgi-specific BFA resistance factor 1; GEF, guanine nucleotide exchange factor; GGA, Golgi-localized, γ-ear–containing, Arf-binding proteins.
References
- Al-Awar, O., H. Radhakrishna, N.N. Powell, and J.G. Donaldson. 2000. Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol. Cell. Biol. 20:5998–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. [DOI] [PubMed] [Google Scholar]
- Amor, J.C., D.H. Harrison, R.A. Kahn, and D. Ringe. 1994. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature. 372:704–708. [DOI] [PubMed] [Google Scholar]
- Antonny, B., S. Beraud-Dufour, P. Chardin, and M. Chabre. 1997. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 36:4675–4684. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and B.S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell. 116:153–166. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and J. Lippincott-Schwartz. 2003. Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 4:409–414. [DOI] [PubMed] [Google Scholar]
- Chavrier, P., and B. Goud. 1999. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11:466–475. [DOI] [PubMed] [Google Scholar]
- Claude, A., B.P. Zhao, C.E. Kuziemsky, S. Dahan, S.J. Berger, J.P. Yan, A.D. Armold, E.M. Sullivan, and P. Melancon. 1999. GBF1: A novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J. Cell Biol. 146:71–84. [PMC free article] [PubMed] [Google Scholar]
- Click, E.S., T. Stearns, and D. Botstein. 2002. Systematic structure-function analysis of the small GTPase Arf1 in yeast. Mol. Biol. Cell. 13:1652–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, N.B., C.L. Smith, N. Sciaky, M. Terasaki, M. Edidin, and J. Lippincott-Schwartz. 1996. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 273:797–801. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., I. Huber, M. Rotman, and D. Cassel. 1995. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 270:1999–2002. [DOI] [PubMed] [Google Scholar]
- Dascher, C., and W.E. Balch. 1994. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269:1437–1448. [PubMed] [Google Scholar]
- Dell'Angelica, E.C., R. Puertollano, C. Mullins, R.C. Aguilar, J.D. Vargas, L.M. Hartnell, and J.S. Bonifacino. 2000. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 149:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, J.G. 2003. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 278:41573–41576. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata, R., and E. Sztul. 2003. The membrane-tethering protein p115 interacts with GBF1, an ARF guanine-nucleotide-exchange factor. EMBO Rep. 4:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata, R., T. Szul, C. Alvarez, and E. Sztul. 2003. ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol. Biol. Cell. 14:2250–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, J. 1998. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 95:237–248. [DOI] [PubMed] [Google Scholar]
- Goldberg, J. 1999. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 96:893–902. [DOI] [PubMed] [Google Scholar]
- Gommel, D.U., A.R. Memon, A. Heiss, F. Lottspeich, J. Pfannstiel, J. Lechner, C. Reinhard, J.B. Helms, W. Nickel, and F.T. Wieland. 2001. Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23. EMBO J. 20:6751–6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J.C. 2001. SNARE complex structure and function. Exp. Cell Res. 271:10–21. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., H. Hirling, and R.H. Scheller. 1996. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J. Biol. Chem. 271:5671–5679. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., D.S. Chao, C.S. Kuo, and R.H. Scheller. 1997. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 89:149–158. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., J. Klumperman, V. Oorschot, M. Steegmaier, C.S. Kuo, and R.H. Scheller. 1998. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol. 141:1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, A., M. Nogami, T. Yokozeki, M. Yamazaki, H. Nakamura, H. Watanabe, K. Kawamoto, K. Nakayama, A.J. Morris, M.A. Frohman, and Y. Kanaho. 1999. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 99:521–532. [DOI] [PubMed] [Google Scholar]
- Icard-Liepkalns, C., P. Ravassard, V.A. Liepkalns, F. Chatail, and J. Mallet. 1997. An ADP-ribosylation-factor(ARF)-like protein involved in regulated secretion. Eur. J. Biochem. 246:388–393. [DOI] [PubMed] [Google Scholar]
- Jackson, C.L., and J.E. Casanova. 2000. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10:60–67. [DOI] [PubMed] [Google Scholar]
- Jones, D.H., B. Bax, A. Fensome, and S. Cockcroft. 1999. ADP ribosylation factor 1 mutants identify a phospholipase D effector region and reveal that phospholipase D participates in lysosomal secretion but is not sufficient for recruitment of coatomer I. Biochem. J. 341:185–192. [PMC free article] [PubMed] [Google Scholar]
- Kawamoto, K., Y. Yoshida, H. Tamaki, S. Torii, C. Shinotsuka, S. Yamashina, and K. Nakayama. 2002. GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic. 3:483–495. [DOI] [PubMed] [Google Scholar]
- Liang, J.O., and S. Kornfeld. 1997. Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J. Biol. Chem. 272:4141–4148. [DOI] [PubMed] [Google Scholar]
- Liang, J.O., T.C. Sung, A.J. Morris, M.A. Frohman, and S. Kornfeld. 1997. Different domains of mammalian ADP-ribosylation factor 1 mediate interaction with selected target proteins. J. Biol. Chem. 272:33001–33008. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., N.B. Cole, and J.G. Donaldson. 1998. Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance. Histochem. Cell Biol. 109:449–462. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., J.G. Donaldson, A. Schweizer, E.G. Berger, H.P. Hauri, L.C. Yuan, and R.D. Klausner. 1990. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 60:821–836. [DOI] [PubMed] [Google Scholar]
- Lu, L., H. Horstmann, C. Ng, and W. Hong. 2001. Regulation of Golgi structure and function by ARF-like protein 1 (Arl1). J. Cell Sci. 114:4543–4555. [DOI] [PubMed] [Google Scholar]
- Macia, E., M. Chabre, and M. Franco. 2001. Specificities for the small G proteins ARF1 and ARF6 of the guanine nucleotide exchange factors ARNO and EFA6. J. Biol. Chem. 276:24925–24930. [DOI] [PubMed] [Google Scholar]
- Majoul, I., M. Straub, S.W. Hell, R. Duden, and H.D. Soling. 2001. KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev. Cell. 1:139–153. [DOI] [PubMed] [Google Scholar]
- Menetrey, J., E. Macia, S. Pasqualato, M. Franco, and J. Cherfils. 2000. Structure of Arf6-GDP suggests a basis for guanine nucleotide exchange factors specificity. Nat. Struct. Biol. 7:466–469. [DOI] [PubMed] [Google Scholar]
- Miura, K., K.M. Jacques, S. Stauffer, A. Kubosaki, K. Zhu, D.S. Hirsch, J. Resau, Y. Zheng, and P.A. Randazzo. 2002. ARAP1: a point of convergence for Arf and Rho signaling. Mol. Cell. 9:109–119. [DOI] [PubMed] [Google Scholar]
- Moss, J., and M. Vaughan. 1995. Structure and function of ARF proteins: activators of cholera toxin and critical components of intracellular vesicular transport processes. J. Biol. Chem. 270:12327–12330. [DOI] [PubMed] [Google Scholar]
- Munro, S. 2002. Organelle identity and the targeting of peripheral membrane proteins. Curr. Opin. Cell Biol. 14:506–514. [DOI] [PubMed] [Google Scholar]
- Nie, Z., D.S. Hirsch, and P.A. Randazzo. 2003. Arf and its many interactors. Curr. Opin. Cell Biol. 15:396–404. [DOI] [PubMed] [Google Scholar]
- Pasqualato, S., J. Menetrey, M. Franco, and J. Cherfils. 2001. The structural GDP/GTP cycle of human Arf6. EMBO Rep. 2:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, P.J., V.W. Hsu, C.E. Ooi, D. Finazzi, S.B. Teal, V. Oorschot, J.G. Donaldson, and R.D. Klausner. 1995. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol. 128:1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley, J.F., T.H. Ward, A.C. Pfeifer, E.D. Siggia, R.D. Phair, and J. Lippincott-Schwartz. 2002. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 417:187–193. [DOI] [PubMed] [Google Scholar]
- Radhakrishna, H., and J.G. Donaldson. 1997. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein, U., U. Andag, R. Duden, H.D. Schmitt, and A. Spang. 2002. ARF-GAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J. Cell Biol. 157:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotsuka, C., S. Waguri, M. Wakasugi, Y. Uchiyama, and K. Nakayama. 2002. a. Dominant-negative mutant of BIG2, an ARF-guanine nucleotide exchange factor, specifically affects membrane trafficking from the trans-Golgi network through inhibiting membrane association of AP-1 and GGA coat proteins. Biochem. Biophys. Res. Commun. 294:254–260. [DOI] [PubMed] [Google Scholar]
- Shinotsuka, C., Y. Yoshida, K. Kawamoto, H. Takatsu, and K. Nakayama. 2002. b. Overexpression of an ADP-ribosylation factor-guanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J. Biol. Chem. 277:9468–9473. [DOI] [PubMed] [Google Scholar]
- Springer, S., and R. Schekman. 1998. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 281:698–700. [DOI] [PubMed] [Google Scholar]
- Stearns, T., M.C. Willingham, D. Botstein, and R.A. Kahn. 1990. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc. Natl. Acad. Sci. USA. 87:1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu, H., K. Yoshino, K. Toda, and K. Nakayama. 2002. GGA proteins associate with Golgi membranes through interaction between their GGAH domains and ADP-ribosylation factors. Biochem. J. 365:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe, Y., M. Seiki, J. Fujisawa, P. Hoy, K. Yokota, K. Arai, M. Yoshida, and N. Arai. 1988. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 8:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, M., T. Ueda, N. Yahara, and A. Nakano. 2002. Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 31:499–515. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., S.H. Low, H.P. Hauri, and W. Hong. 1995. Segregation of ERGIC53 and the mammalian KDEL receptor upon exit from the 15 degrees C compartment. Eur. J. Cell Biol. 68:398–410. [PubMed] [Google Scholar]
- Vasudevan, C., W. Han, Y. Tan, Y. Nie, D. Li, K. Shome, S.C. Watkins, E.S. Levitan, and G. Romero. 1998. The distribution and translocation of the G protein ADP-ribosylation factor 1 in live cells is determined by its GTPase activity. J. Cell Sci. 111:1277–1285. [DOI] [PubMed] [Google Scholar]
- Ward, T.H., R.S. Polishchuk, S. Caplan, K. Hirschberg, and J. Lippincott-Schwartz. 2001. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji, R., R. Adamik, K. Takeda, A. Togawa, G. Pacheco-Rodriguez, V.J. Ferrans, J. Moss, and M. Vaughan. 2000. Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc. Natl. Acad. Sci. USA. 97:2567–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., T.K. Lasell, and P. Melancon. 2002. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol. Biol. Cell. 13:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]