Abstract
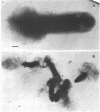
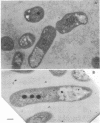
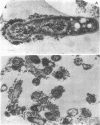
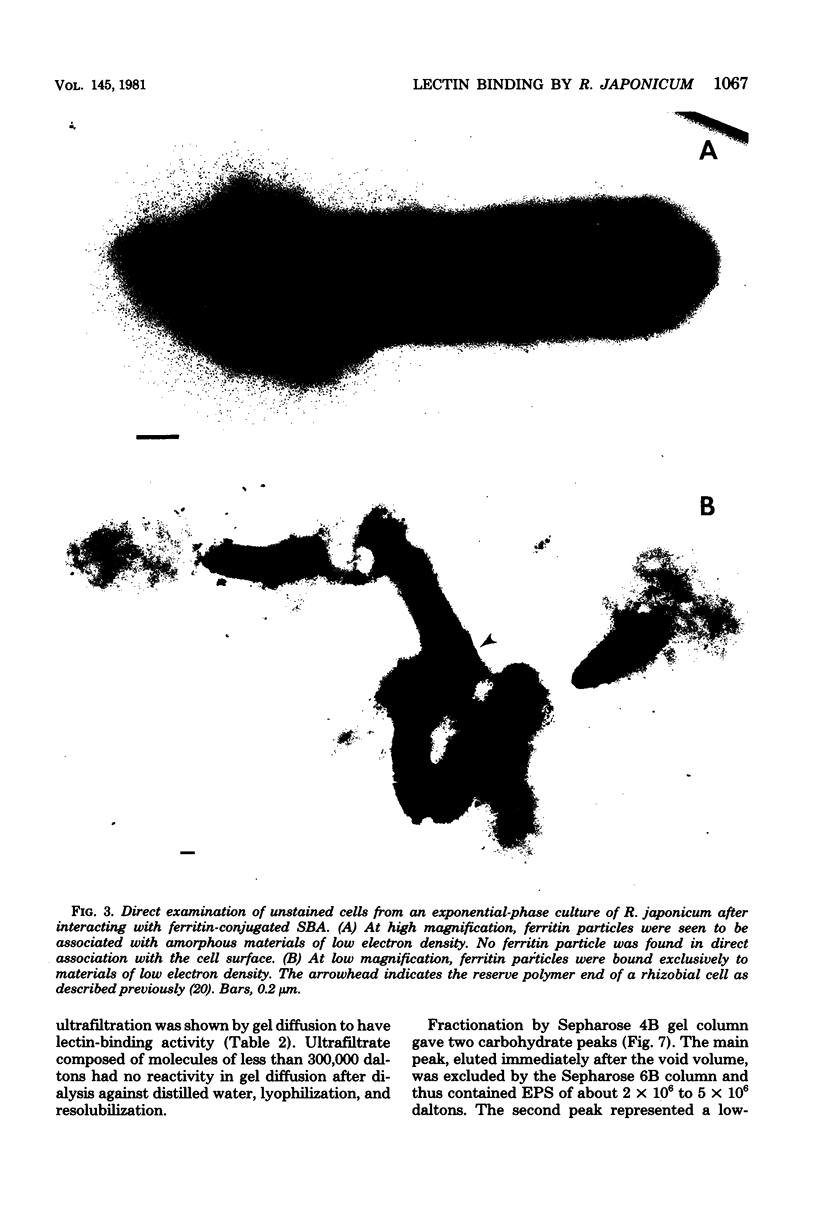
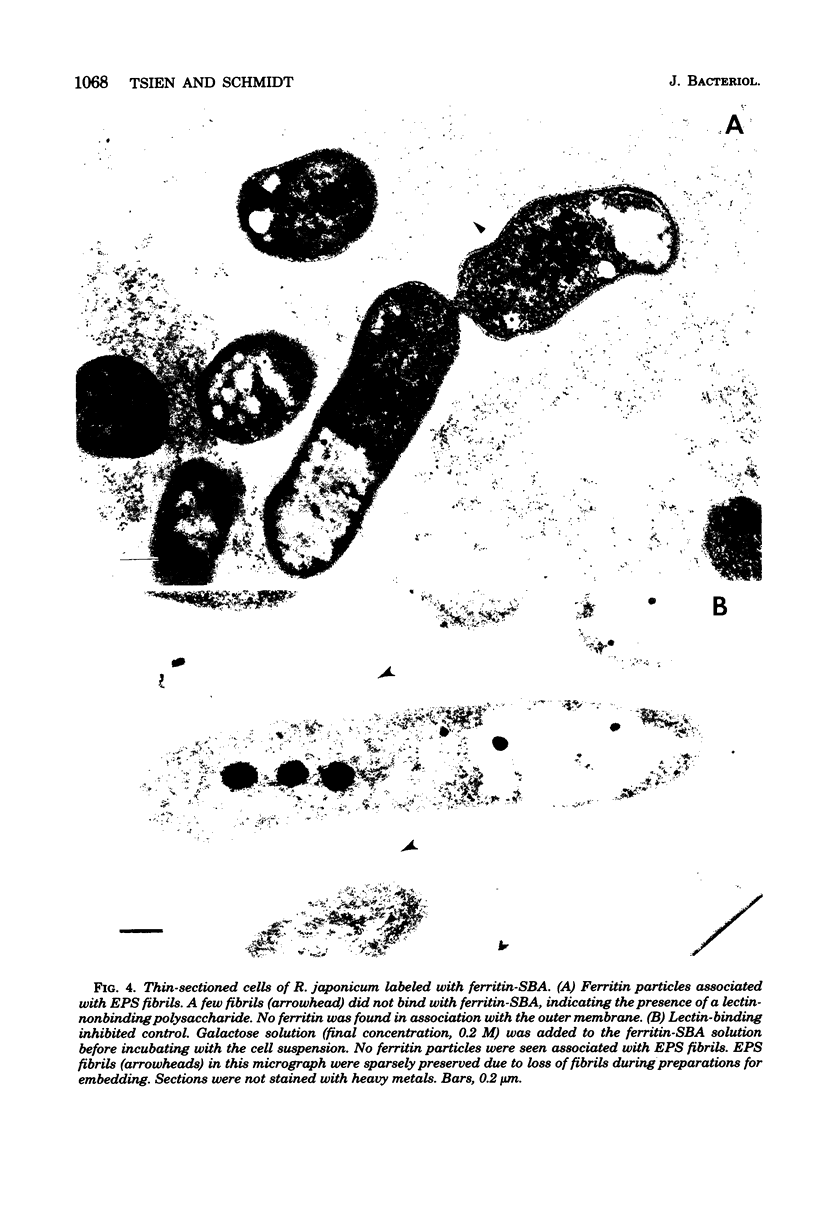
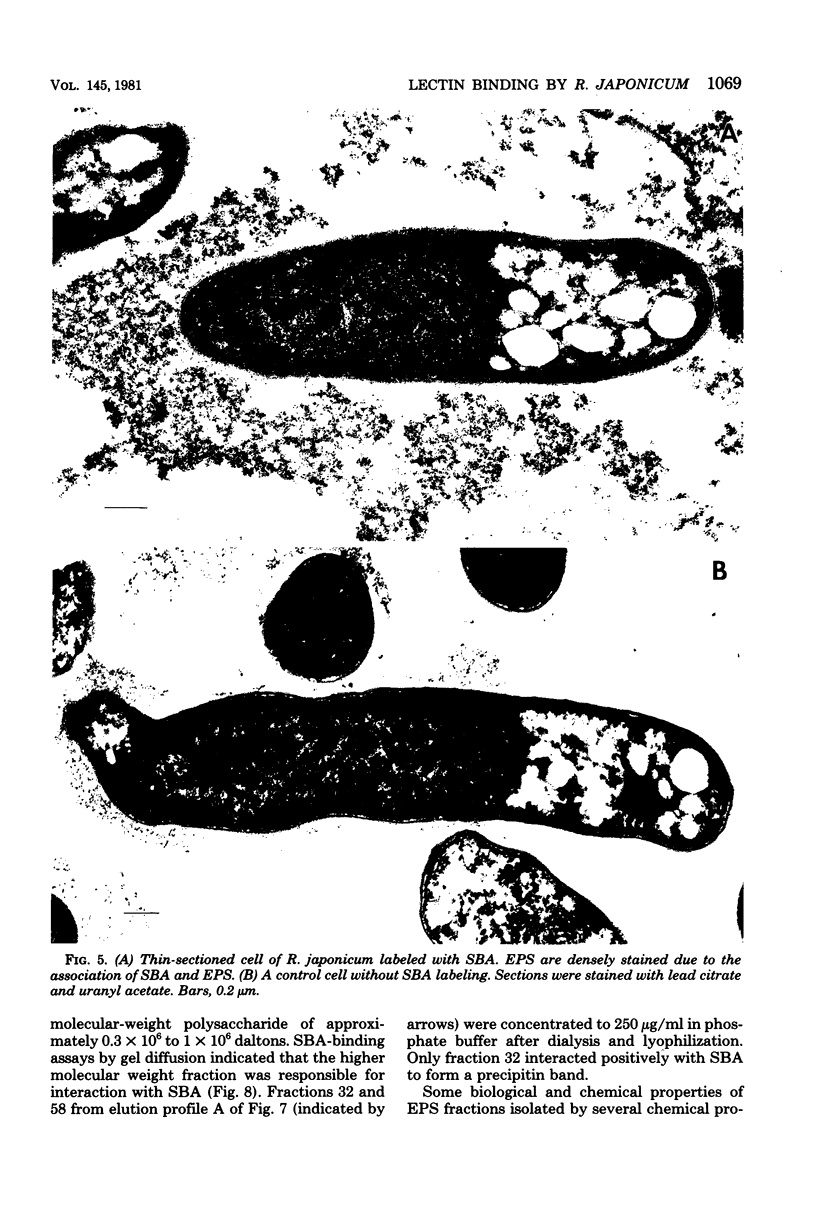
Immunoelectron microscopy was combined with partial characterization of isolated exopolysaccharide to study binding of soybean lectin by Rhizobium japonicum strain USDA 138. Lectin-binding activity resided in two forms of exopolysaccharide produced during growth: an apparently very high-molecular-weight capsular form and a lower-molecular-weight diffusible form. At low-speed centrifugation, the capsular form cosedimented with cells to form a viscous, white, cell-gel complex which was not diffusible in 1% agar, and the diffusible form remained in the cell-free supernatant. Electron microscopic observation of the cell-gel complex after labeling with soybean lectin-ferritin conjugate revealed that capsular polysaccharides, frequently attached to one end of the cells, were receptors for lectin. The outer membrane of the cell bound no lectin. Various preparations of exopolysaccharide isolated from the culture supernatant were tested for lectin binding, interaction with homologous somatic antigen, and the presence of 2-keto-3-deoxyoctonate and were chromatographed in Sepharose 4B and 6B gel beds. Lectin binding was restricted to a polysaccharide component designated as lectin-binding polysaccharide. This polysaccharide, as present in the cell-free culture supernatant, was a diffusible acidic polysaccharide devoid of 2-keto-3-deoxyoctonate, with a molecular weight of 2 X 10(6) to 5 X 10(6). It was concluded that the soybean lectin-binding component of R. japonicum is an extracellular polysaccharide and not a lipopolysaccharide and that the diffusible lectin-binding polysaccharide probably differs from the very high-molecular-weight lectin-binding polysaccharide of the loose capsule (slime) only in the degree of polymerization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H. J., Johnson E. A. The isolation of lectins on acid-treated agarose. Carbohydr Res. 1976 Aug;50(1):121–131. doi: 10.1016/s0008-6215(00)84089-6. [DOI] [PubMed] [Google Scholar]
- Bal A. K., Shantharam S., Ratnam S. Ultrastructure of Rhizobium japonicum in relation to its attachment to root hairs. J Bacteriol. 1978 Mar;133(3):1393–1400. doi: 10.1128/jb.133.3.1393-1400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Immunofluorescent polar tips of Rhizobium japonicum: possible site of attachment or lectin binding. J Bacteriol. 1976 Mar;125(3):1188–1194. doi: 10.1128/jb.125.3.1188-1194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- LIENER I. E., PALLANSCH M. J. Purification of a toxic substance from defatted soy bean flour. J Biol Chem. 1952 May;197(1):29–36. [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. The distribution and asymmetry of mammalian cell surface saccharides utilizing ferritin-conjugated plant agglutinins as specific saccharide stains. J Cell Biol. 1974 Jan;60(1):236–248. doi: 10.1083/jcb.60.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantharam S., Gow J. A., Bal A. K. Fractionation and characterization of two morphologically distinct types of cells in Rhizobium japonicum broth culture. Can J Microbiol. 1980 Feb;26(2):107–114. doi: 10.1139/m80-016. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., KOLB J. J. CARBOHYDRATE ANALYSIS OF BACTERIAL SUBSTANCES BY A NEW ANTHRONE PROCEDURE. Anal Biochem. 1964 May;8:54–69. doi: 10.1016/0003-2697(64)90168-x. [DOI] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Accumulation of Soybean Lectin-Binding Polysaccharide During Growth of Rhizobium japonicum as Determined by Hemagglutination Inhibition Assay. Appl Environ Microbiol. 1980 Jun;39(6):1100–1104. doi: 10.1128/aem.39.6.1100-1104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977 Sep;23(9):1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. 1,4-Butanediol diglycidyl ether coupling of carbohydrates to Sepharose: affinity adsorbents for lectins and glycosidases. Anal Biochem. 1977 Jul;81(1):98–107. doi: 10.1016/0003-2697(77)90602-9. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]