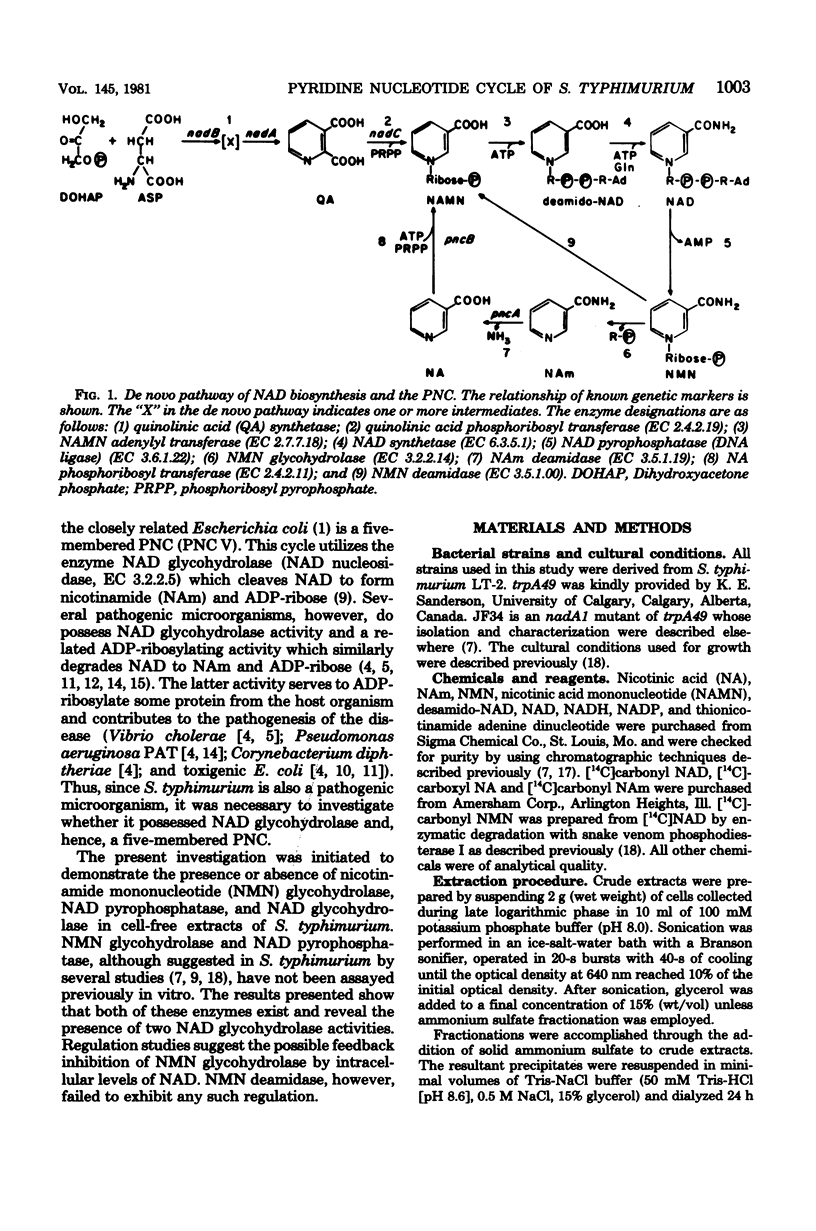
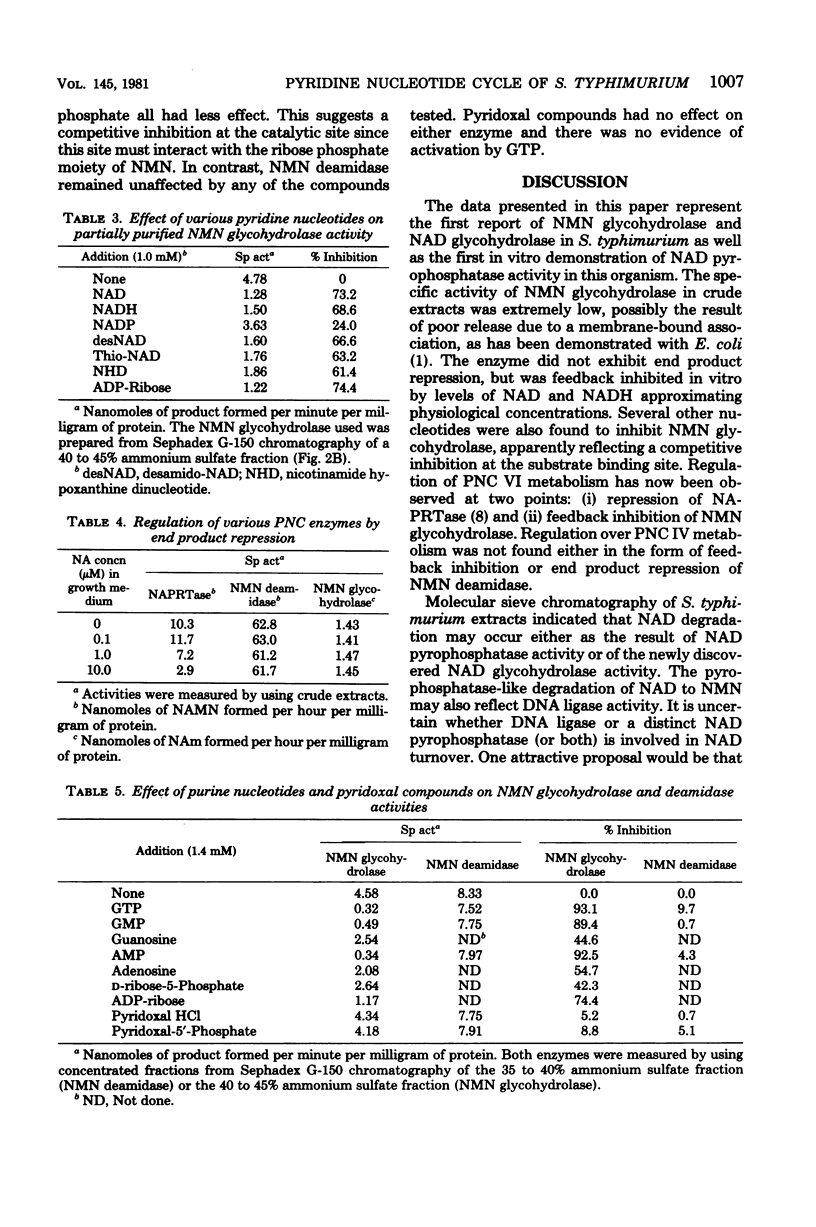
Abstract
Extracts of Salmonella typhimurium were chromatographed by using Sephadex G-150 to separate the various enzymes involved with pyridine nucleotide cycle metabolism. This procedure revealed a previously unsuspected nicotinamide adenine dinucleotide (NAD) glycohydrolase (EC 3.2.2.5) activity, which was not observed in crude extracts. In contrast to NAd glycohydrolase, NAD pyrophosphatase (EC 3.6.1.22) was readily measured in crude extracts. This enzyme possessed a native molecular weight of 120,000. Other enzymes examined included nicotinamide mononucleotide (NMN) deamidase (EC 3.5.1.00), molecular weight of 43,000; NMN glycohydrolase (EC 3.2.2.14), molecular weight of 67,000; nicotinic acid phosphoribosyl transferase (EC 2.4.2.11), molecular weight of 47,000; and nicotinamide deamidase (EC 3.5.1.19), molecular weight of 35,000. NMN deamidase and NMN glycohydrolase activities were both examined for end product repression by measuring their activities in crude extracts prepared from cells grown with and without 10(-5) M nicotinic acid. No repression was observed with either activity. Both activities were also examined for feedback inhibition by NAD, reduced NAD, and NADP. NMN deamidase was unaffected by any of the compounds tested. NMN glycohydrolase was greatly inhibited by NAD and reduced NAD, whereas NADP was much less effective. Inhibition of NMN glycohydrolase was found to level off at an NAD concentration of ca. 1 mN, the approximate intracellular concentration of NAD.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli A. J., Okita T. W., Bloom R., Grover T. A. The pyridine nucleotide cycle: presence of a nicotinamide mononucleotide-specific glycohydrolase in Escherichia coli. Biochem Biophys Res Commun. 1972 Oct 6;49(1):264–269. doi: 10.1016/0006-291x(72)90039-3. [DOI] [PubMed] [Google Scholar]
- Baecker P. A., Yung S. G., Rodriguez M., Austin E., Andreoli A. J. Periplasmic localization of nicotinate phosphoribosyltransferase in Escherichia coli. J Bacteriol. 1978 Mar;133(3):1108–1112. doi: 10.1128/jb.133.3.1108-1112.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. Studies on the biosynthesis of NAD in Escherichia coli. 3. Precursors of quinolinic acid in vitro. Biochim Biophys Acta. 1972 Apr 21;264(2):311–318. doi: 10.1016/0304-4165(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Welsh K. M., Bayer M. E. Characterization of membrane-bound nicotinamide adenine dinucleotide glycohydrolase of Vibrio cholerae. J Biol Chem. 1979 Sep 25;254(18):9254–9261. [PubMed] [Google Scholar]
- Foster J. W., Baskowsky-Foster A. M. Pyridine nucleotide cycle of Salmonella typhimurium: in vivo recycling of nicotinamide adenine dinucleotide. J Bacteriol. 1980 Jun;142(3):1032–1035. doi: 10.1128/jb.142.3.1032-1035.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: isolation and characterization of pncA, pncB, and pncC mutants and utilization of exogenous nicotinamide adenine dinucleotide. J Bacteriol. 1979 Mar;137(3):1165–1175. doi: 10.1128/jb.137.3.1165-1175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: regulation of nicotinic acid phosphoribosyltransferase and nicotinamide deamidase. J Bacteriol. 1979 Jun;138(3):957–961. doi: 10.1128/jb.138.3.957-961.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Richardson S. H. Adenosine diphosphate-ribosylation of adenylate cyclase catalyzed by heat-labile enterotoxin of Escherichia coli: comparison with cholera toxin. J Infect Dis. 1980 Jan;141(1):64–70. doi: 10.1093/infdis/141.1.64. [DOI] [PubMed] [Google Scholar]
- Gopinathan K. P., Sirsi M., Vaidyanathan C. S. Nicotinamide-adenine dinucleotide glycohydrolase of Mycobacterium tuberculosis H37Rv. Biochem J. 1964 May;91(2):277–282. doi: 10.1042/bj0910277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushoff P. S., Shany S., Bernheimer A. W. Purification and properties of streptococcal nicotinamide adenine dinucleotide glycohydrolase. J Bacteriol. 1975 May;122(2):599–605. doi: 10.1128/jb.122.2.599-605.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T. Isolation and properties of a glycohydrolase specific for nicotinamide mononucleotide from Azotobacter vinelandii. J Biochem. 1979 Apr;85(4):887–899. doi: 10.1093/oxfordjournals.jbchem.a132420. [DOI] [PubMed] [Google Scholar]
- Kasărov L. B., Moat A. G. Convenient method for enzymic synthesis of 14 C-nicotinamide riboside. Anal Biochem. 1972 Mar;46(1):181–186. doi: 10.1016/0003-2697(72)90410-1. [DOI] [PubMed] [Google Scholar]
- Kinney D. M., Foster J. W., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: in vitro demonstration of nicotinamide mononucleotide deamidase and characterization of pnuA mutants defective in nicotinamide mononucleotide transport. J Bacteriol. 1979 Nov;140(2):607–611. doi: 10.1128/jb.140.2.607-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupal L. R., Deibel R. H. Assay, characterization, and localization of an enterotoxin produced by Salmonella. Infect Immun. 1975 Jan;11(1):14–22. doi: 10.1128/iai.11.1.14-22.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman I. R. DNA ligase: structure, mechanism, and function. Science. 1974 Nov 29;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- Nozawa R., Mizuno D. Replication and properties of DNA in nicotinamide adenine dinucleotide deficiency of Escherichia coli cells. Proc Natl Acad Sci U S A. 1969 Jul;63(3):904–910. doi: 10.1073/pnas.63.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Benz E. J., Jr, St Peter D. A., Krieger J. N., Meuth M., Trieshmann H. W., Jr Hyperproduction and purification of nicotinamide deamidase, a microconstitutive enzyme of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):6792–6796. [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Isolation of skin permeability factors from culture filtrates of Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):671–679. doi: 10.1128/iai.14.3.671-679.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlock D. M., Koupal L. R., Deibel R. H. Production and partial purification of Salmonella enterotoxin. Infect Immun. 1978 May;20(2):375–380. doi: 10.1128/iai.20.2.375-380.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


