Abstract
Plant cells communicate with each other via channels called plasmodesmata (PD). PD are not passive channels, but critical players in gene regulation, controlling intercellular transport of macromolecules between particular cells during development.
Keywords: transport; cell-to-cell transport; plasmodesmata; plant; development
Plant cells are encased in cell walls that form the plant skeleton, enabling and stabilizing three-dimensional growth. As plant cells physically do not touch, plants have evolved cytoplasmic bridges, called plasmodesmata (PD), spanning cell walls and linking the cytoplasm between adjacent cells. Intercellular communication, as well as temporal and spatial regulation of gene expression, are global mechanisms to control developmental programming in multicellular organisms. Signals can be transmitted via receptor–ligand interactions in both plant and animal cells. However, PD provide plants with a unique means of intercellular communication, where each plant cell can form direct conduits to its neighbors, forming domains of cells sharing common components. Historically, PD were seen as facilitating traffic of low molecular weight growth regulators and nutrients, such as the ample products of photosynthesis. Evidence for macromolecular transport via PD was based largely on pirating of these channels by plant viruses during infection. Now there is abundant evidence that PD transport endogenous macromolecules.
Generic PD have two major components, membranes and spaces (Zambryski and Crawford, 2000; Roberts and Oparka, 2003) (Fig. 1). Membranes form the boundaries of the PD channel through which transport may occur. The plasma membrane (PM) between adjacent cells defines the outer limit of PD. The axial center of the PD, the desmotubule (D), is appressed ER. The region between the D and the PM is the cytoplasmic sleeve (CS), the major conduit for molecular passage. The CS likely contains components, perhaps anchored to the D and PM, that regulate and facilitate PD transport. Ultrastructural studies of PD reveal actin and myosin along the length and centrin nanofilaments at the neck region that may provide contractile elements to regulate PD aperture. To date, no unique PD components have been identified.
Figure 1.
Diagram of longitudinal section of PD spanning the cell wall between adjacent cells. CR, central rod; CW, cell wall; SP, spoke-like connections between the D and PM that may control aperture. Blue and orange circles represent hypothetical PD-specific proteins. Figure from Roberts and Oparka, 2003.
In the last few years, there have been two major breakthroughs in PD research. First, native (nonvirally perturbed) PD are no longer considered static but instead fluctuate in aperture in different cell types during development and in response to the environment. Second, evidence that PD can transport (nonviral) macromolecules increased dramatically. Numerous reports demonstrate intercellular movement of both exogenous proteins (such as GFP) and endogenous transcription factors (cited below) and RNAs, such as mRNA (Ruiz-Medrano et al., 1999; Kim et al., 2001), and gene silencing signals (Baulcombe, 1999; Voinnet, 2001). Recent studies on protein and fluorescent tracer movement during the three major stages of plant development are summarized below.
PD function during vegetative development
The vegetative phase is the most prolonged and easiest to access. After seed germination, plants continuously produce new organs, such as leaves and roots. A large literature documents PD function in mature leaf tissues using plant viral probes (Lazarowitz and Beachy, 1999). Specific viral movement proteins enable infectious spread. As viruses and their proteins were above the (then) predicted 1-kD size exclusion limit of PD, such studies revealed that PD could function as dilated channels. It was assumed that plant viruses were uniquely able to manipulate PD to achieve their ends. The advent of improved fluorescent tracers, and noninvasive means for their introduction, dramatically shifted this view, revealing an innate complexity of PD function.
Studies with GFP and its fusions revealed that proteins of at least 50 kD could traffic through cells of the leaf, and the extent of such movement was highly dependent on leaf age (Oparka et al., 1999). Younger leaves exhibit more extensive cell-to-cell transport than older leaves. Quantitative studies demonstrate that the leaf consists of different populations of cells exhibiting no, low, or high efficiency of GFP movement. Thus, there is no single size exclusion limit for leaf PD; instead the leaf contains a heterogeneous mixture of cells where PD apertures fluctuate according to changes in the environment (Crawford and Zambryski, 2001).
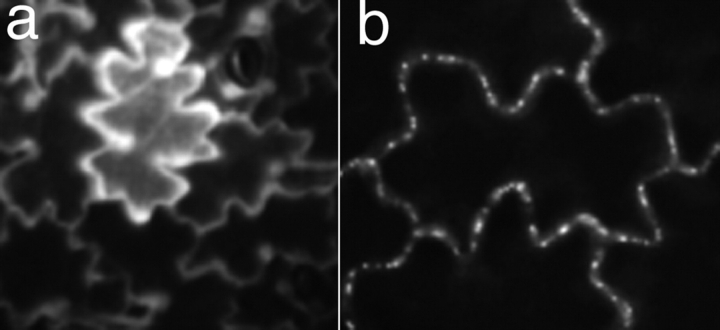
There are two general types of protein movement, nontargeted and targeted (Fig. 2) (Crawford and Zambryski, 2000). Nontargeted movement is exemplified by the diffusive pattern of GFP movement following its expression in a single cell; GFP is detected as a gradient from high in the initial cell to low in surrounding cells to which it has moved. GFP is not targeted to particular cellular sites, but rather displays uniform fluorescence throughout the cell. To date, targeted movement is best illustrated by fusing GFP to proteins known to interact specifically with PD, such as viral movement proteins; such GFP fusions localize to punctate foci around cell perimeters that correspond to PD.
Figure 2.
Nontargeted and targeted cell-to-cell protein movement. Panel a exemplifies nontargeted movement of GFP, and panel b shows targeted movement, GFP fused to a plant viral protein. Figure from Zambryski and Crawford, 2000.
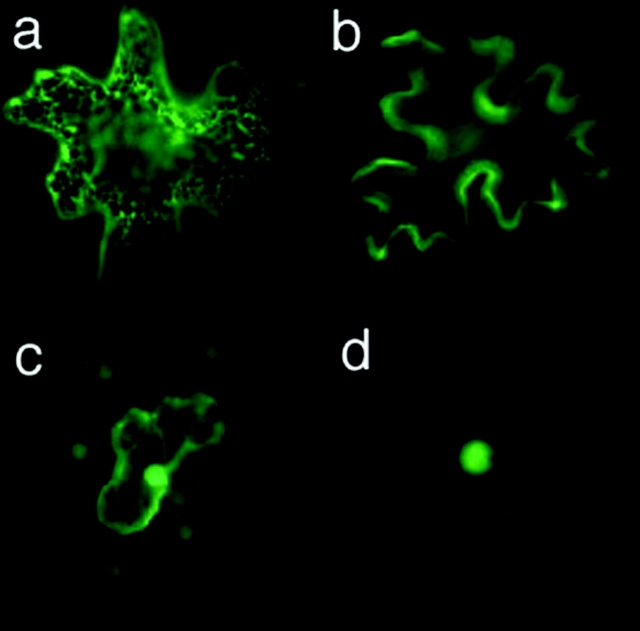
Obviously protein cargo size and the aperture of PD physically govern macromolecular traffic. Given these two criteria are met, can all macromolecules move cell to cell like GFP? If such rampant exchange occurs, how are cells protected against loss of cell-specific components? While GFP is a soluble cytoplasmic protein that can move freely via PD, GFP fusions targeted to the ER or anchored to actin filaments do not move cell to cell (Crawford and Zambryski, 2000) (Fig. 3). Further, as the nucleus is a soluble compartment, GFP carrying an NLS localizes to the nucleus while retaining the ability to move between cells. Double-sized GFP carrying an NLS did not move cell to cell, presumably as nuclear–cytoplasmic shuttling is limited to proteins <40 kD (transcription factors likely are retained in the nucleus by binding to specific chromatin sites; Fig. 4). While nontargeted movement of macromolecules between cells may be the default state, cells likely protect against loss by specifically anchoring or sequestering proteins within the cell.
Figure 3.
Subcellular localization determines the availability of proteins for PD transport. GFP fused to an ER retention signal (a), the actin filament binding domain of talin (b), or an NLS (c). (d) Double-sized GFP fused to an NLS. Figure from Crawford and Zambryski, 2000.
Figure 4.
Shr protein localization in Arabidopsis roots. Transgenic plants expressing Shr fused to GFP reveal Shr in the inner cells of the stele (Ste) as well as adjacent endodermal (End) cells, while SHR transcripts (GFP under the control of the SHR promoter in inset) are only present in stele cells. Cor, cortex; Epi, epidermis; Cei, cortex/endodermis initial; QC, quiescent center. Bars: 50 and 25 μm (inset). Figure from Nakajima et al., 2001.
The vascular system, immediately connected to surrounding tissues by PD, provides an exceptionally valuable conduit for macromolecular dispersal. Elegant studies to induce GFP expression specifically in cells immediately abutting the vascular system reveal that GFP generally moves toward regions of new growth, such as new leaves and newly emerging floral organs (Imlau et al., 1999). Such movement implies that endogenous macromolecular signals may traffic the vascular highway to facilitate new development.
PD transport in other vegetative tissues highlights amazing precision to intercellular trafficking. The maize KNOTTED1 transcript is expressed in the inner cell layers of the shoot meristem, but not the outermost cell layer. However, immunolocalization studies reveal KN1 protein in outermost cells (Lucas et al., 1995). Recent studies with GFP-KN1 fusions reveal directional traffic of KN1 in different tissues (Kim et al., 2003). SHORT-ROOT (SHR) mRNA and protein are expressed in the stele cells of the central region of the root tip. Remarkably, the Shr protein moves to a single file of adjacent cells (Nakajima et al., 2001) (Fig. 4). While Shr has a diffuse cytoplasmic localization in stele cells, it nuclear localizes after transport to adjacent cells. This latter localization then allows Shr to induce expression of a related gene (SCARECROW) that induces differentiation of two cell types.
PD function during reproductive development
One of the major differences between plants and animals is their reproductive strategy. In animals, reproductive organs are set aside early, during embryogenesis. In contrast, plants mature to adults before producing reproductive organs. During vegetative development, roots and leaves are produced continuously from meristematic regions. The shoot meristem, at the plant apex, contains a dome of undifferentiated cells that give rise to new leaf primordia off its flanks. Late in life, in response to developmental and environmental signals, the adult plant reprograms this apical meristem to stop making leaves and instead make floral organs. This developmental switch results in a dramatic reorganization of the meristem; instead of giving rise to single leaf primordia, the meristem now produces floral meristems, which in turn produce multiple primordia corresponding to the four floral organ types.
The important resource of developmentally competent cells in the meristem is expected to be highly sensitive to signals that traffic cell to cell. Indeed, temporal and spatial changes in cell-to-cell transport occur at the shoot apex as a function of developmental age (Gisel et al., 2002). Apices of vegetative plants reveal tracer (8-hydroxypyrene 1,3,6 trisulfonic acid [HPTS]; 520 D) in the outer cell layers of the meristem and in very young primordia. Surprisingly, fluorescence signal in the entire shoot apical meristem region decreases before the onset of flowering. After floral morphogenesis is underway, floral apices again traffic tracer. Such studies suggest that cell-to-cell transport via PD is an additional parameter of the multifactorial control of the transition to reproductive development, and support a model where reduction of transport of a floral repressor(s) contributes to the induction of flowering. As the apical meristem undergoes profound changes in architecture and concomitant signaling and expression pathways, it may be advantageous to be sequestered from intercellular input during establishment of the reproductive program.
Independent studies on the initiation and control of floral development predominantly aim to define which genes control this pathway. Many floral regulatory genes encode transcription factors that affect floral patterning depending on their specific temporal and spatial patterns of expression. However, when and where their specific RNAs are expressed is not the end point of their regulation. It is now clear that critical floral transcriptional factors transit PD. Such traffic is highly regulated as some, but not all, meristem-expressed factors move cell to cell (Sessions et al., 2000; Kim et al., 2003; Wu et al., 2003). Two essential transcription factors, Apetala1 (Ap1) and Leafy (Lfy), were each expressed from a promoter driving outer cell layer–specific expression. While Lfy could move down into internal cell layers, Ap1 remained in the outer cell layer. As suggested above, nontargeted movement (Lfy) may be the default state for macromolecules, unless they are specifically retained (Ap1). Perhaps nontargeted protein movement provides coordination between large groups of cells, whereas proteins with more limited movement program more specific pathways. Such studies implicate PD not only as regulators of overall traffic potential, but also as regulators of the specificity of “cargo” macromolecules that interact with and transit these channels.
PD function during embryonic development
The correspondence between levels of cell-to-cell transport and differentiation noted above predicts that other essential programs, such as embryogenesis, also alter their intercellular transport capacity to induce or regulate development. While small fluorescent tracer (HPTS) traffics uniformly during all stages of embryogenesis in Arabidopsis, larger tracer (10-kD fluorescent dextran) traffics only during early embryogenesis. F-dextran ceases traffic during mid-embryogenesis when primitive root and leaves begin to form. Thus, there is a developmental down-regulation of PD aperture at this stage (Kim et al., 2002). Embryogenesis provides a simple accessible system to dissect PD function.
Identification of PD components
PD research is at a juncture. The dynamic and critical roles of PD are established. It is now essential to uncover the intricacies of the mechanisms involved in PD structure and function during development. To understand PD requires the identification of specific structural or regulatory components that control PD function. Several recent approaches are highlighted.
A biochemical approach used proteins known to traffic via PD as bait for the affinity purification of interaction partners contained within PD-enriched cell wall extracts (Lee et al., 2003). One protein, NCAPP1, is a highly basic protein with an ER trans-membrane domain that localizes to the cell periphery.
The generation of plant cDNA-GFP fusions and the subsequent observation of the expression patterns of GFP in vivo reveals cellular addresses of the protein fusions (Cutler et al., 2000). A high throughput plant viral expression system allowed the screening of infection foci expression of >20,000 independent GFP fusions. 12 fusion proteins specifically localized to PD (Escobar et al., 2003). Localization was observed as puncta (as in Fig. 2 b), strongly supporting that candidates are bone fide PD structural or regulatory components. Half of the encoded proteins exhibit no similarity to known proteins and may represent novel PD factors.
Finally, a classic method to identify PD-specific components is genetics. A major obstacle to this approach is that alterations of PD function likely will have severe defects in growth and not produce viable plants. However, PD defects are expected to manifest first as defects during embryo development. Embryo-lethal lines can be propagated as heterozygotes that segregate wild-type and embryo-lethal phenotypes in their seedpods. Determination of developmental transitions in PD function during embryogenesis provides entry points to identify mutants with altered cell-to-cell transport. Indeed, screening embryo-defective lines individually by fluorescence microscopy identified lines altered in their ability to traffic tracers during mid-embryogenesis (Kim et al., 2002). Map-based cloning of the affected genes and their characterization should provide insights into intercellular communication in plants.
Perspectives
PD are essential gatekeepers for plant cell-to-cell transport. As PD have the innate ability to transport macromolecules, developmental transitions in PD function and aperture likely play critical roles in the transmission of macromolecular signals to coordinate differentiation pathways. Obvious questions for the future are: what regulatory molecules signal PD to open or close; what structural elements of PD respond to such regulatory molecules; do PD in different cell types have different specific components; what changes in PD components/architecture occur as a cell matures; how is the number of PD per specific cell type determined; and how is PD formation synchronized with cell division?
Acknowledgments
P. Zambryski thanks colleagues and the National Institutes of Health (GM45244) for support.
Abbreviations used in this paper: Ap1, Apetala1; CS, cytoplasmic sleeve; D, desmotubule; Lfy, Leafy; PD, plasmodesmata; PM, plasma membrane; SHR, SHORT-ROOT.
References
- Baulcombe, D.C. 1999. Gene silencing: RNA makes RNA makes no protein. Curr. Biol. 9:R599–R601. [DOI] [PubMed] [Google Scholar]
- Crawford, K.M., and P.C. Zambryski. 2000. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 10:1032–1040. [DOI] [PubMed] [Google Scholar]
- Crawford, K.M., and P.C. Zambryski. 2001. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125:1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S.R., D.W. Ehrhardt, J.S. Griffitts, and C.R. Somerville. 2000. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA. 97:3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar, N.M., S. Haupt, G. Thow, P. Boevink, S. Chapman, and K. Oparka. 2003. High throughput viral expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell. 15:1507–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisel, A., F.D. Hempel, S. Barella, and P. Zambryski. 2002. Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis. Proc. Natl. Acad. Sci. USA. 99:1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlau, A., E. Truernit, and N. Sauer. 1999. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 11:309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I., F.D. Hempel, K. Sha, J. Pfluger, and P.C. Zambryski. 2002. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development. 129:1261–1272. [DOI] [PubMed] [Google Scholar]
- Kim, J.-Y., Z. Yuan, and D. Jackson. 2003. Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development. 130:4351–4362. [DOI] [PubMed] [Google Scholar]
- Kim, M., W. Canio, S. Kessler, and N. Sinha. 2001. Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science. 293:287–289. [DOI] [PubMed] [Google Scholar]
- Lazarowitz, S.G., and R.N. Beachy. 1999. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell. 11:535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.Y., B.C. Yoo, M.R. Rojas, N. Gomez-Ospina, L.A. Staehelin, and W.J. Lucas. 2003. Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science. 299:392–396. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., S. Bouche-Pillon, D.P. Jackson, L. Nguyen, L. Baker, B. Ding, and S. Hake. 1995. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. 270:1980–1983. [DOI] [PubMed] [Google Scholar]
- Nakajima, K., G. Sena, T. Nawy, and P.N. Benfey. 2001. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 413:307–311. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J., A.G. Roberts, P. Boevink, S. Santa Cruz, I. Roberts, K.S. Pradel, A. Imlau, G. Kotlizky, N. Sauer, and B. Epel. 1999. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell. 97:743–754. [DOI] [PubMed] [Google Scholar]
- Roberts, A.G., and K.J. Oparka. 2003. Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26:103–124. [Google Scholar]
- Ruiz-Medrano, R., B. Xoconostle-Cazares, and W.J. Lucas. 1999. Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development. 126:4405–4419. [DOI] [PubMed] [Google Scholar]
- Sessions, A., M.F. Yanofsky, and D. Weigel. 2000. Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science. 289:779–782. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. 2001. RNA silencing as a plant immune system against viruses. Trends Genet. 17:449–459. [DOI] [PubMed] [Google Scholar]
- Wu, X., J.R. Dinnemy, K.M. Crawford, Y. Rhee, V. Citovsky, P.C. Zambryski, and D. Weigel. 2003. Modes of intercellular transcription factor movement in the Arabidopsis apex. Development. 130:3735–3745. [DOI] [PubMed] [Google Scholar]
- Zambryski, P., and K. Crawford. 2000. Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu. Rev. Cell Dev. Biol. 16:393–421. [DOI] [PubMed] [Google Scholar]