Abstract
Polyamine pools were measured under various conditions of high and low concentrations of cytosolic ornithine with the wild-type and mutant strains of Neurospora crassa. In minimal medium, the wild-type strain has 1 to 2 nmol of putrescine and approximately 14 nmol of spermidine per mg (dry weight); no spermine is found in N. crassa. Exogenous ornithine was found to cause a rapid, but quickly damped, increase in the rate of polyamine synthesis. This effect was greater in a mutant (ota) unable to catabolize ornithine. No turnover of polyamines was detected during exponential growth. Exogenous spermidine was not taken up efficiently by N. crassa; thus, the compound could not be used directly in studies of regulation. However, by nutritional manipulation of a mutant strain, aga, lacking arginase, cultures were starved for ornithine and thus ultimately for putrescine and spermidine. During ornithine starvation, the remaining putrescine pool was not converted to spermidine. The pattern of polyamine synthesis after restoration of ornithine to the polyamine-deprived aga strain indicated that, in vivo, spermidine regulates polyamine synthesis at the ornithine decarboxylase reaction. The results suggest that the regulatory process is a form of negative control which becomes highly effective when spermidine exceeds its normal level. The possible relationship between the regulation of polyamine synthesis and the ratio of free to bound spermidine is discussed.
Full text
PDF

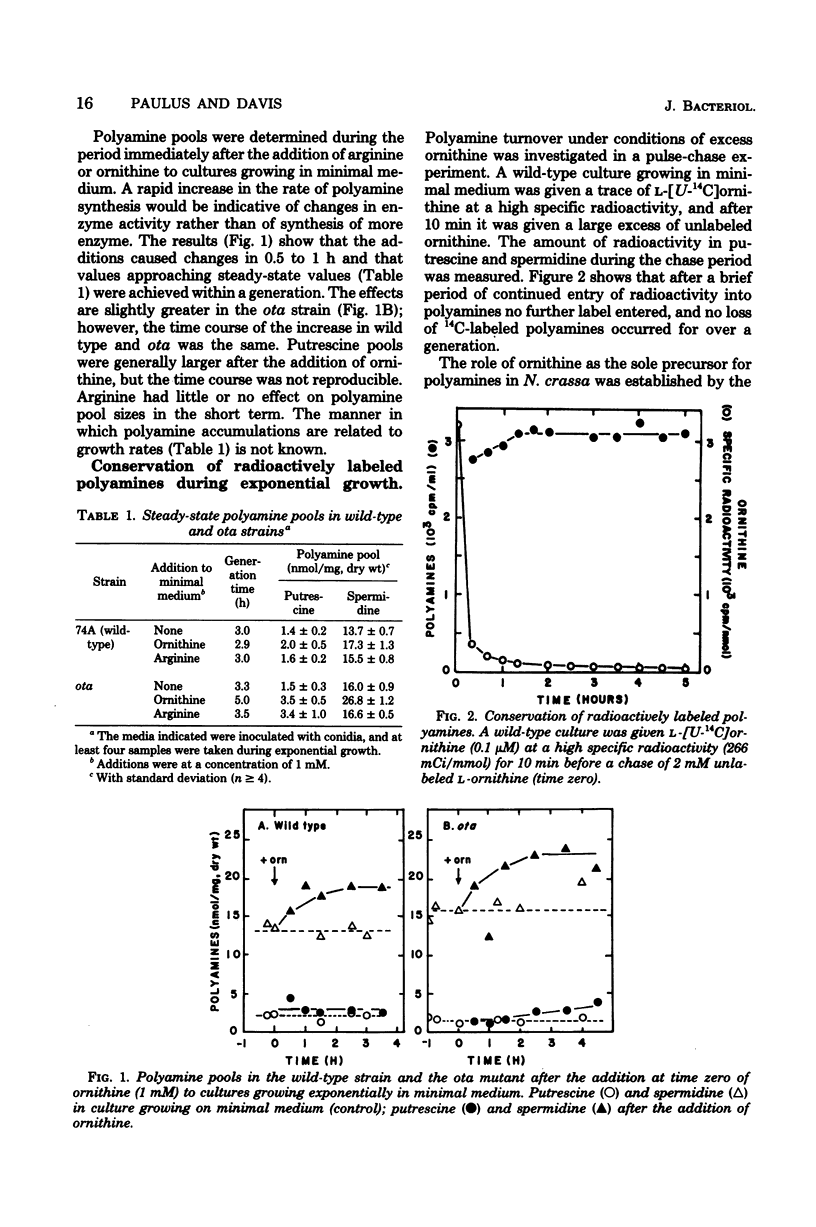

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
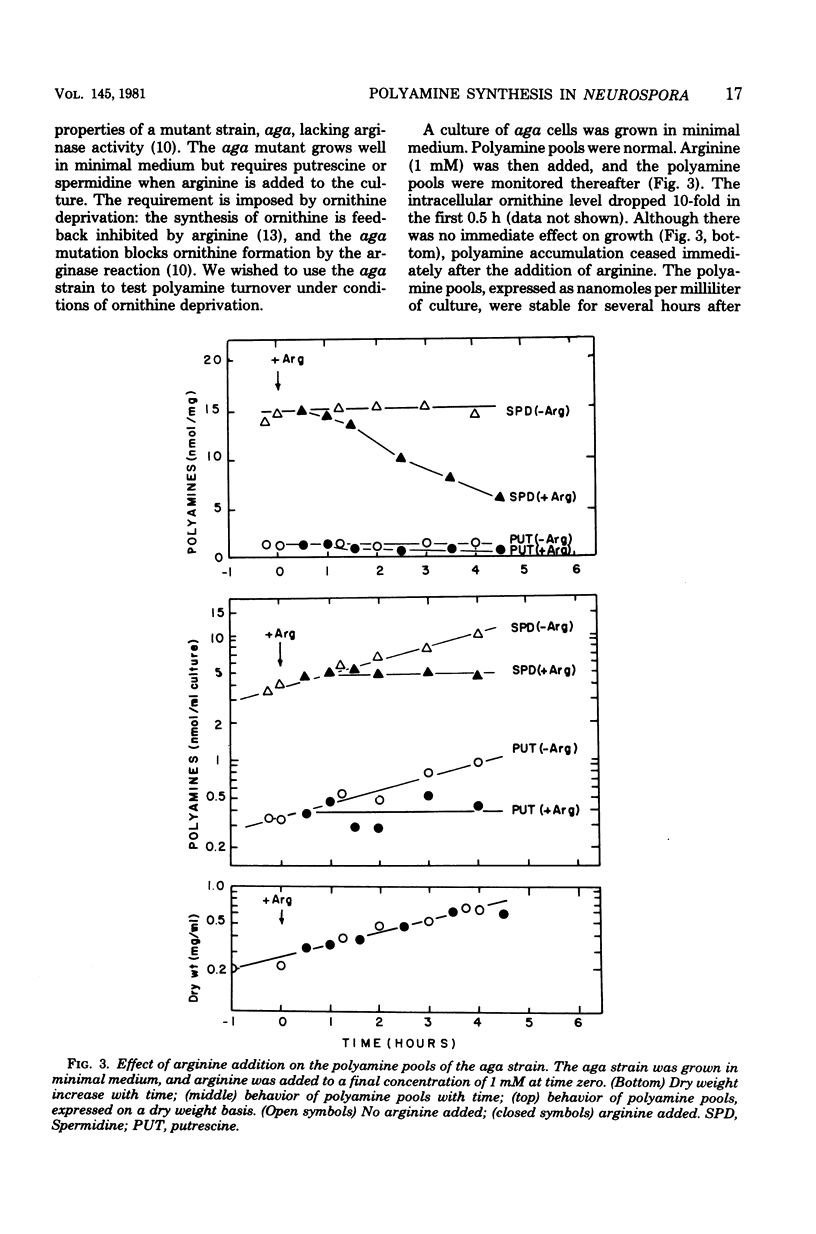
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Arginine catabolism in Neurospora: cycling of ornithine. J Bacteriol. 1977 Apr;130(1):285–291. doi: 10.1128/jb.130.1.285-291.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Cellular distribution of ornithine in Neurospora: anabolic and catabolic steady states. J Bacteriol. 1977 Apr;130(1):274–284. doi: 10.1128/jb.130.1.274-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
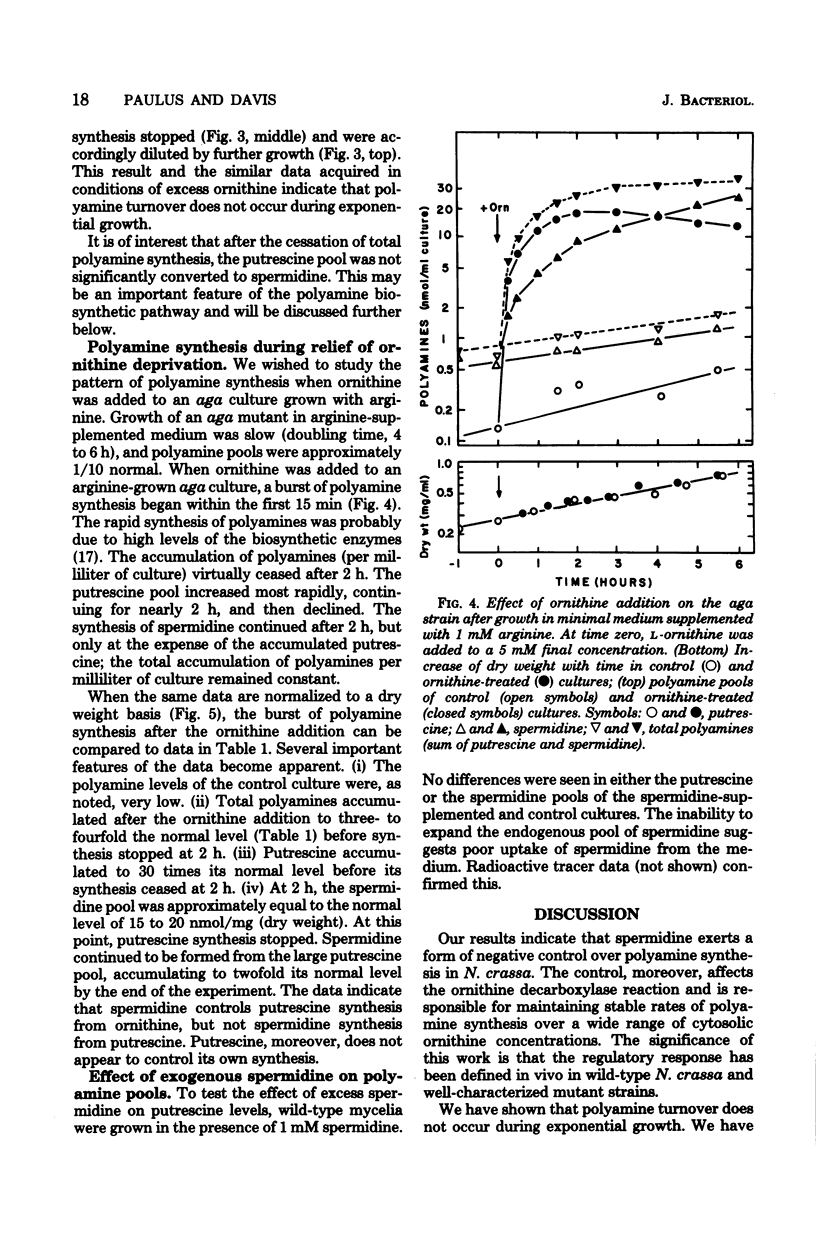
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Pyridoxal 5'-phosphate and the regulation of ornithine decarboxylase activity and stability. Eur J Biochem. 1976 Aug 1;67(1):303–314. doi: 10.1111/j.1432-1033.1976.tb10662.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. What do the polyamines do? Nature. 1978 Jul 20;274(5668):209–210. doi: 10.1038/274209a0. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
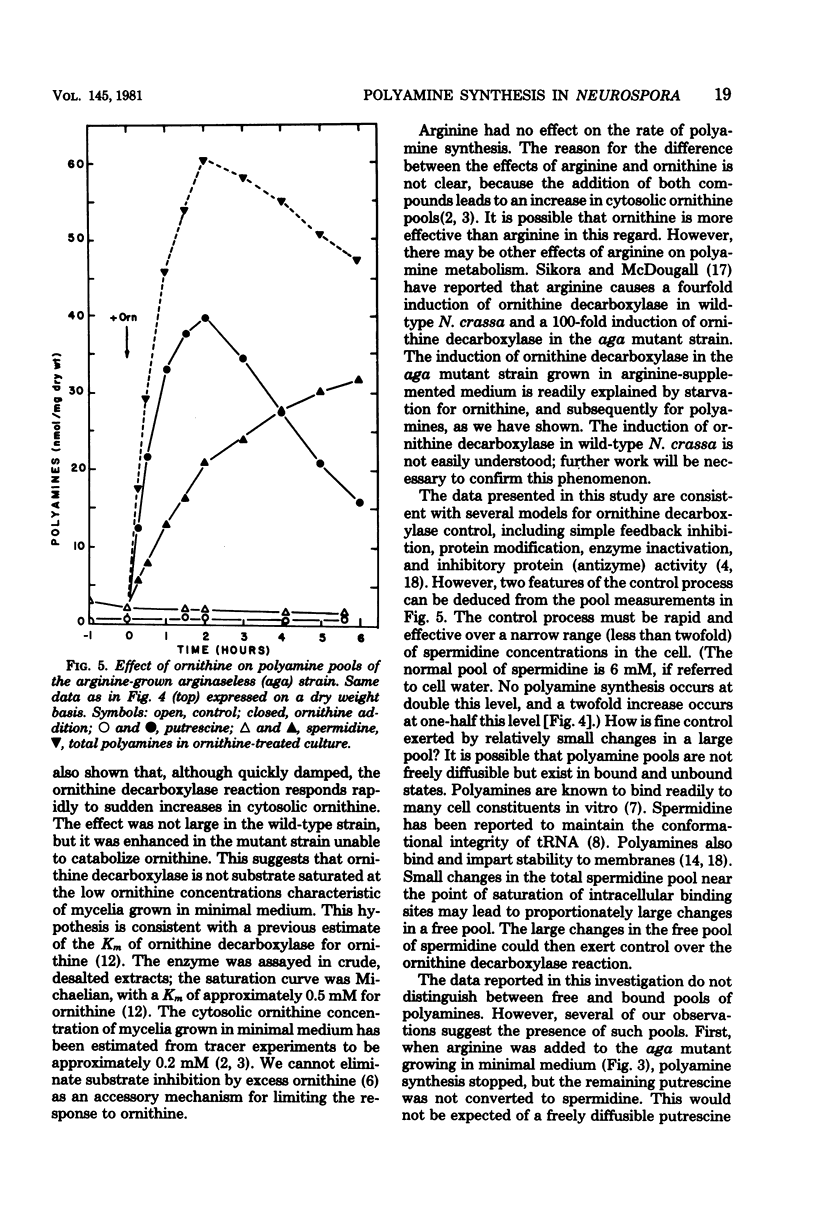
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Utilization of exogenous and endogenous ornithine by Neurospora crassa. J Bacteriol. 1968 Aug;96(2):389–395. doi: 10.1128/jb.96.2.389-395.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman I., Weiss R. L. Control of arginine metabolism in Neurospora: flux through the biosynthetic pathway. J Bacteriol. 1980 Jan;141(1):227–234. doi: 10.1128/jb.141.1.227-234.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Chen K. Y., Kyriakidis D. A., Fong W. F., Canellakis E. S. The modulation of the induction of ornithine decarboxylase by spermine, spermidine and diamines. J Cell Physiol. 1978 Aug;96(2):225–234. doi: 10.1002/jcp.1040960211. [DOI] [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- Nickerson K. W., Dunkle L. D., Van Etten J. L. Absence of spermine in filamentous fungi. J Bacteriol. 1977 Jan;129(1):173–176. doi: 10.1128/jb.129.1.173-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]


