Abstract
The members of the Toc159 family of GTPases act as the primary receptors for the import of nucleus-encoded preproteins into plastids. Toc159, the most abundant member of this family in chloroplasts, is required for chloroplast biogenesis (Bauer, J., K. Chen, A. Hiltbunner, E. Wehrli, M. Eugster, D. Schnell, and F. Kessler. 2000. Nature. 403:203–207) and has been shown to covalently cross-link to bound preproteins at the chloroplast surface (Ma, Y., A. Kouranov, S. LaSala, and D.J. Schnell. 1996. J. Cell Biol. 134:1–13; Perry, S.E., and K. Keegstra. 1994. Plant Cell. 6:93–105). These reports led to the hypothesis that Toc159 functions as a selective import receptor for preproteins that are required for chloroplast development. In this report, we provide evidence that Toc159 is required for the import of several highly expressed photosynthetic preproteins in vivo. Furthermore, we demonstrate that the cytoplasmic and recombinant forms of soluble Toc159 bind directly and selectively to the transit peptides of these representative photosynthetic preproteins, but not representative constitutively expressed plastid preproteins. These data support the function of Toc159 as a selective import receptor for the targeting of a set of preproteins required for chloroplast biogenesis.
Keywords: chloroplasts; protein transport; protein binding; cross-linking; GTPase
Introduction
The biogenesis of chloroplasts relies on the import of ∼3,000 nucleus-encoded preproteins. Targeting of the majority of these preproteins to the organelle is mediated by interactions between their intrinsic NH2-terminal transit peptides and Toc159 and Toc33/34, two GTPase subunits of the preprotein translocon at the outer envelope membrane of chloroplasts (Toc; Keegstra and Froehlich, 1999; Jarvis and Soll, 2002). Toc159 and Toc33/34 associate with Toc75, a component of the translocation pore, to constitute the core of the outer envelope translocation machinery (Bauer et al., 2001).
The import of preproteins into chloroplasts requires GTP hydrolysis, implicating the two Toc GTPases as regulators of transit peptide recognition and/or the translocation reaction. Toc159 is proposed to serve as the primary site of transit peptide recognition during import into isolated chloroplasts, based on the observations that the transit peptides of chloroplast-bound preproteins covalently cross-link to Toc159 (Perry and Keegstra, 1994; Ma et al., 1996; Kouranov and Schnell, 1997), and anti-Toc159 antibodies inhibit preprotein binding and import (Hirsch et al., 1994). In vivo, Toc159 partitions approximately equally between a soluble cytoplasmic form and a membrane-bound form that is integrated into the Toc complex (Hiltbrunner et al., 2001b; Lee et al., 2003). Targeting of the putative soluble receptor to Toc complexes involves a direct interaction between the G domains of Toc159 and Toc33/34 and is regulated by GTP hydrolysis (Bauer et al., 2002; Smith et al., 2002b; Lee et al., 2003; Wallas et al., 2003). These observations have led to the proposal that the protein functions as a cycling receptor that delivers newly synthesized preproteins to the Toc complex during the import reaction (Hiltbrunner et al., 2001b; Smith et al., 2002b).
In Arabidopsis thaliana, the Toc159 gene family consists of four members: atToc159, atToc132, atToc120, and atToc90 (Bauer et al., 2000; Hiltbrunner et al., 2001a). A null mutant of atToc159, ppi2, exhibits an albino phenotype and is not viable on soil beyond the cotyledon stage of development (Bauer et al., 2000). Remarkably, ppi2 plants survive when grown on sucrose-supplemented media, indicating that although ppi2 is defective in photosynthetic capacity, other essential constitutive functions of plastids remain intact. On the basis of the analysis of the ppi2 mutant and the in vitro data supporting a receptor role for Toc159 in peas, we hypothesized that Toc159 functions as a specific transit peptide receptor for the import of a subclass of nucleus-encoded preproteins that are required for the assembly of the photosynthetic apparatus during photomorphogenesis (Bauer et al., 2000; Hiltbrunner et al., 2001b; Smith et al., 2002b). This hypothesis predicts that other members of the Toc159 family mediate targeting of constitutively expressed plastid proteins.
In this work, we have investigated two essential elements of this hypothesis. First, we investigate the targeting of different preproteins to plastids in the ppi2 mutant to test directly whether atToc159 is specifically required for the import of light-induced chloroplast-specific proteins. We provide in vivo and in vitro evidence that atToc159 is required for the import of several photosynthetic preproteins, but not representative constitutively expressed proteins. Second, we examine the proposal that atToc159 functions as a soluble receptor by testing its ability to specifically bind transit peptides. We demonstrate that soluble atToc159 binds specifically to chloroplast preproteins via an interaction between transit peptides and the G domain of the receptor. These data provide direct evidence for the function of Toc159 as a selective preprotein receptor and suggest a possible mechanism for the role of the Toc159 GTPase in preprotein targeting to the Toc complex.
Results
In vivo targeting of photosynthetic versus constitutive preproteins to ppi2 plastids
The specific defect in the accumulation of light-induced photosynthetic proteins in the ppi2 mutant (Bauer et al., 2000) is consistent with the proposal that atToc159 functions as a selective protein import receptor. However, this interpretation is complicated by the fact that the transcriptional expression of a wide array of chloroplast proteins is down-regulated in response to many types of disruptions in organelle integrity. As a result, the ppi2 defect could reflect a secondary effect on gene expression rather than a direct effect on preprotein import (Yu and Li, 2001).
To test whether the ppi2 phenotype results from a direct or indirect effect on import, we examined import of the precursor to the small subunit of Rubisco (pSSU) and the precursor to the pyruvate dehydrogenase E1α subunit (pE1α), proteins whose accumulation is dramatically reduced or unaffected in the mutant, respectively (Fig. 1 A). We generated genes encoding the pSSU and pE1α transit peptides fused to GFP and introduced them into ppi2 plants under the control of the constitutive [35S]CaMV promoter. Under these conditions, the expression of the transit peptide–GFP fusions is independent of both light and the physiological state of the chloroplast, thereby eliminating the complications of distinguishing between effects on transcription and protein import. As a control, plants were also transformed with a GFP construct lacking a transit peptide.
Figure 1.
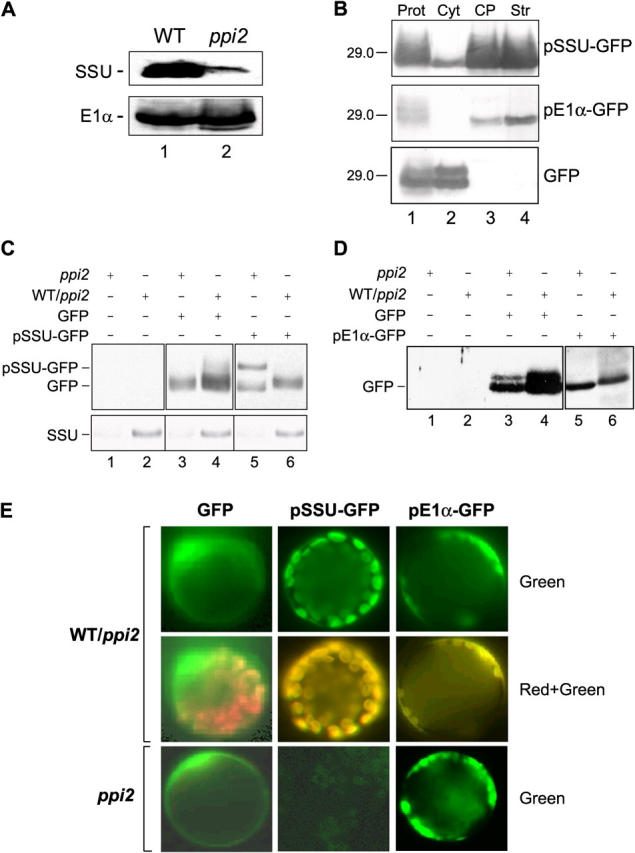
In vivo targeting of transit peptide–GFP fusion proteins to plastids in heterozygous or homozygous ppi2 seedlings. Heterozygous ppi2 plants were transformed with binary vector constructs encoding pSSU-GFP, pE1 α-GFP, or GFP. (A) Levels of endogenous SSU and E1α in wild-type (WT) and ppi2 plants. Extracts from 3-wk-old plants (75 μg protein) were resolved by SDS-PAGE and immunoblotted with antisera to each protein. (B) Enrichment of pSSU-GFP and pE1α-GFP in chloroplast fractions. Protoplasts (Prot) from heterozygous ppi2 plants expressing either pSSU-GFP, pE1α-GFP, or GFP alone were fractionated into cytoplasmic (Cyt), total chloroplast (CP), and chloroplast stroma (Str) fractions. The fractions (12 μg protein) were immunoblotted with anti-GFP antibodies. The 29-kD marker is indicated to the left of each panel. (C and D) Immunoblot analysis of total protein extracts from heterozygous ppi2 (WT/ppi2) and homozygous ppi2 (ppi2) plants expressing GFP, pSSU-GFP, or pE1α-GFP with anti-GFP antibodies (top panels) or with an anti-SSU serum (C, bottom). Lanes 1 and 2 (C and D) contain protein extracts from plants not transformed with GFP constructs. Black lines indicate grouping of images from different portions of the same gel. Images from different gels are in separate boxes. (E) Confocal laser scanning microscopy of protoplasts isolated from heterozygous ppi2 (WT/ppi2) or homozygous ppi2 (ppi2) plants expressing GFP, pSSU-GFP, or pE1α-GFP. GFP fluorescence (Green) and the merge of chlorophyll autofluorescence and GFP fluorescence (Red + Green) are shown.
The distribution of the GFP constructs in phenotypically normal heterozygous ppi2 plants was assessed to confirm their proper import and processing in vivo. Extracts of the transformants were separated into intact chloroplasts and a soluble fraction containing cytoplasm, and the fractions were immunoblotted with an anti-GFP mAb (Fig. 1 B). Mature GFP has a molecular mass of ∼27 kD, whereas pSSU-GFP and pE1α-GFP are 33.5 and 36.4 kD, respectively. Heterozygous ppi2 plants expressing the transit peptide–GFP fusions or GFP alone contain immunoreactive bands at ∼27 kD, indicating that the GFP fusions were imported into plastids and properly processed (Fig. 1 B). The fusion proteins were enriched in the chloroplast fraction of the extracts, confirming their localization to the organelle (Fig. 1 B). In contrast, GFP lacking a transit peptide was localized exclusively in the soluble cytoplasmic fraction (Fig. 1 B). The sizes of the imported products are identical to the sizes of imported products observed in in vitro import assays using isolated Arabidopsis chloroplasts (unpublished data). Thus, all of the fusions are competent for import and processing in vitro and in vivo.
The pattern of pE1α-GFP processing in homozygous ppi2 plants is indistinguishable from heterozygous plants, indicating that it is imported in vivo in the absence of atToc159 (Fig. 1 D, compare lane 5 with lane 6). In contrast, homozygous ppi2 plants accumulate a higher mol wt polypeptide in the pSSU-GFP transformed line (Fig. 1 C, compare lane 5 with lane 6). This polypeptide is the same size as its corresponding in vitro–translated fusion protein (unpublished data), indicating that it is not imported or processed in the mutant. The expression levels of the GFP construct in all genotypes of both lines is approximately equivalent, discounting the possibility that varying levels of expression account for the differences in processing.
To establish that the immunoblots of the transit peptide–GFP fusions represented the state of plastid localization and not aberrant processing, we determined the subcellular distribution of the GFP fusions by direct fluorescence microscopy in protoplasts derived from the leaves of transformed plants. GFP lacking a transit peptide gave a diffuse cytoplasmic and nuclear fluorescence pattern in both heterozygous and homozygous ppi2 plants (Fig. 1 E, left). In contrast, both transit peptide–GFP fusions expressed in heterozygous ppi2 (WT/ppi2) plants gave a distinct patched fluorescence pattern characteristic of chloroplast localization (Fig. 1 E, top). Moreover, the green fluorescence pattern for the fusion proteins overlaps with the red autofluorescence of chlorophyll (Fig. 1 E, middle), confirming the localization of both fusion proteins to chloroplasts. However, only plants expressing pE1α-GFP exhibit a punctate fluorescence pattern characteristic of plastid localization in homozygous ppi2 protoplasts (Fig. 1 E, bottom). The fluorescence pattern of ppi2 homozygous plants expressing pSSU-GFP is markedly distinct from wild-type plants (Fig. 1 E, compare top and bottom panels of middle column). Although expression levels of the construct are similar to those in control plants (Fig. 1 C, compare lane 5 with lane 6), there is no detectable green fluorescence in the ppi2 protoplasts (Fig. 1 E). The unprocessed pSSU-GFP fusion does not fluoresce in these plants because the pSSU transit peptide prevents proper GFP folding and/or fluorophore acquisition (unpublished data). On the basis of these data, we conclude that the lack of atToc159 results in the inability of plastids to import pSSU-GFP, consistent with the proposal that ppi2 plants are specifically affected in their ability to import photosynthetic preproteins.
Preprotein binding by soluble atToc159
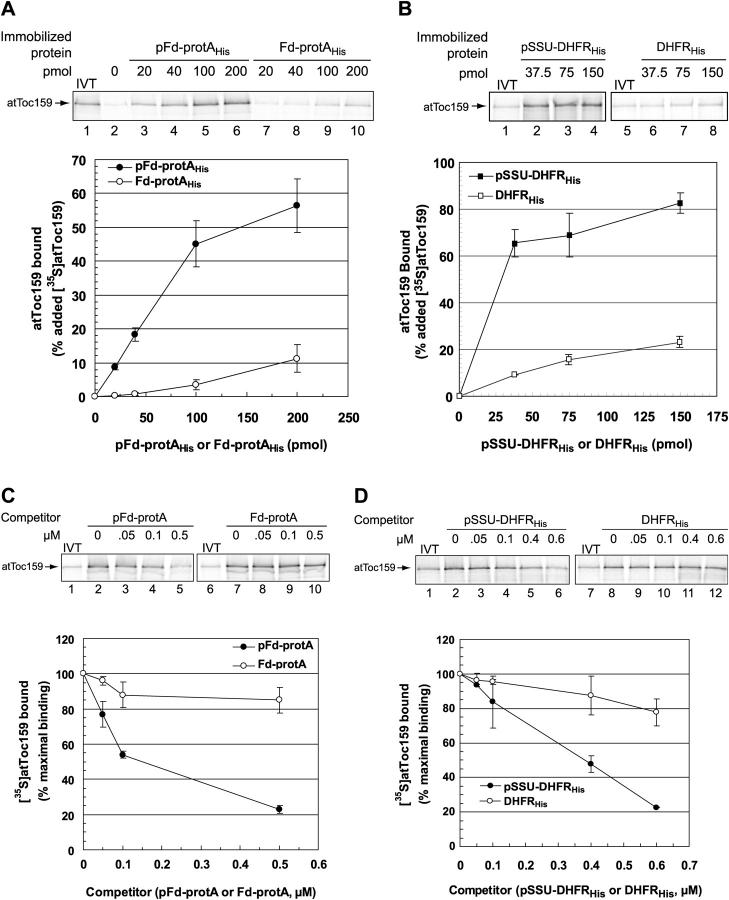
To directly examine the potential role of atToc159 as a receptor, we tested its ability to specifically and selectively bind transit peptides. As a first step in this analysis, we tested binding to two hybrid preproteins: pSSU-DHFRHis corresponding to the transit peptide of pSSU fused to dihydrofolate reductase (DHFR), and pFd-protAHis corresponding to preferredoxin fused to Staphylococcal protein A (protA). The transit peptides of both proteins were previously shown to cross-link to Toc159 when bound to isolated chloroplasts (Ma et al., 1996; Kouranov and Schnell, 1997). As controls we generated the comparable fusion proteins lacking the transit peptides (Fd-protAHis and DHFRHis). The fusion proteins were immobilized on nickel-nitrilotriacetic acid (Ni-NTA) matrix via their COOH-terminal hexahistidine tags and were incubated with in vitro–translated [35S]atToc159. Binding was measured as the fraction of [35S]atToc159 that cosedimented with the immobilized fusion proteins.
As shown in Fig. 2, [35S]atToc159 bound efficiently to both immobilized pFd-protAHis and pSSU-DHFRHis. Binding was dose dependent, reaching a maximum at ∼50 and ∼75% of added [35S]atToc159 for pFd-protAHis (Fig. 2 A, lanes 3–6) and pSSU-DHFRHis (Fig. 2 B, lanes 2–4), respectively. In contrast, the Fd-protAHis and DHFRHis controls bound <10% of [35S]atToc159 when tested at levels where maximum binding was observed with the transit peptide fusions (Fig. 2 A, compare lane 5 with lane 9; Fig. 2 B, compare lane 2 with lane 6). [35S]atToc159 exhibited no significant binding to the Ni-NTA matrix alone (Fig. 2 A, lane 2). Therefore, the interaction of atToc159 with the fusion proteins is dependent on the presence of a functional transit peptide.
Figure 2.
Soluble atToc159 binds specifically to chloroplast preprotein transit peptides. [35S]atToc159 was incubated with increasing amounts of immobilized pFd-protAHis or Fd-protAHis (A), or with pSSU-DHFRHis or DHFRHis (B). Lane 2 in A contains [35S]atToc159 that bound to the Ni-NTA resin alone. Binding is presented as the percentage of added [35S]atToc159 recovered in each reaction. (C) 100 pmol immobilized pFd-protAHis was incubated with soluble [35S]atToc159 in the absence or presence of increasing concentrations of soluble pFd-protA or Fd-protA. Binding is presented as the percentage of maximal [35S]atToc159 binding. (D) [35S]atToc159 was incubated with 100 pmol IgG-Sepharose–immobilized pFd-protA in the absence or presence of increasing concentrations of soluble pSSU-DHFRHis or DHFRHis. Binding is presented as in C. Error bars represent SEM from triplicate experiments. Lanes labeled IVT in all panels contain 10% of the [35S]atToc159 in vitro translation product added to each reaction.
To further establish the specificity of binding, we tested the ability of the soluble preproteins and their mature counterparts to compete for the binding of [35S]atToc159 to the preferredoxin fusion proteins. [35S]atToc159 was incubated with immobilized pFd-protAHis in the presence of soluble pFd-protA, Fd-protA, pSSU-DHFRHis, or DHFRHis. pFd-protA (Fig. 2 C) and pSSU-DHFRHis (Fig. 2 D) effectively competed for binding of the receptor in a dose-dependent manner. Both preproteins reduced binding to ∼20% of maximum binding at 0.5–0.6 μM of competitor (Fig. 2 C, lane 5). The control proteins lacking transit peptides, Fd-protA (Fig. 2 C) and DHFRHis (Fig. 2 D), were ineffective as competitors for receptor binding. These data demonstrate that atToc159 binds preproteins via a specific interaction with their transit peptides, and support the proposal that the soluble protein can function as a receptor.
atToc159 binds with low affinity to the transit peptides of nonphotosynthetic preproteins
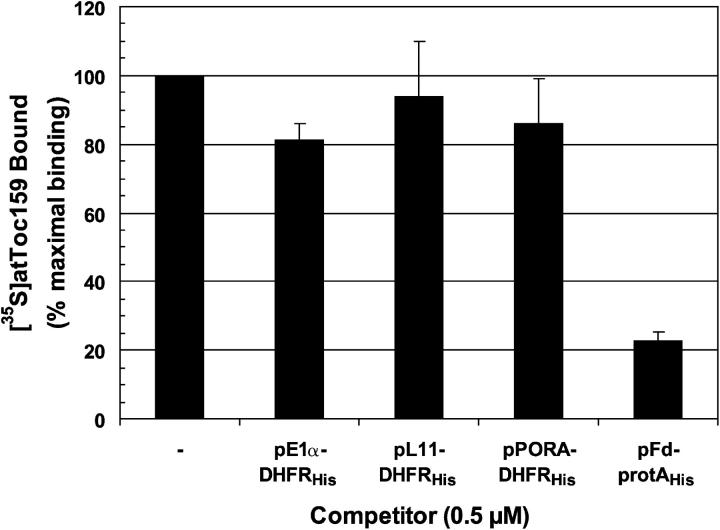
Having established that [35S]atToc159 interacts specifically with the transit peptides of two chloroplast-specific photosynthetic proteins, we next investigated whether the selective import defect observed in ppi2 was due to differential preprotein binding by atToc159. To this end, we overexpressed fusion proteins containing the transit peptides of three different nonphotosynthetic plastid proteins and tested their abilities to compete with immobilized pFd-protA for binding to [35S]atToc159. The transit peptides were derived from pE1α (pE1α-DHFRHis), the precursor to plastid ribosomal subunit L11 (pL11-DHFRHis), and the precursor to protochlorophyllide oxidoreductase A (pPORA-DHFRHis). As shown in Fig. 3, none of the three constructs compete significantly for binding of [35S]atToc159 to pFd-protA at concentrations where pFd-protAHis inhibits binding by ∼80% (Fig. 2 C). These data correlate with the selective import defect observed in the ppi2 mutant and suggest that the differential accumulation of different plastid proteins in these plants (Fig. 1) is due to a requirement for atToc159 as a specific receptor for at least a subset of essential photosynthetic proteins.
Figure 3.
atToc159 specifically recognizes the transit peptides of photosynthetic preproteins. [35S]atToc159 was incubated with 50 pmol IgG-Sepharose–immobilized pFd-protA in the absence or presence of 0.5 μM pE1α-DHFRHis, pL11-DHFRHis, or pPORA- DHFRHis, or with 50 pmol immobilized pFd-protAHis in the absence or presence of 0.5 μM pFd-protA. The data from triplicate experiments are presented as the percentage of maximal binding of [35S]atToc159 to pFd-protA or pFd-protAHis in the absence of competitor. Error bars represent SEM.
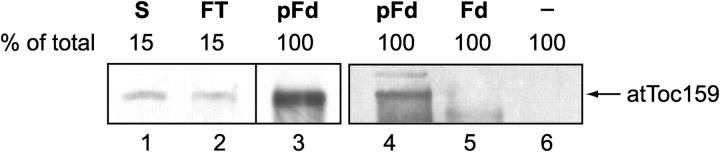
Endogenous soluble atToc159 binds preprotein
Given the results of our analysis of import in the ppi2 mutant and the ability of in vitro–translated atToc159 to bind transit peptides, we wished to investigate whether the soluble, cytoplasmic form of atToc159 could function as a transit peptide receptor by testing its ability to interact with preproteins. To this end, we isolated a soluble Arabidopsis extract containing cytoplasm and applied it to columns containing immobilized pFd-protAHis or Fd-protAHis. atToc159 binding was detected by immunoblotting eluates with an anti-atToc159 serum. As is shown in Fig. 4, cytoplasmic atToc159 bound to pFd-protAHis (lanes 3 and 4), but not to control columns either lacking immobilized protein (lane 6) or containing Fd-protAHis (lane 5). These data demonstrate that endogenous soluble atToc159 is able to recognize and bind specifically to preprotein transit peptides.
Figure 4.
Endogenous soluble atToc159 binds to the transit peptide of preferredoxin. A soluble Arabidopsis protoplast extract containing cytoplasm was applied to Ni-NTA columns containing 75 μg immobilized pFd-protAHis (pFd, lanes 3 and 4) or Fd-protAHis (Fd, lane 5), or Ni-NTA matrix alone (lane 6). Bound proteins were eluted, resolved using SDS-PAGE, and immunoblotted with anti-atToc159 antibodies. Lanes 1 and 2 show 15% of the starting material (S) and unbound fractions (FT) for the pFd-protAHis column. The dividing line indicates grouping of lanes from different parts of the same gel.
Transit peptide binding maps to the G and M domains of atToc159
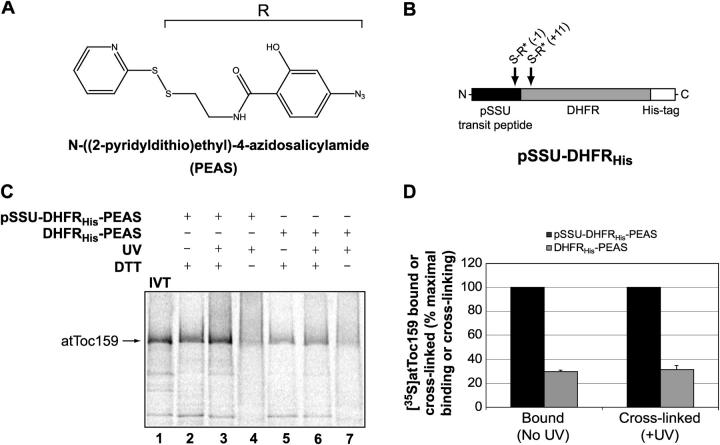
Upon establishing the specific interaction of atToc159 with preproteins, we wished to examine the regions of the receptor that form the transit peptide–binding site. Toc159 consists of three structurally distinct segments: an NH2-terminal acidic domain (A domain), a central GTPase domain (G domain), and a COOH-terminal membrane anchor domain (M domain). As a first step in identifying the segments required for transit peptide binding, we used a covalent cross-linking strategy in which we incorporated a photoactivatable cross-linker into pSSU-DHFRHis or DHFRHis. The proteins were modified at cysteine residues with the cleavable, photoactivatable cross-linker, N-((2-pyridyldithio)ethyl)-4-azidosalicylamide (PEAS) by disulfide exchange (Fig. 5 A). pSSU-DHFRHis was chosen as the cross-linking substrate because it has one cysteine at the last residue of the transit peptide (position −1) and one cysteine 11 residues into the DHFR sequence (position +11; Fig. 5 B). Previous reports have shown that modification of the cysteine within the transit peptide of pSSU does not inhibit preprotein binding or import into isolated chloroplasts, and therefore is unlikely to block receptor binding (Ma et al., 1996; Kouranov and Schnell, 1997). DHFRHis contains only the cysteine within DHFR and was used as the control for the cross-linking reactions. The modified substrates are referred to as pSSU-DHFRHis-PEAS and DHFRHis-PEAS.
Figure 5.
Chemical cross-linking of atToc159 to the transit peptide of the small subunit of Rubisco. (A) Structure of the heterobifunctional PEAS cross-linker. The photoactivatable phenyl azido group and linker arm that are transferred to a cysteine residue in a disulfide exchange reaction are labeled as “R.” (B) Schematic representation of the pSSU-DHFRHis construct used in the cross-linking reactions. Arrows point to cysteines at positions −1 and +11 of pSSU-DHFRHis that, when fully reduced, undergo a disulfide exchange with PEAS (indicated by R*). (C) [35S]atToc159 was incubated with immobilized pSSU-DHFRHis-PEAS or DHFRHis-PEAS. After the incubation, reactions were treated without (−) or with (+) UV light to activate the cross-linker. Resin-bound proteins were eluted from the Ni-NTA and treated with (+) or without (−) DTT before being resolved by SDS-PAGE. [35S]atToc159 was detected in dried gels using a phosphorimager. Lane 1 contains 30% of the [35S]atToc159 in vitro translation product (IVT) added to each reaction. (D) [35S]atToc159 bound or cross-linked to pSSU-DHFRHis-PEAS or DHFRHis-PEAS in samples treated with DTT was quantitated using a phosphorimager. Data are presented as the percentage of maximal binding or cross-linking. Quantitation of the data from two replicates is shown.
Soluble [35S]atToc159 was incubated with pSSU-DHFRHis-PEAS or DHFRHis-PEAS in the in vitro pull-down assay and the reactions were exposed to UV light to induce cross-linking or retained in the dark to prevent covalent coupling. The samples were treated without or with DTT to cleave the cross-linked products and the proteins were resolved by SDS-PAGE. As shown in Fig. 5 C, soluble [35S]atToc159 binds with similar efficiency to pSSU-DHFRHis-PEAS as it does to pSSU-DHFRHis (compare Fig. 5 C, lane 2 with Fig. 2 B). Furthermore, [35S]atToc159 binding to DHFRHis-PEAS is fourfold lower than to pSSU-DHFRHis-PEAS (Fig. 5 C, compare lane 2 and lane 5; Fig. 5 D, Bound), as is the case for the nonderivitized proteins (see Fig. 2 B). Therefore, derivitization of pSSU-DHFRHis with PEAS does not affect the interaction with soluble [35S]atToc159. Illumination with UV light also does not alter the efficiency of the interaction of atToc159 with pSSU-DHFRHis-PEAS (Fig. 5 C, compare lane 2 with lane 3; see also Fig. 5 D). However, irradiation does appear to result in covalent coupling of the receptor to pSSU-DHFRHis-PEAS because of the apparent shift in [35S]atToc159 to a higher mol wt smear (Fig. 5 C, compare lane 3 with lane 4). The shift is drastically reduced when DHFRHis-PEAS is used as the substrate (Fig. 5 C, compare lane 3 with lane 6, lane 4 with lane 7). These data indicate that pSSU-DHFRHis-PEAS specifically and efficiently cross-links to soluble [35S]atToc159, and is therefore a suitable substrate for mapping the transit peptide–binding site.
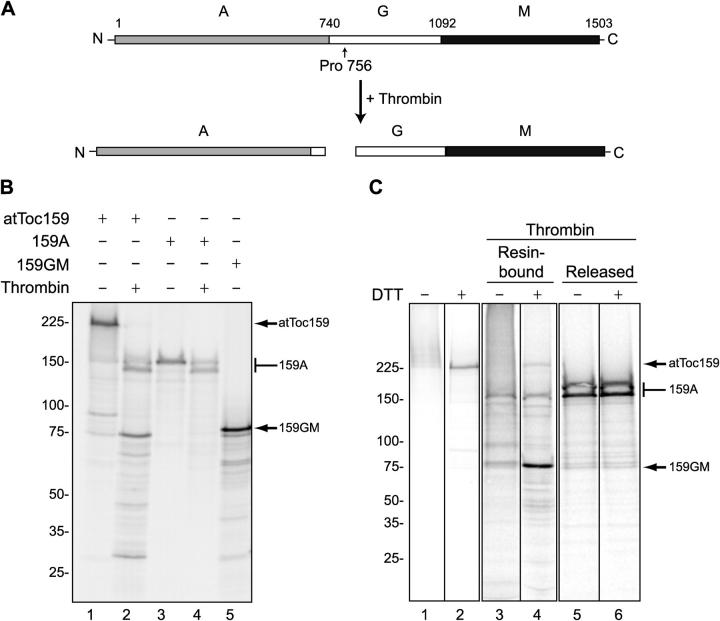
To distinguish which regions of atToc159 interact with the transit peptide, we used a selective proteolysis strategy to cleave atToc159 after the cross-linking reaction. atToc159 contains a consensus cleavage site for thrombin between Pro 756 and Arg 757. Digestion is predicted to generate two fragments approximately corresponding to the A domain (159A) and the combined G and M domains (159GM; Fig. 6 A). To confirm the specific cleavage of atToc159, we incubated in vitro–translated [35S]atToc159 with thrombin and separated the fragments using SDS-PAGE. The digestion produced a doublet at ∼150 kD and a third fragment at 75 kD (Fig. 6 B, lane 2). The 75-kD cleavage product comigrates with authentic in vitro–translated [35S]159GM, confirming its identity (Fig. 6 B, compare lane 2 with lane 5). In vitro–translated [35S]159A comigrates with the upper band of the ∼150-kD doublet (Fig. 6 B, compare lane 2 with lane 3), suggesting that this domain might have an additional cryptic thrombin site. This was confirmed by treatment of [35S]159A with thrombin. This treatment revealed an identical pattern to the ∼150-kD doublet observed with intact [35S]atToc159 (Fig. 6 B, compare lane 3 with lane 4). Therefore, there is one additional thrombin cleavage site within 159A, which gives rise to the doublet at ∼150 kD (Fig. 6 B, compare lane 2 with lane 4).
Figure 6.
Transit peptides cross-link to the GM domain of atToc159. (A) Schematic representation of the proteolysis strategy for mapping the transit peptide–binding site on atToc159. Thrombin is predicted to cleave atToc159 between Pro756 and Asp757. (B) [35S]atToc159, [35S]atToc159A, and [35S]atToc159GM were treated without (−) or with (+) thrombin for 1 h at 37°C and resolved by SDS-PAGE. (C) pSSU-DHFRHis was modified with PEAS as described in the legend to Fig. 5, immobilized on Ni-NTA resin, and incubated with [35S]atToc159 in the presence of GTP. After cross-linking, reactions were treated without (lanes 1 and 2) or with (lanes 3–6) thrombin and were separated into resin-bound and released fractions. The samples were then treated without (−) or with (+) DTT, separated by SDS-PAGE, and analyzed using a phosphorimager. The positions of mol wt markers (kD) are indicated to the left, and atToc159, atToc159A (159A), and atToc159GM (159GM) to the right of each figure. Black lines indicate grouping of images from different portions of the same gel. Images from different gels are in separate boxes.
We performed our standard in vitro binding and cross-linking assay, and incubated the cross-linked products with thrombin. UV irradiation resulted in cross-linking of [35S]atToc159 to pSSU-DHFRHis-PEAS, as demonstrated by the shift of the intact receptor to a lower mobility smear in the absence of DTT compared with the presence of DTT (Fig. 6 C, compare lane 1 with lane 2). After thrombin treatment of the cross-linked mixture, the resin was recovered by centrifugation to yield a supernatant containing any thrombin-released fragments of the receptor that were not covalently bound to pSSU-DHFRHis-PEAS (Fig. 6 C, Released). The cross-linked fragments of the receptor were subsequently eluted from the matrix together with pSSU-DHFRHis-PEAS using imidazole (Fig. 6 C, Resin-bound). The vast majority of the 159GM fragment generated by thrombin remains covalently bound to immobilized pSSU-DHFRHis-PEAS, whereas the majority of the 159A is released (Fig. 6 C, compare lane 4 with lane 6). This result indicates that the preprotein specifically cross-links to regions within the G and M domains of the receptor.
When the samples are resolved by SDS-PAGE in the absence of a reducing agent, the mobility of the 159GM shifts to higher mol wt species (Fig. 6 C, compare lane 3 with lane 4), whereas the mobility of 159A in the resin-bound and released fractions is unaffected (Fig. 6 C, compare lane 3 with lane 4, and lane 5 with lane 6), providing additional evidence that the GM domain has indeed been cross-linked, whereas the A domain has not. We conclude that the transit peptide of the Rubisco small subunit cross-links specifically to regions within the GM domains of atToc159 and that the A domain is not involved directly in preprotein binding.
The G domain of atToc159 interacts specifically with transit peptides
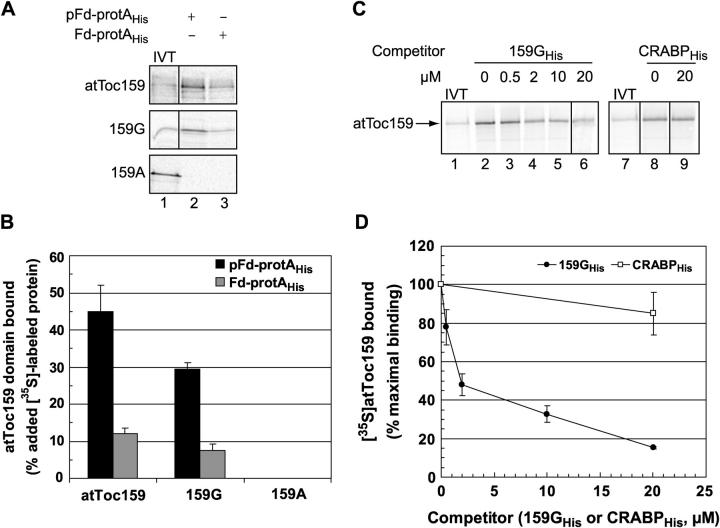
The covalent cross-linking data implicate the G and/or M domains of the atToc159 receptor in transit peptide binding. To test whether the G domain itself might comprise the transit peptide–binding domain of the atToc159 receptor, we expressed the G domain (159G) as a [35S]-labeled in vitro translation product and tested the ability of the fragment to bind to pFd-protAHis or Fd-protAHis in the solid phase binding assay. As a control we examined binding of the atToc159 A domain (159A) because the cross-linking experiments suggest that it does not play a direct role in substrate binding. As shown in Fig. 7 A, [35S]159G exhibits a similar pattern of binding to pFd-protAHis and Fd-protAHis as that of the full-length receptor, albeit with slightly lower efficiency (lane 2, compare top and middle panels; see also Fig. 7 B). The binding of [35S]159G to pFd-protAHis is threefold higher than to Fd-protAHis (Fig. 7 A, middle panel, compare lane 2 with lane 3; see also Fig. 7 B), suggesting that it recognizes and binds specifically to the transit peptide of preferredoxin. On the other hand, 159A does not bind detectably to pFd-protAHis or Fd-protAHis (Fig. 7 A, lanes 2 and 3, bottom), confirming that it is not involved directly in transit peptide binding.
Figure 7.
The GTPase domain of atToc159 binds transit peptides selectively. (A) [35S]atToc159, [35S]atToc159G (159G), or [35S]atToc159A (159A) was incubated with 100 pmol Ni-NTA–immobilized pFd-protAHis (lane 2) or Fd-protAHis (lane 3). Bound proteins were eluted, separated by SDS-PAGE and analyzed using a phosphorimager. (B) Quantitation of the data from triplicate experiments including those in A. (C) 100 pmol IgG-Sepharose–immobilized pFd-protA was incubated with [35S]atToc159 in the absence or presence of increasing concentrations of atToc159GHis or CRABPHis. Bound proteins were eluted, resolved by SDS-PAGE, and analyzed using a phosphorimager. (D) Quantitation of the data from replicate experiments including those presented in C. Error bars represent SEM. Lanes labeled IVT in all panels contain 10% of the [35S]atToc159, [35S]atToc159G, or [35S]atToc159A in vitro translation products added to each reaction. Dividing lines in figures indicate grouping of images from different parts of the same gel.
To confirm that the G domain does contain a transit peptide–binding site, Escherichia coli–expressed 159GHis (Smith et al., 2002b) was added as a cold competitor of soluble [35S]atToc159 binding to pFd-protA that had been immobilized on IgG-Sepharose. Fig. 7 C shows that increasing concentrations of 159GHis effectively compete with [35S]atToc159 for binding to pFd-protA (Fig. 7 C, lanes 2–6; see also Fig. 7 D). This is in contrast to an unrelated control protein, CRABPHis (Clark et al., 1998), which does not compete for binding (Fig. 7 C, compare lane 6 with lane 9; see also Fig. 7 D). Collectively, the data indicate that the G domain of atToc159 specifically recognizes and binds transit peptides, and therefore comprises at least part of the preprotein binding site of the atToc159 receptor.
Nucleotide requirements for preprotein binding by atToc159
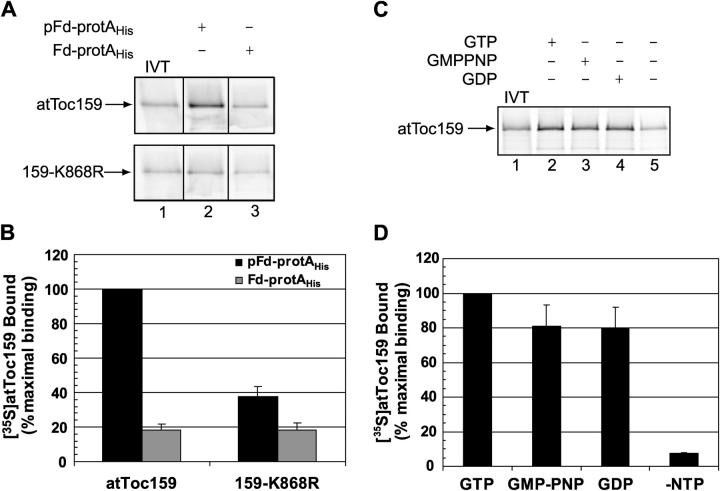
The identification of the G domain as part of the preprotein binding site of the atToc159 receptor raises the possibility that nucleotide binding/hydrolysis plays a role in transit peptide recognition. To investigate whether the guanine nucleotide status of atToc159 affects binding of preprotein, we made use of atToc159-K868R, a mutant form of atToc159 that contains a single point mutation in the consensus G1 GTP-binding motif (P-loop) that prevents nucleotide binding (Smith et al., 2002b). The [35S]atToc159-K868R mutant binds ∼60% less pFd-protAHis than does wild-type atToc159 in the in vitro pull-down assay (Fig. 8 A, lane 2, compare top and bottom panels; see also Fig. 8 B). This level of binding is only slightly more than the low level of binding to Fd-protAHis (Fig. 8 A, compare lane 2 with lane 3, bottom; see also Fig. 8 B). These data suggest that atToc159 requires bound nucleotide to specifically bind transit peptides.
Figure 8.
Preprotein binding by atToc159 requires nucleotide. (A) Nucleotide-depleted [35S]atToc159 or [35S]atToc159-K868R was incubated with 100 pmol immobilized pFd-protAHis (lane 2) or Fd-protAHis (lane 3) in the presence of GTP. Bound proteins were eluted, separated by SDS-PAGE, and analyzed using a phosphorimager. Lane 1 contains 10% of the [35S]atToc159 or [35S]atToc159-K868R in vitro translation products (IVT) added to each reaction. Dividing lines indicate grouping of lanes from different portions of the same gel. (B) Quantitation of data from three experiments including those presented in A. (C) pFd-protAHis was immobilized on Ni-NTA resin and incubated with nucleotide-depleted [35S]atToc159 in the absence or presence of 50 μM GTP, GMP-PNP, or GDP. Bound proteins were eluted, resolved by SDS-PAGE, and analyzed using a phosphorimager. Lane 1 contains 20% of the [35S]atToc159 added to each reaction. (D) Quantitation of the data from triplicate experiments, including those presented in C. Error bars represent SEM.
To further examine the nucleotide dependence of preprotein binding, immobilized pFd-protAHis was incubated with nucleotide-depleted [35S]atToc159 in the presence or absence of GTP, GDP, or the nonhydrolyzable GTP analogue guanyl-5′-yl imidodiphosphate (GMP-PNP). Fig. 8 C shows that preprotein binding by [35S]atToc159 in the absence of nucleotide is dramatically reduced compared with binding in the presence of GTP. In contrast, binding in the presence of GMP-PNP or GDP is reduced only by ∼20% (Fig. 8 C, compare lanes 2, 3, and 4). Together, the data in Fig. 8 suggest that atToc159 requires bound nucleotide to stably associate with transit peptides, but that transit peptide binding is not strictly regulated by the phosphorylation state of the nucleotide.
Discussion
In the current paper, we provide several pieces of evidence that fulfill the criteria for the assignment of Toc159 as a soluble preprotein receptor. First, atToc159 preferentially binds chimeric proteins containing functional transit peptides versus those lacking transit peptides in a solid phase binding assay (Fig. 2). Second, transit peptide binding maps to a specific domain of atToc159, the GTPase domain (Fig. 6 and Fig. 7). Third, transit peptide binding is dependent upon nucleotide binding at the receptor (Fig. 8). Finally, both recombinant atToc159 from an in vitro translation mixture and soluble atToc159 from Arabidopsis cytoplasm exhibit specific transit peptide binding (Fig. 2 and Fig. 4). The latter observation provides compelling evidence that Toc159 can bind to chloroplast preproteins in the cytoplasm and can potentially function as a soluble targeting receptor.
We also provide evidence to support the proposal that atToc159 is a selective receptor required for the import of a class of preproteins that is necessary for chloroplast biogenesis. We demonstrate that the ppi2 mutation results in the cytoplasmic accumulation of pSSU-GFP, whereas pE1α-GFP is imported and processed normally (Fig. 1). These data confirm that the reduced accumulation of some photosynthesis-related proteins in ppi2 is due to a direct import defect and not only a secondary effect of disrupting chloroplast integrity (Yu and Li, 2001). The selective defect observed in vivo with the ppi2 mutant was further substantiated by the observation that recombinant atToc159 bound to the pSSU and pFd transit peptides (Fig. 2) with much higher relative affinity than to pE1α, pL11, or pPORA transit peptides in an in vitro pull-down assay (Fig. 3). pE1α and pL11 are expressed in all plastid types and pPORA is reported to use a Toc-independent pathway for import (Reinbothe et al., 2004). This observation provides direct evidence for the selective binding of atToc159 to different preproteins and supports the conclusion that the ppi2 phenotype is due to a defect in the receptor function of atToc159. As such, atToc159 defines a specific pathway for protein import that is required for chloroplast biogenesis. Such a pathway could be required to accommodate the relatively large influx of this subclass of preproteins during photomorphogenesis, thereby avoiding competition for import between the precursors of major photosynthetic proteins and constitutively expressed plastid proteins. It remains to be determined whether atToc159 is required for the import of all highly expressed light-induced proteins, but our data suggest that at least an essential subset of these proteins use the atToc159 pathway. atToc90, atToc120, and/or atToc132 could define additional targeting pathways responsible for the import of other plastid proteins. These preproteins presumably possess functionally distinct transit peptides that are selectively recognized by these alternate receptors.
Our covalent cross-linking experiments demonstrate that the transit peptide–binding site of atToc159 is contained within the G and M domains of the receptor (Fig. 6). The analysis of atToc159 deletion mutants in the solid phase binding assay confirmed that the A domain does not interact with preproteins and indicated that the G domain alone binds with a similar specificity as the full-length receptor (Fig. 7). Furthermore, the isolated G domain can compete with the full-length receptor for binding to the preferredoxin transit peptide, suggesting that this domain of the protein represents an authentic transit peptide–binding site on the receptor (Fig. 7). The participation of the G domain in preprotein recognition suggested a possible role for GTP binding/hydrolysis in the interaction as well. Indeed, the interaction of the receptor with the preferredoxin transit peptide is disrupted by a single point mutation in atToc159 that inhibits nucleotide binding (atToc159-K868R), indicating that bound nucleotide is a prerequisite for preprotein binding (Fig. 8). Interestingly, it does not appear that the form of bound nucleotide is critical in regulating the interaction with transit peptides, as GDP and the nonhydrolyzable analogue of GTP, GMP-PNP, can support binding at ∼80% of the levels observed with GTP (Fig. 8).
When combined with the notable import defect of the ppi2 mutant, the preprotein binding and import data presented here, together with previous reports on the preprotein binding activity of Toc33/34, suggest a scenario for the coordinate action of the two GTPases in the targeting and import of preproteins into chloroplasts. In this model, soluble Toc159 would serve as the primary receptor for preproteins. Binding could be facilitated by the previously described guidance complex that includes a chaperone activity (May and Soll, 2000). Docking of the Toc159–preprotein complex at the chloroplast surface would be mediated by an interaction between the GTPase domains of Toc159 and Toc33/34 (Bauer et al., 2002; Smith et al., 2002b). Although we picture the initial interaction between Toc159 and preproteins occurring in the cytoplasm during or shortly after translation, it is clear from the analysis of protein import in vitro that Toc159 also can bind transit peptides at the chloroplast surface (Perry and Keegstra, 1994; Kouranov and Schnell, 1997), and therefore can function as a membrane-bound receptor. It remains to be determined which is the major pathway in vivo or whether both might operate simultaneously.
Subsequent to docking at the Toc complex, GTP hydrolysis at Toc159 and/or Toc33/34 would trigger two events. First, it would promote high affinity binding and insertion of Toc159 into the membrane to form a multimeric complex including Toc75 (Bauer et al., 2002; Smith et al., 2002b; Wallas et al., 2003). Second, hydrolysis of GTP at the two receptor components would also trigger insertion of the preprotein into the translocon channel (Young et al., 1999; Schleiff et al., 2003b). In this scenario, GTP hydrolysis would serve as a switch to ensure unidirectional targeting of preproteins. Furthermore, GTP hydrolysis at one or both Toc GTPases could provide the energetic driving force for translocation across the outer membrane translocon. The general aspects of this model can be extended to include the other members of the Toc159 receptor family, albeit with different classes of preproteins involved.
Previous works have indicated that Toc33/34 also binds preproteins (Sveshnikova et al., 2000; Jelic et al., 2002, 2003; Schleiff et al., 2002). Interestingly, the nucleotide state of Toc33/34 appears to affect its interaction with some, but not all, preproteins. For example, Toc34 binding to pSSU is strictly GTP dependent, although binding to pOE23 is nucleotide independent (Schleiff et al., 2002). Recent data also suggest that preprotein binding stimulates the GTPase activities of both Toc159 (Becker et al., 2004) and Toc34 (Jelic et al., 2002, 2003). Interestingly, the stimulation of Toc159 GTPase activity is strictly dependent on the transit peptide, whereas the stimulation of the Toc34 GTPase requires additional elements of the preprotein (Becker et al., 2004). Thus, the transit peptide–dependent recognition of preproteins by Toc159 at the initial stages of translocation might initiate the cascade of GTPase-dependent reactions that regulate the import process.
The data presented here and those of previous papers indicate that the domains of Toc159 participate in multiple steps in the import reaction. The G domain appears to mediate transit peptide binding and docking of the receptor at the translocon (Bauer et al., 2002; Smith et al., 2002b; Wallas et al., 2003). It should be noted that the binding efficiency of the isolated G domain is slightly lower than that of intact atToc159, suggesting that the M domain might also participate in the binding reaction (Fig. 7). Several observations suggest that the M domain plays a role in preprotein translocation across the outer membrane. Preproteins cross-link to the M domain of Toc159 during translocation through the Toc complex (Kouranov and Schnell, 1997). Furthermore, chloroplasts treated with thermolysin such that the A and G domains of Toc159 are cleaved, but Toc34 and Toc75 are left intact, can still import preproteins, albeit at a reduced rate when compared with untreated chloroplasts (Chen et al., 2000). Schleiff et al. (2003a) have shown that a fragment of Toc159 corresponding to the G and M domains together with Toc75 form the minimal unit required for translocation of a preprotein into reconstituted proteoliposomes in the absence of Toc34. Recently, Lee and colleagues (2003) were able to partially rescue the ppi2 mutant with only the M domain of atToc159. These data suggest that the M domain participates in the formation of the protein-conducting channel of the Toc complex and has led to the proposal that it functions as part of a GTP-driven translocation motor (Schleiff et al., 2003a). As such, Toc159 is emerging as a multifunctional translocon component that participates both in transit peptide recognition and membrane translocation.
Materials and methods
DNA constructs
Plasmids encoding atToc159, atToc159-K868R, atToc159A, atToc159GHis, atToc159G, atToc159GM, pFd-protA, Fd-protA, pFd-protAHis, and Fd-protAHis have been described previously (Ma et al., 1996; Bauer et al., 2000; Smith et al., 2002b). The pET21d-DHFRHis plasmid encoding DHFRHis was generated by modifying the coding region of DHFR by PCR such that it could be inserted into pET21d in-frame with a COOH-terminal histidine tag. Coding sequences for the transit peptides of pSSU, pPORA, pL11, and pE1α were amplified from A. thaliana cDNA and fused in-frame with the coding sequence of DHFRHis to generate pET21d-pSSU-DHFRHis, pET21d-pPORA-DHFRHis, pET21d-pL11-DHFRHis, and pET21d-pE1α-DHFRHis, respectively.
Constructs encoding pE1α-GFPHis and pSSU-GFPHis were generated by amplifying the coding sequences for the transit peptides plus the first four residues of the mature portions of pE1α and pSSU from Arabidopsis cDNA using RT-PCR such that they could be fused in-frame to the 3′ end of the coding sequence of GFP in pBluescript®-GFP (a gift from Dr. A.Y. Cheung, University of Massachusetts, Amherst, MA). For expression of the GFP fusions in Arabidopsis, the coding regions of GFP, pSSU-GFP, and pE1α-GFP were inserted into the binary vector, pSMB (Mylne and Botella, 1998), to generate pSMB-GFP, pSMB-pSSU-GFP, and pSMB-pE1α-GFP. Purified recombinant CRABPHis was a gift from Dr. L. Gierasch, University of Massachusetts, Amherst, MA.
In vitro translation and protein expression in E. coli
All [35S]methionine-labeled in vitro translation products were generated in a coupled transcription–translation system containing reticulocyte lysate according to the manufacturer's instructions (Promega). When noted, the mixture was depleted of free nucleotides by gel filtration as described previously (Chen and Schnell, 1997).
Bacterial expression of all constructs was performed in E. coli BL21 (DE3) using 0.4 mM IPTG for 3 h at 37°C. pSSU-DHFRHis, DHFRHis, pFd-protAHis, Fd-protAHis, pE1α-DHFRHis, pPORA-DHFRHis, pL11-DHFRHis, and atToc159GHis were purified using Ni-NTA chromatography (Novagen). pFd-protA and Fd-protA without COOH-terminal hexahistidine tags were purified from E. coli lysates using IgG-Sepharose chromatography as described previously (Schnell and Blobel, 1993).
Solid phase binding assays
For assays using Ni-NTA resin, urea-denatured pFd-protAHis, Fd-protAHis, pSSU-DHFRHis, or DHFRHis was rapidly diluted 50-fold into 50 mM Hepes-KOH, pH 7.5, 2 mM MgCl2, and 40 mM KOAc (HMK buffer), incubated for 30 min at RT, and centrifuged at 18,000 g for 10 min to remove insoluble aggregates. The soluble protein was bound to ∼7 μl of packed Ni-NTA resin and washed with HMK buffer containing 10 mM imidazole and 0.1% Triton X-100 (binding buffer), and 0.1 mM GTP, GMP-PNP, or GDP as indicated. The resin was incubated with 1–3 μl [35S]atToc159, [35S] atToc159-K868R, [35S]atToc159G, or [35S]atToc159A in binding buffer with the appropriate nucleotide in a final volume of 100 μl for 30 min at RT. After washing, resin-bound proteins were eluted with SDS-PAGE sample buffer containing 0.75 M imidazole.
For assays using IgG-Sepharose, purified pFd-protA was bound to 5 μl packed IgG Sepharose. The resin was washed with HMK buffer containing 0.1 mM GTP and 0.1% Triton X-100, and incubated with [35S]atToc159 in the absence or presence of increasing concentrations of pSSU-DHFRHis, DHFRHis, atToc159GHis, CRABPHis (Clark et al., 1998), pE1α-DHFRHis, pL11-DHFRHis, or pPORA-DHFRHis in a final volume of 100 μl for 30 min at RT. After washing, bound proteins were eluted using 0.2 M glycine, pH 2.2. All proteins from in vitro pull-down assays were resolved using SDS-PAGE, and radiolabeled proteins were detected in dried gels using a phosphorimager (Storm 840; Molecular Dynamics) and quantitated using ImageQuant version 5.2 software.
Preparation of chloroplasts and soluble extracts from Arabidopsis
Chloroplasts were isolated from Arabidopsis protoplasts as described previously (Smith et al., 2002a). For the purpose of isolating a soluble extract containing cytoplasm, protoplasts were first evacuolated using a method adapted from Newell et al. (1998). Specifically, protoplasts were resuspended in 20 mM MES-KOH, pH 6.0, 0.4 M mannitol, and 1 mM CaCl2, layered onto a cushion of 30% (vol/vol) percoll, 20 mM MES-KOH, pH 6.8, and 0.5 M mannitol, and evacuolated by centrifugation at 100,000 g for 30 min at 21°C in a swinging bucket rotor (SW41Ti; Beckman Coulter). The evacuolated protoplasts, which formed a band at the interface with the silica pellet, were diluted into 50 ml HMK buffer containing 330 mM sorbitol (HMKS) and were collected by centrifugation at 100 g for 4 min in an HB-4 rotor (Sorvall). The protoplasts were resuspended in 1 ml HMKS containing 0.02% Triton X-100 and 0.2% (vol/vol) protease inhibitor cocktail (P9599; Sigma-Aldrich), and were ruptured by forcing them twice through layers of 20- and 10-μm nylon mesh. The lysate was immediately centrifuged at 1,000 g for 4 min to pellet intact chloroplasts, and the supernatant containing cytoplasm was removed and centrifuged at 100,000 g for 20 min to remove residual membranes. The resulting supernatant, containing membrane-free cytoplasm, was used for further analysis. Immunoblotting was performed as described previously (Ma et al., 1996).
Affinity chromatography
The soluble extract obtained from evacuolated protoplasts was applied to columns containing 75 μg of pFd-protAHis or Fd-protAHis immobilized on 250 μl of packed Ni-NTA resin under gravity at 4°C. The resin was washed with 20 column volumes of binding buffer, and bound proteins were eluted in the same buffer containing 500 mM imidazole. All fractions were precipitated with 10% TCA, resolved using SDS-PAGE, transferred to nitrocellulose, and immunoblotted with affinity-purified atToc159 antibody as described previously (Chen et al., 2002).
Modification of pSSU-DHFRHis and DHFRHis with PEAS
All precursor modification and cross-linking assays were performed in the dark. Purified pSSU-DHFRHis and DHFRHis in 6 M urea were incubated with 2% (vol/vol) β-mercaptoethanol for 15 min at 37°C, and were gel filtered using Sephadex G-25 equilibrated in HMK buffer containing 6 M urea (immobilization buffer) to remove the β-mercaptoethanol. The filtered proteins were mixed with PEAS (Molecular Probes, Inc.) at a 1:100 (protein/PEAS) molar ratio and incubated for 3 h at RT. The modified proteins were used immediately or stored at −80°C for later use.
Cross-linking assays
Cross-linking between pSSU-DHFRHis-PEAS or DHFRHis-PEAS and [35S] atToc159 was performed using a modified solid phase binding assay. In brief, 37.5 pmol pSSU-DHFRHis-PEAS or DHFRHis-PEAS was bound to ∼50 μl packed Ni-NTA resin in immobilization buffer. The resin was incubated with 7–10 μl nucleotide-depleted [35S]atToc159 containing 1 mM GTP in a final volume of 400 μl binding buffer for 30 min at RT with constant mixing.
The reaction was divided into three equivalent samples and was held on ice. Two were irradiated from above with UV light at a distance of ∼5 cm using a Chromato-Vue transilluminator (Ultra-Violet Products) at 312 nm for 5 min with constant shaking, whereas the third was kept in the dark. All three samples were washed with binding buffer, eluted directly into SDS-PAGE sample buffer containing 0.75 M imidazole, and resolved by reducing or nonreducing SDS-PAGE as indicated. Gels were stained with Coomassie blue to ensure equal loading of pSSU-DHFRHis-PEAS or DHFRHis-PEAS, and [35S]atToc159 was detected in dried gels using a phosphorimager (Storm 840; Molecular Dynamics).
Selective proteolysis of cross-linked [35S]atToc159
After cross-linking of [35S]atToc159 to pSSU-DHFRHis-PEAS, the resin was washed with PBS containing 0.1 mM GTP. 2 U thrombin was added to the resin in a final volume of 400 μl PBS and resin was incubated for 1 h at 37°C. The resin was collected by centrifugation, and the supernatant was saved as the “thrombin-released” fraction. The resin was washed with immobilization buffer containing 0.1% Triton X-100 and 6 M urea and was separated into two equal fractions. Bound proteins from one fraction were eluted with SDS-PAGE sample buffer containing 0.75 M imidazole and 80 mM DTT, and from the second with SDS-PAGE sample buffer containing 0.75 M imidazole without DTT. Proteins were resolved using SDS-PAGE and were analyzed using a phosphorimager (Storm 840; Molecular Dynamics).
Transformation of Arabidopsis with GFP constructs and microscopy
The pSMB-GFP, pSMB-pSSU-GFPHis, and pSMB-pE1α-GFPHis constructs were transformed into heterozygous ppi2 Arabidopsis plants (Bauer et al., 2000) using the Agrobacterium-mediated floral dip method (Clough and Bent, 1998). ppi2 plants carrying the GFP transgenes were grown on agar plates containing Murashige and Skoog growth medium, 1% sucrose, 50 μg/ml kanamycin (a marker linked to ppi2), and 50 μg/ml glufosinate ammonium (BASTA, a marker linked to the GFP transgenes).
Proteins were extracted in boiling SDS-PAGE sample buffer from the total above ground tissue of ∼4-wk-old plate-grown plants (Bauer et al., 2000), resolved using SDS-PAGE, and analyzed by immunoblotting using anti-GFP mAb (CLONTECH Laboratories, Inc.). For microscopy, protoplasts were isolated as described previously (Bauer et al., 2002) from plants stably transformed with GFP constructs, and were viewed in buffer containing 5 mM MES, pH 5.7, 0.4 M mannitol, and 20 mM CaCl2. Confocal laser scanning microscopy was performed on a confocal system (MRC-600; Bio-Rad Laboratories) using an inverted microscope (Diaphot 200; Nikon) and a 60× 1.4 NA PlanApo objective lens. Image acquisition was performed at RT with Confocal Assistant version 4.02 software (Bio-Rad Laboratories). Merged images were generated using the Image J image-processing program (National Institutes of Health, Bethesda, MD).
Acknowledgments
We would like to thank Caroline Robinson and Tanya Wallas for technical assistance and Dr. Alice Cheung and Dr. Hen-ming Wu for their expert technical assistance with fluorescence microscopy.
This work is supported by National Institutes of Health grant GM61893 (to D.J. Schnell).
M. Afitlhile's present address is Kentucky State University, 400 East Main St., Frankfort, KY 40601.
Abbreviations used in this paper: DHFR, dihydrofolate reductase; GMP-PNP, guanyl-5′-yl imidodiphosphate; Ni-NTA, nickel-nitrilotriacetic acid; pE1α, precursor to the E1α subunit of plastid pyruvate dehydrogenase; PEAS, N-((2-pyridyldithio)ethyl)-4-azidosalicylamide; pL11, precursor to the plastid ribosomal L11 subunit; pPORA, precursor to protochlorophyllide oxidoreductase A; protA, protein A; pSSU, precursor to the small subunit of Rubisco; Toc, translocon at the outer envelope membrane of chloroplasts.
References
- Bauer, J., K. Chen, A. Hiltbunner, E. Wehrli, M. Eugster, D. Schnell, and F. Kessler. 2000. The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature. 403:203–207. [DOI] [PubMed] [Google Scholar]
- Bauer, J., A. Hiltbrunner, and F. Kessler. 2001. Molecular biology of chloroplast biogenesis: gene expression, protein import and intraorganellar sorting. Cell. Mol. Life Sci. 58:420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, J., A. Hiltbrunner, P. Weibel, P.A. Vidi, M. Alvarez-Huerta, M.D. Smith, D.J. Schnell, and F. Kessler. 2002. Essential role of the G-domain in targeting of the protein import receptor atToc159 to the chloroplast outer membrane. J. Cell Biol. 159:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, T., M. Jelic, A. Vojta, A. Radunz, J. Soll, and E. Schleiff. 2004. Preprotein recognition by the Toc complex. EMBO J. 23:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., and D.J. Schnell. 1997. Insertion of the 34-kDa chloroplast protein import component, IAP34, into the chloroplast outer membrane is dependent on its intrinsic GTP-binding capacity. J. Biol. Chem. 272:6614–6620. [DOI] [PubMed] [Google Scholar]
- Chen, K., X. Chen, and D.J. Schnell. 2000. Initial binding of preproteins involving the Toc159 receptor can be bypassed during protein import into chloroplasts. Plant Physiol. 122:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., M.D. Smith, L. Fitzpatrick, and D.J. Schnell. 2002. In vivo analysis of the role of atTic20 in protein import into chloroplasts. Plant Cell. 14:641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, P.L., B.F. Weston, and L.M. Gierasch. 1998. Probing the folding pathway of a β-clam protein with single-tryptophan constructs. Fold. Des. 3:401–412. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and A.F. Bent. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16:735–743. [DOI] [PubMed] [Google Scholar]
- Hiltbrunner, A., J. Bauer, M. Alvarez-Huerta, and F. Kessler. 2001. a. Protein translocon at the Arabidopsis outer chloroplast membrane. Biochem. Cell Biol. 79:629–635. [DOI] [PubMed] [Google Scholar]
- Hiltbrunner, A., J. Bauer, P.A. Vidi, S. Infanger, P. Weibel, M. Hohwy, and F. Kessler. 2001. b. Targeting of an abundant cytosolic form of the protein import receptor at Toc159 to the outer chloroplast membrane. J. Cell Biol. 154:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, S., E. Muckel, F. Heemeyer, G. von Heijne, and J. Soll. 1994. A receptor component of the chloroplast protein translocation machinery. Science. 266:1989–1992. [DOI] [PubMed] [Google Scholar]
- Jarvis, P., and J. Soll. 2002. Toc, tic, and chloroplast protein import. Biochim. Biophys. Acta. 1590:177–189. [DOI] [PubMed] [Google Scholar]
- Jelic, M., N. Sveshnikova, M. Motzkus, P. Horth, J. Soll, and E. Schleiff. 2002. The chloroplast import receptor Toc34 functions as preprotein-regulated GTPase. Biol. Chem. 383:1875–1883. [DOI] [PubMed] [Google Scholar]
- Jelic, M., J. Soll, and E. Schleiff. 2003. Two Toc34 homologues with different properties. Biochemistry. 42:5906–5916. [DOI] [PubMed] [Google Scholar]
- Keegstra, K., and J.E. Froehlich. 1999. Protein import into chloroplasts. Curr. Opin. Plant Biol. 2:471–476. [DOI] [PubMed] [Google Scholar]
- Kouranov, A., and D.J. Schnell. 1997. Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts. J. Cell Biol. 139:1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.H., S.J. Kim, Y.J. Lee, J.B. Jin, and I. Hwang. 2003. The M domain of atToc159 plays an essential role in the import of proteins into chloroplasts and chloroplast biogenesis. J. Biol. Chem. 278:36794–36805. [DOI] [PubMed] [Google Scholar]
- Ma, Y., A. Kouranov, S. LaSala, and D.J. Schnell. 1996. Two components of the chloroplast protein import apparatus, IAP86 and IAP75, interact with the transit sequence during the recognition and translocation of precursor proteins at the outer envelope. J. Cell Biol. 134:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, T., and J. Soll. 2000. 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell. 12:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylne, J., and J.R. Botella. 1998. Binary vectors for sense and antisense expression of Arabidopsis ESTs. Plant Mol. Biol. Rep. 16:257–262. [Google Scholar]
- Newell, J.M., R.A. Leigh, and J.L. Hall. 1998. Vacuole development in cultured evacuolated oat mesophyll protoplasts. J. Exp. Bot. 49:817–827. [Google Scholar]
- Perry, S.E., and K. Keegstra. 1994. Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell. 6:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe, S., F. Quigley, J. Gray, A. Schemenewitz, and C. Reinbothe. 2004. Identification of plastid envelope proteins required for import of protochlorophyllide oxidoreductase A into the chloroplast of barley. Proc. Natl. Acad. Sci. USA. 101:2197–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff, E., J. Soll, N. Sveshnikova, R. Tien, S. Wright, C. Dabney-Smith, C. Subramanian, and B.D. Bruce. 2002. Structural and guanosine triphosphate/diphosphate requirements for transit peptide recognition by the cytosolic domain of the chloroplast outer envelope receptor, toc34. Biochemistry. 41:1934–1946. [DOI] [PubMed] [Google Scholar]
- Schleiff, E., M. Jelic, and J. Soll. 2003. a. A GTP-driven motor moves proteins across the outer envelope of chloroplasts. Proc. Natl. Acad. Sci. USA. 100:4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff, E., J. Soll, M. Kuchler, W. Kuhlbrandt, and R. Harrer. 2003. b. Characterization of the translocon of the outer envelope of chloroplasts. J. Cell Biol. 160:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell, D.J., and G. Blobel. 1993. Identification of intermediates in the pathway of protein import into chloroplasts and their localization to envelope contact sites. J. Cell Biol. 120:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.D., L.M. Fitzpatrick, K. Keegstra, and D.J. Schnell. 2002a. In vitro analysis of chloroplast protein import. Current Protocols in Cell Biology. J.S. Bonifacino, J. Lippincott-Schwartz, J.B. Harford, and K.M. Yamada, editors. John Wiley & Sons, Inc., New York. 11.16.1–11.16.21. [DOI] [PubMed]
- Smith, M.D., A. Hiltbrunner, F. Kessler, and D.J. Schnell. 2002. b. The targeting of the atToc159 preprotein receptor to the chloroplast outer membrane is mediated by its GTPase domain and is regulated by GTP. J. Cell Biol. 159:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveshnikova, N., J. Soll, and E. Schleiff. 2000. Toc34 is a preprotein receptor regulated by GTP and phosphorylation. Proc. Natl. Acad. Sci. USA. 97:4973–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallas, T.R., M.D. Smith, S. Sanchez-Nieto, and D.J. Schnell. 2003. The roles of toc34 and toc75 in targeting the toc159 preprotein receptor to chloroplasts. J. Biol. Chem. 278:44289–44297. [DOI] [PubMed] [Google Scholar]
- Young, M.E., K. Keegstra, and J.E. Froehlich. 1999. GTP promotes the formation of early-import intermediates but is not required during the translocation step of protein import into chloroplasts. Plant Physiol. 121:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, T.S., and H. Li. 2001. Chloroplast protein translocon components atToc159 and atToc33 are not essential for chloroplast biogenesis in guard cells and root cells. Plant Physiol. 127:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]