Abstract
Rud3p is a coiled-coil protein of the yeast cis-Golgi. We find that Rud3p is localized to the Golgi via a COOH-terminal domain that is distantly related to the GRIP domain that recruits several coiled-coil proteins to the trans-Golgi by binding the small Arf-like GTPase Arl1p. In contrast, Rud3p binds to the GTPase Arf1p via this COOH-terminal “GRIP-related Arf-binding” (GRAB) domain. Deletion of RUD3 is lethal in the absence of the Golgi GTPase Ypt6p, and a screen of other mutants showing a similar genetic interaction revealed that Golgi targeting of Rud3p also requires Erv14p, a cargo receptor that cycles between the endoplasmic reticulum and Golgi. The one human protein with a GRAB domain, GMAP-210 (CEV14/Trip11/Trip230), is known to be on the cis-Golgi, but the COOH-terminal region that contains the GRAB domain has been reported to bind to centrosomes and γ-tubulin (Rios, R.M, A. Sanchis, A.M. Tassin, C. Fedriani, and M. Bornens. 2004. Cell. 118:323–335). In contrast, we find that this region binds to the Golgi in a GRAB domain–dependent manner, suggesting that GMAP-210 may not link the Golgi to γ-tubulin and centrosomes.
Introduction
The organization of the secretory and endocytic pathways requires the accurate recruitment of diverse peripheral membrane proteins to specific organelles and vesicles. These proteins include vesicle coats, motor proteins, and the tethering factors that attach vesicles to their destinations before membrane fusion. In general, these proteins are recruited by recognition of specific lipid species and activated GTP-binding proteins of the Arf and Rab families that have a restricted spatial distribution (Munro, 2004). One important class of peripheral membrane proteins are the large coiled-coil proteins or “golgins” that are found on the Golgi apparatus (Barr and Short, 2003; Gillingham and Munro, 2003). These proteins appear to play several roles in vesicle transport and structural organization of the Golgi. For example, p115 (Uso1p in yeast) tethers ER-derived vesicles to the cis-Golgi, whereas golgin-84 is required for the integrity of the ribbon of Golgi stacks (Cao et al., 1998; Diao et al., 2003). In addition, a recent study has found that the protein kinase YSK1 binds to the golgin GM130, indicating that golgins can also serve as scaffolds to bring regulatory proteins into proximity with Golgi membranes (Preisinger et al., 2004).
Individual golgins generally have a restricted distribution within the Golgi, being found only on a subset of cisternae. Although a few have a single transmembrane domain, the majority are peripheral membrane proteins (Barr and Short, 2003; Gillingham and Munro, 2003). This means that their distribution within the stack must reflect their recruitment by determinants generated only on a subset of Golgi membranes. Moreover, if, as is widely believed, the cisternae of the Golgi progress through the stack by a process of maturation, then these determinants must be continuously relocated from later to earlier cisternae to ensure that the golgins maintain a polarized distribution. Given these complexities, it is perhaps not surprising that the targeting of those golgins so far examined involves multiple components. For example, a set of golgins targeted to the trans-Golgi of mammals and yeast share a COOH-terminal GRIP domain, a short motif that mediates binding to the GTP-bound form of the Arf-like GTPase Arl1 (Gangi Setty et al., 2003; Lu and Hong, 2003; Panic et al., 2003b). This GTPase is recruited to the trans-Golgi by a pathway that contains a second GTPase, Arl3p/ARFRP1, and a small transmembrane protein, Sys1 (Behnia et al., 2004; Setty et al., 2004). In contrast, GM130 is recruited to the cis-Golgi of mammalian cells by binding via its COOH terminus to the lipid-anchored protein GRASP65 and also to the GTPase Rab1 (Barr et al., 1997; Weide et al., 2001).
The yeast Saccharomyces cerevisiae does not contain an obvious homologue of GM130, but the coiled-coil protein Rud3p has been found to be on the cis-Golgi. Rud3p (also known as Grp1p) was identified as a suppressor of temperature-sensitive mutations in Uso1p (the yeast homologue of the golgin p115) and Sec34p (a subunit of the COG complex; Kim et al., 1999; VanRheenen et al., 1999). Deletion of Rud3p results in defects in the Golgi processing of N-linked glycans, suggesting that it is involved in the recycling of glycosylation enzymes (Kim, 2003). Rud3p was shown to colocalize with the cis-Golgi enzyme Och1p, although how it is targeted to this compartment is unknown (Kim, 2003). We noted a short COOH-terminal domain in Rud3p that is conserved in coiled-coil proteins in eukaryotes ranging from mammals to trypanosomes. Moreover, the human protein that contains this domain, GMAP-210 (CEV14/Trip11/Trip230), is known to be localized to the cis-Golgi (Rios et al., 1994; Chen et al., 1999). GMAP-210 is a long coiled-coil protein that has been suggested to contribute to the maintenance of the Golgi around the centrosomes by binding to Golgi membranes via its NH2-terminal portion and γ-tubulin via its COOH-terminal portion (Infante et al., 1999; Rios et al., 2004). However, we find that the COOH-terminal domains from both the yeast and human protein are sufficient for Golgi targeting. Alignment of the COOH-terminal domain from many species revealed that this domain is distantly related to the GRIP domain, but instead of binding Arl1p, Rud3p binds to the Golgi GTPase Arf1p. Interestingly, we find that the targeting of Rud3p also requires the ER to Golgi cargo receptor Erv14p. This finding suggests that the cis-Golgi localization of Rud3p reflects the combinatorial action of Arf1p and Erv14p, or its cargo, and suggests that recruitment of Rud3p to the Golgi could be coupled to arrival of vesicles from the ER.
Results
The COOH terminus of Rud3p contains a Golgi localization motif
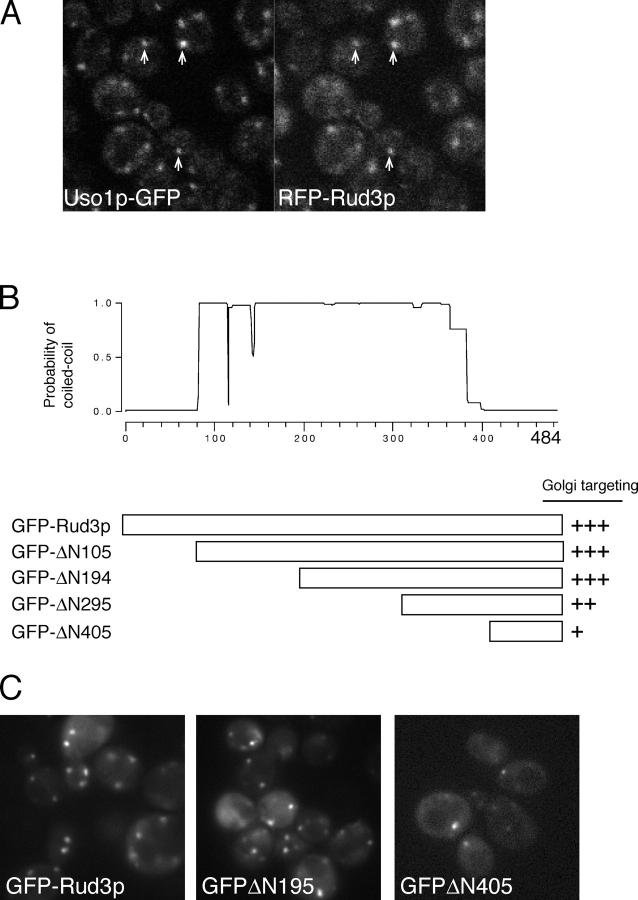
To investigate how Rud3p is recruited to the Golgi, we initially expressed the full-length protein tagged at the NH2 terminus with a nonmultimerizing form of DsRed (“RFP,” tdimer2(12); Campbell et al., 2002). RFP-Rud3p accumulated in punctate structures that colocalized with a GFP-tagged form of the vesicle tethering protein Uso1p (Fig. 1 A). Uso1p apparently tethers ER-derived vesicles to Golgi membranes, and hence is a marker for the earliest, or cis, compartment of the Golgi (Cao et al., 1998). To map the region of Rud3p required for this cis-Golgi targeting, a GFP cassette was introduced at a series of locations through the genomic copy of the RUD3 gene (Fig. 1 B). This approach allows targeting to be examined in the absence of full-length endogenous protein. Removal of the NH2-terminal 194 of the 484 residues of Rud3p did not detectably affect Rud3p targeting (Fig. 1 C). Further NH2-terminal truncation led to a partial cytoplasmic distribution of the tagged protein. However, protein blotting indicated that this relocalization was most likely due to increased protein instability (unpublished data). Nonetheless, detectable membrane targeting was still observed with GFP fused to residue 405 of Rud3p (405–484; Fig. 1 C). These results indicate that Rud3p is recruited to the cis-Golgi, and that targeting information is encoded within the COOH-terminal 80 residues of the protein.
Figure 1.
The COOH terminus of Rud3p mediates Golgi association. (A) Fluorescent micrographs of live yeast expressing the indicated fusion proteins. Uso1p was tagged with GFP at the COOH terminus in the genome of the wild-type strain BY4741, whereas RFP-Rud3p was expressed from a CEN plasmid under the control of a constitutive version of the PHO5 promoter. The two proteins were found to be localized to the same punctate structures, as illustrated by those marked with arrows. (B) Schematic diagram of truncations made in the RUD3 ORF in the yeast strain SEY6210 (Robinson et al., 1988) by insertion by homologous recombination of a cassette encoding a PHO5 promoter and an NH2-terminal GFP tag. Also shown is a coiled-coil prediction for Rud3p (Lupas, 1996). (C) Fluorescent micrographs of live yeast expressing the indicated GFP-Rud3p truncations as in B.
Specific residues within the Rud3p COOH terminus are critical for its localization
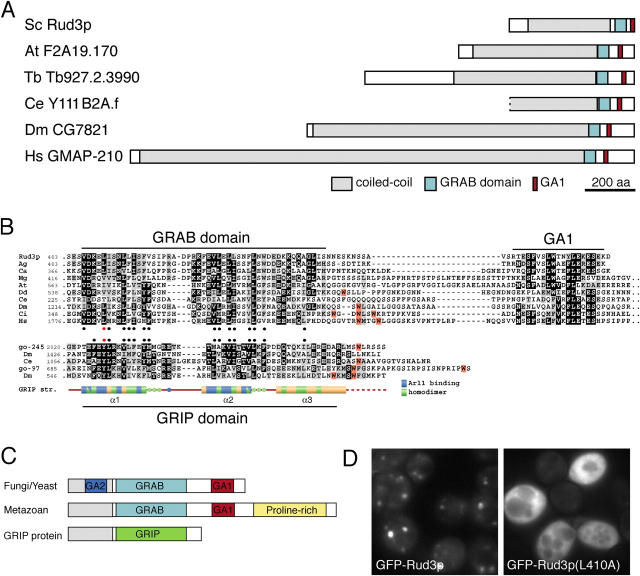
The majority of Rud3p is predicted to form a coiled-coil (Fig. 1 B), but the last 80 residues that mediate targeting lie outside this region. When this COOH-terminal region was used to search the GenBank database using the iterative program PSI-BLAST, it revealed that a large coiled-coil protein with a COOH-terminal domain related to the COOH-terminal 80 residues of Rud3p is present in the genomes of most eukaryotes including mammals, Drosophila melanogaster, Caenorhabditis elegans, plants, fungi, and Dictyostelium discoideum (Fig. 2, A and B). In all but one of these species there is a single member of the family present, the exception being Arabidopsis thaliana, which has two such proteins that are very closely related. The only one of these proteins from other species to have been previously characterized is that from humans, which is called GMAP-210, CEV14, Trip11, or Trip230. This protein was independently identified by either screening an expression library with a human auto-antiserum (GMAP-210, p210; Rios et al., 1994) or by yeast two-hybrid screens with thyroid receptor (Trip11; Lee et al., 1995) and retinoblastoma protein (Trip230; Chang et al., 1997), or as a gene found fused to that of the platelet-derived growth factor receptor by an oncogenic chromosomal translocation (CEV14; Abe et al., 1997). Immunoelectron microscopy has shown that GMAP-210 is localized to the cis side of the Golgi apparatus (Rios et al., 1994).
Figure 2.
Rud3p is a member of a family of coiled-coil proteins with a conserved COOH-terminal domain. (A) Schematic representation of S. cerevisiae Rud3p and its relatives from the indicated species. At, A. thaliana; Ce, C. elegans; Dm, D. melanogaster; Hs, Homo sapiens; Sc, S. cerevisiae; Tb, Trypanosoma brucei. (B) Alignment of the COOH-terminal regions of the GRAB domain proteins with those of GRIP domain proteins golgin-245 and golgin-97, and the structure of the GRIP domain of human golgin-245. The two sets of sequences were independently aligned with CLUSTAL W and shaded where more than half the residues are related (gray) or identical (black). Hydrophobic residues conserved in each set are marked with filled circles and with a red circle for the critical tyrosine in the GRIP domain, and the leucine is in the equivalent position in Rud3p. In both alignments the tryptophans are shaded orange and cluster downstream of the conserved region. In the case of golgin-245, the tryptophan apparently stabilizes the interaction of the GRIP domain with Golgi membranes (Panic et al., 2003a). (C) A schematic representation of the GRAB domain proteins from metazoans and yeasts, along with a GRIP domain protein. All contain either a GRIP or GRAB domain, and the latter have a downstream GA1 motif. Metazoan GRAB proteins also have an extended, proline-rich, COOH-terminal region, whereas GRAB proteins from yeasts and filamentous fungi have an upstream region of sequence conservation (GA2, blue). (D) Fluorescent micrographs of rud3Δ cells expressing GFP-tagged wild-type Rud3p or the mutant L410A as in Fig. 1 A.
In all of these Rud3p-related proteins, the Rud3p homology domain is close to the COOH terminus, although in both plants and metazoans this is followed by a poorly conserved proline-rich extension of 100–150 residues (Fig. 2, B and C). Alignment of the region shared by all the proteins suggests that it is bi-partite, with two well conserved sections separated by a spacer of variable length and sequence. The first of these sections shows a striking similarity to the GRIP domain that is present at the COOH terminus of a number of golgins, and which binds Arl1-GTP on the trans-Golgi (Fig. 2 B). Moreover, the GRIP domain forms a dimer with many hydrophobic contacts formed between well conserved hydrophobic residues (Panic et al., 2003a; Wu et al., 2004). The pattern of conserved hydrophobic residues in the Rud3p-related family closely matches that seen in the GRIP domain (Fig. 2 B). This observation suggests that this region of the protein has a related structure to the GRIP domain, and because it binds to the Arf1 GTPase (see the section Rud3p binds to the GTP-binding proteins Arf1p and Arf3p) we suggest that it be called a GRIP-related Arf-binding (GRAB) domain, and we will call the COOH-terminal conserved region (which has no equivalent in GRIP proteins) the GRAB-associated 1 (GA1) motif.
The GRIP domain contains an invariant tyrosine residue that forms extensive contact with Arl1-GTP, and has been shown to be essential for Golgi targeting in various species from humans to protozoa (Munro and Nichols, 1999; McConville et al., 2002). A leucine residue in the GRAB domain of Rud3p (L410) lies in an equivalent position to this tyrosine residue. Mutation of L410 to A led to a complete loss of Golgi targeting of GFP-Rud3p with the tagged protein appearing instead diffusely distributed in the cytoplasm (Fig. 2 D). Western blot analysis confirmed that the mutant protein was intact and expressed at equivalent levels to the wild type (unpublished data). This observation confirms that the GRAB domain of Rud3p is necessary for Golgi targeting.
Rud3p binds to the GTP-binding proteins Arf1p and Arf3p
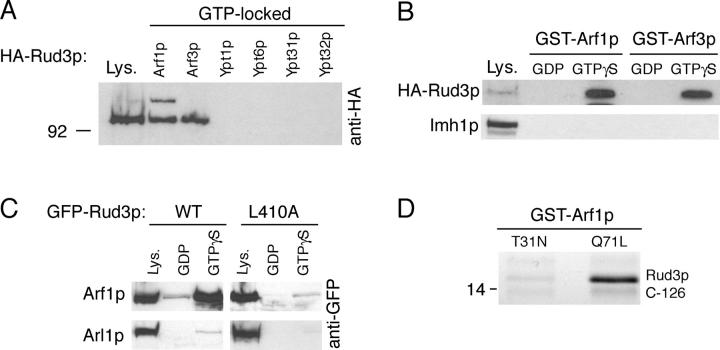
GRIP domain proteins are recruited to the Golgi by interaction with the small GTP-binding protein Arl1. Because the GRIP and GRAB domains appear related, we examined whether or not Rud3p interacts with a small GTP-binding protein on the Golgi. In yeast there are seven small GTP-binding proteins that have been found on Golgi membranes, Arf1p, Arl1p, Arl3p, Ypt1p, Ypt6p, Ypt31p, and Ypt32p. In addition, Arf3p (the closest yeast relative of mammalian Arf6) was analyzed as its location was unknown at the time, although it was subsequently reported to be on the plasma membrane (Huang et al., 2003). Of these GTPases, Arl1p and Arl3p are unlikely candidates to bind Rud3p as they are localized to the late Golgi for the recruitment of the GRIP domain protein Imh1p (Gangi Setty et al., 2003; Panic et al., 2003a). Thus, the other six proteins were expressed in Escherichia coli as fusions to GST, with a Q to L mutation in the GTPase motif. This mutation has been found in other Ras family GTPases to lock the proteins in a GTP-bound state. The GST fusions were immobilized on beads and incubated with lysates from yeast expressing HA-tagged Rud3p. HA-Rud3p bound to both the Golgi-specific GTPase Arf1p and the plasma membrane GTPase Arf3p, but not to the other Golgi GTPases (Fig. 3 A).
Figure 3.
Rud3p interacts with the small GTPase Arf1p. (A) Anti-HA protein blot of total cell lysate (Lys.) from a strain expressing Rud3p tagged in the genome with an NH2-terminal HA epitope tag (AGY10) and of proteins that bound to GST fusions of the GTP-locked versions of Arf1p, Arf3p, Ypt1p, Ypt6, Ypt31p, and Ypt32p. For the GTP-locked forms of Arf1p (Q71L) and Arf3p (Q71L), the first 14 amino acids that form an amphipathic helix were removed and replaced with an NH2-terminal GST tag. For Ypt1p (Q67L), Ypt6p (Q67L), Ypt31p (Q72L), and Ypt32p (Q72L), the COOH-terminal cysteine residues were replaced with a COOH-terminal GST tag. Lysate is 10% of material applied to beads. (B) Anti-HA protein blot of total cell lysate (Lys.; 10% of material loaded) and proteins that bound to GST fusions of wild-type Arf1p and Arf3p preloaded with GDP or a nonhydrolysable analogue of GTP, GTPγS. The blot was stripped and reprobed with a rabbit antibody against Imh1p. (C) As for GST-Arf1p in B, except cell lysates were prepared from a strain expressing either GFP-Rud3p or the mutant proteins L410A expressed under the control of a constitutively active PHO5 promoter from the CEN plasmid pRS416. (D) Binding of the COOH-terminal 126 amino acids of Rud3p to Arf1p (T31N) or Arf1p (Q71L). The indicated forms of GST-Arf1p were coexpressed in E. coli with the COOH terminus of Rud3p, and after cell lysis isolated on glutathione Sepharose beads. Bound proteins were analyzed by gel electrophoresis, and the indicated band was identified as the COOH terminus of Rud3p by matrix-assisted laser desorption ionization mass spectrometry of tryptic peptides (Shevchenko et al., 1996).
To investigate if the interaction between Arf1p and Rud3p was nucleotide dependent, GST fusions to wild-type Arf1p and Arf3p were immobilized on beads and loaded with either GDP or the nonhydrolysable analogue GTP-γ-S. Epitope-tagged Rud3p bound to both Arf1p and Arf3p only in the GTP-bound state, whereas the GRIP domain protein Imh1p did not bind to these proteins irrespective of their nucleotide status (Fig. 3 B). In addition, the L410A mutation in the GRAB domain of Rud3p greatly reduced binding to Arf1p, whereas neither wild-type nor mutant Rud3p bound to Arl1p (Fig. 3 C).
To determine if the interaction between Rud3p and activated Arf1p was direct, the COOH-terminal 126 amino acids of Rud3p were coexpressed in E. coli with either GDP-locked GST-Arf1p (T31N) or GTP-locked GST-Arf1p (Q71L). Fig. 3 D shows that when the Arf1p fusions were purified on glutathione Sepharose beads the Rud3p COOH-terminal domain was detected only in association with the activated Arf1p GTPase (Fig. 3 D). These results demonstrate that Rud3p binds directly to GTP-bound Arf1p and Arf3p and that this interaction is mediated by the GRAB domain that is required for Golgi targeting.
Rud3p is required for viability in the absence of active Ypt6p
To test the importance of the GRAB domain for the function of Rud3p, we took advantage of a recently reported genetic interaction between Rud3p and Ric1p, a subunit of the exchange factor for Ypt6p (yeast Rab6; Tong et al., 2004). Deletion of the genes for Ypt6p or for its exchange factor subunit Ric1p, does not affect growth, but causes partial defects in sorting to the vacuole. However, when a number of other nonessential genes are deleted in combination with ypt6Δ or ric1Δ, viability is severely impaired or lost (Tsukada et al., 1999; Siniossoglou et al., 2000). This “synthetic lethality” probably reflects the existence of two or more recycling pathways between endosomes and Golgi, with at least one of these being required for sufficient recycling to the Golgi to sustain exocytosis. To identify more proteins that show such an interaction, both YPT6 and RIC1 were included in a large scale synthetic genetic array analysis in which 132 mutant query strains were mated against a genome-wide set of viable deletions and then the double mutations tested for viability after sporulation. This approach revealed that deletion of RUD3 is synthetically lethal with loss of either YPT6 or RIC1 (Tong et al., 2004).
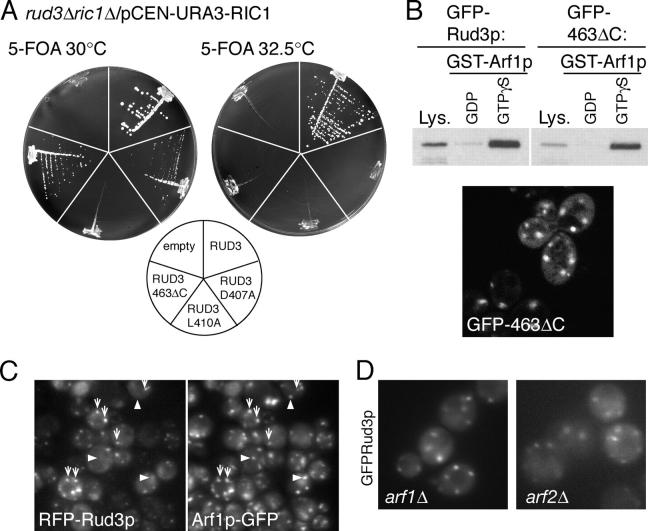
To examine this further, a strain (AGY24) was created in which RUD3 and RIC1 were deleted from the genome, with viability maintained by a CEN plasmid expressing Ric1p. When GFP-Rud3p was expressed in this strain it was able to maintain growth upon loss of the RIC1 plasmid, indicating that the fusion protein is functional. However, GFP-Rud3p (L410A) could not sustain growth in the absence of Ric1p (Fig. 4 A). In addition, mutation of another conserved residue, D407A, resulted in slow growth after loss of Ric1p, with this mutation causing a partial delocalization from the Golgi (unpublished data). These data indicate that the GRAB domain is required not only for Rud3p targeting but also for its function.
Figure 4.
COOH-terminal mutants of Rud3p are not functional. (A) Growth at the indicated temperatures of yeast lacking genomic copies of both RIC1 and RUD3, but containing the either an empty plasmid or expressing wild-type or mutant GFP-Rud3p (indicated on the diagram) from a constitutive PHO5 promoter. Cells lacking RUD3 and with only copy of RIC1 on a CEN, URA3 plasmid were transformed with CEN, LEU2 plasmids expressing GFP-Rud3p or mutants, and the yeast plated onto plates containing 5-fluoroorotic acid (5-FOA) to remove the RIC1 containing URA3 plasmid. (B) A yeast strain expressing GFP-tagged Rud3p lacking the GA1 motif (463ΔC) was used in a binding assay as in Fig. 3 C with GST-Arf1p loaded with either GDP or GTP-γ-S. Also shown is a fluorescent micrograph of live yeast lacking genomic RUD3 and expressing GFP-Rud3p463ΔC. (C) Fluorescent micrographs of a rud3Δ strain with the ARF1 ORF tagged in the genome with a COOH-terminal GFP tag (AGY26) and expressing RFP-Rud3p under the constitutive PHO5 promoter from the CEN plasmid pRS416. Some structures contain both RFP-Rud3p and Arf1p-GFP (arrows) and some contain just the latter (arrowheads). (D) Fluorescent micrographs of live BY4741 yeast with the indicated genes deleted and expressing GFP-Rud3p as in Fig. 1 C.
Mutations in the GA1 motif affect the function of Rud3p
As noted in the section Specific residues within the Rud3p COOH terminus are critical for its localization, Rud3p and its relatives share an additional region of similarity COOH-terminal of the GRAB domain, which we have termed the GA1 motif (Fig. 2 B). We used the synthetical lethality between ric1Δ and rud3Δ to examine the contribution this motif makes to Rud3p function, Golgi targeting, and Arf1p binding. A mutant form of GFP-Rud3p lacking the COOH-terminal 21 amino acids including the GA1 motif (GFP-Rud3p463ΔC) was not detectably delocalized to the cytoplasm in vivo and bound GST-Arf1p in a GTP-dependent manner in vitro (Fig. 4 B). However, expression of this mutant lacking the GA1 motif in the double knockout strain (ric1Δrud3Δ) gave only slow growth at 30°C and no growth at all at 32.5°C, indicating that this GA1 motif is not required for Arf1p binding or Golgi targeting but is important for Rud3p function (Fig. 4 A).
Rud3p colocalizes with Arf1p
Because Rud3p interacts in vitro with Arf1p-GTP, we compared the localization of the two proteins in living cells. As expected, both RFP-Rud3p and Arf1p-GFP localized to punctate structures and some, but not all, of these puncta contained both proteins, with RFP-Rud3p always being present with Arf1p-GFP, but with the latter also being present in structures that lacked detectable Rud3p (Fig. 4 C). The in vitro binding assay had also shown that Rud3p could bind the GTPase Arf3p, but as recently reported by Huang et al. (2003), Arf3p-GFP was not in cytoplasmic puncta like Rud3p, but rather localized to the cytoplasm and to specific regions of the cell surface including the emerging bud (unpublished data). S. cerevisiae also contains a second gene, ARF2, that is very closely related to ARF1, and has overlapping functions as both must be deleted for loss of viability (Stearns et al., 1990). Deletion of either gene alone does not affect the distribution of GFP-Rud3p, indicating that, as with other functions, either is sufficient for Rud3p targeting (Fig. 4 D). These results indicate that Rud3p colocalizes in vivo with membranes containing Arf1p, but suggest that other factors may affect recruitment as the protein is only localized to a subset of membrane that contains Arf1p (and Arf2p) and does not colocalize at all with Arf3p.
The ER cargo receptor Erv14p is required for Rud3p localization
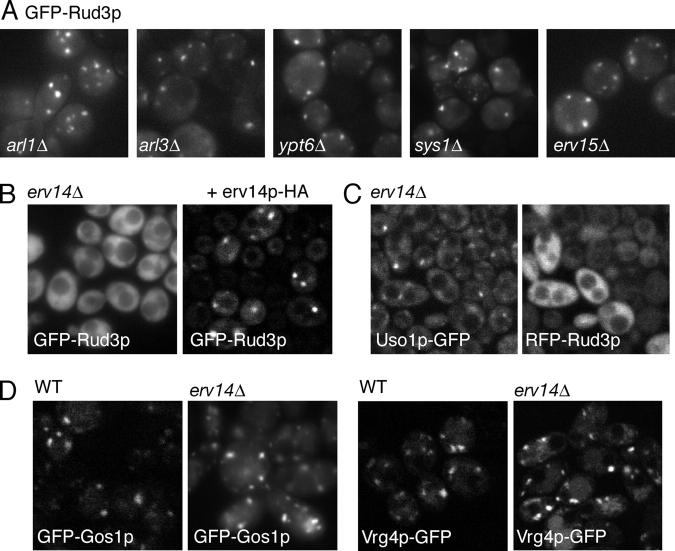
Because the RUD3 gene displays a synthetic lethal interaction with the RIC1 gene, it seemed likely that genes encoding proteins involved in Rud3p targeting will also be synthetically lethal when deleted in combination with the RIC1 gene. Thus, we examined the localization of GFP-Rud3p in deletion strains for all of the 113 genes whose deletion had been found to be synthetically lethal with ric1Δ by synthetic genetic array analysis (Tong et al., 2004; Fig. 5 A and Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200407088/DC1). GFP-Rud3p showed a typical punctate distribution in all of the mutants, with one exception. In the mutant lacking the gene ERV14, GFP-Rud3p was instead found diffusely distributed throughout the cytosol (Fig. 5 B). This phenotype could be rescued by expression of untagged Erv14p or Erv14p tagged at the COOH terminus with two copies of the HA epitope, indicating that it was caused by loss of Erv14p and not due to an indirect effect of the deletion (Fig. 5 B). Moreover, the mislocalization of Rud3p did not reflect a general disturbance of the Golgi in the erv14Δ strain, as Uso1p-GFP remained largely localized to puncta (Fig. 5 C). In addition, the distributions of the Golgi SNARE protein Gos1p and the Golgi GDP-mannose transporter Vrg4p were also unaffected in the erv14Δ strain (Fig. 5 D).
Figure 5.
Erv14p is required for the Golgi localization of Rud3p. (A) Fluorescent micrographs of live yeast (BY4741) expressing GFP-Rud3p from plasmid pE1, with the indicated genes deleted. (B) Fluorescent micrographs of an erv14Δ strain with the RUD3 ORF NH2-terminally GFP tagged in the genome and containing either an empty CEN plasmid or a similar plasmid encoding Erv14p-HA as indicated. (C) Fluorescent micrographs of a yeast strain lacking ERV14 and with USO1 COOH-terminally GFP tagged in the genome and containing a CEN plasmid expressing RFP-Rud3p as in Fig. 1 A. (D) Distribution of the Golgi markers Gos1p and Vrg4p expressed as GFP-fusions in either wild-type cells or a mutant lacking ERV14 as indicated. GFP-tagged proteins expressed as in A.
Erv14p is an integral membrane protein that was isolated as a component of COPII vesicles and was shown to cycle between the ER and the Golgi and to act as a cargo receptor for the ER exit of the plasma membrane protein Axl2p (Powers and Barlowe, 1998, 2002). Therefore, we examined the localization of GFP-Rud3p in deletion strains for the other nonessential ER vesicle proteins (Erp1p, Erp2p, Erv25p, Erv29p, Erv46p, Emp24, Emp47p, Rer1p, and Yip3p). In none of these deletions strains did GFP-Rud3p show a diffuse distribution, and indeed only one (Rer1p) had appeared in the initial RIC1 synthetic lethal screen. In addition, Erv15p is 63% identical to Erv14p, but loss of ERV15 had no effect on the localization of GFP-Rud3p (Fig. 5 A). This finding is consistent with the finding that Erv15p cannot substitute for Erv14p as a cargo receptor for Axl2p, suggesting that it has a function distinct to that of Erv14p (Powers and Barlowe, 1998).
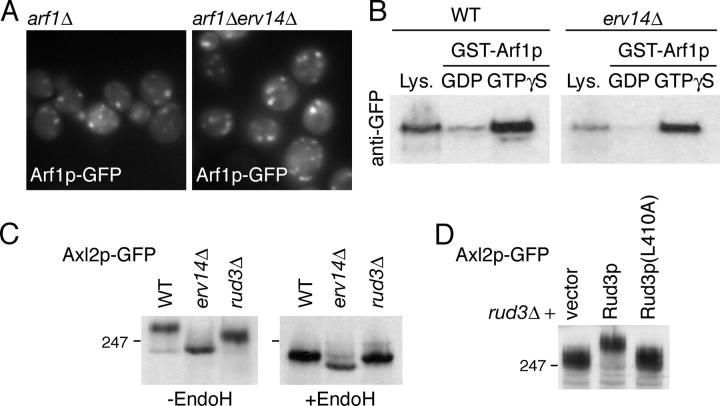
The redistribution of Rud3p in cells lacking Erv14p is not due to mislocalization of Arf1p as the GTPase is not relocalized to the cytoplasm in an erv14Δ strain (Fig. 6 A). In addition, loss of Erv14p does not appear to cause an alteration in Rud3p that renders it incapable of binding Arf1p, as GFP-Rud3p from strains lacking Erv14p was still able to associate with Arf1p-GTP in vitro (Fig. 6 B). Finally, we examined whether or not Rud3p is required for Erv14p to function. Deletion of Erv14p causes the cargo protein Axl2p to accumulate in the ER, resulting in increased gel mobility due to the absence of Golgi processing of N- and O-linked glycans (Powers and Barlowe, 1998). However, in a rud3Δ strain the ER form of Axl2p did not accumulate, indicating that Rud3p is not required for the Erv14p-dependent ER exit of Axl2p (Fig. 6 C). However, in this strain the N-linked glycans on Axl2p were not as extensively modified as in wild-type, which is consistent with the partial defect in Golgi glycan modification previously reported with a different substrate (Kim, 2003). Fig. 6 D shows that this phenotype could not be rescued by Rud3p carrying the L410A mutant that prevents Arf1p binding, indicating that interaction between Rud3p and Arf1p is required for normal Golgi function.
Figure 6.
Erv14p and Rud3p in Golgi function. (A) Fluorescent micrographs of yeast (BY4741) expressing Arf1p-GFP from a CEN, URA3 plasmid, with the genomic copies of ARF1 and ERV14 deleted as indicated. (B) Anti-GFP protein blot of lysates (Lys.; 10% of material loaded) from wild-type BY4741 (WT) or erv14Δ cells expressing GFP-Rud3p or of the proteins bound when the lysates were applied to immobilized GST-Arf1p loaded with the indicated nucleotides, as in Fig. 3 C. (C) Anti-GFP protein blot of total cellular proteins from BY4741 (WT) or the indicated strains expressing Axl2p-GFP from a CEN plasmid, with or without endoglycosidase H digestion. (D) As C, except that the cells were rud3Δ and contained either an empty CEN vector or the same with the indicated forms of GFP-Rud3p.
The GRAB domain of the mammalian protein GMAP-210 mediates Golgi targeting
As described in the Specific residues within the Rud3p COOH terminus are critical for its localization, the GRAB domain identified in Rud3p is also present at the COOH terminus of the mammalian protein GMAP-210. This protein has been reported independently by two groups to be Golgi localized, although there is conflicting data as to how it interacts with the Golgi membranes (Rios et al., 1994; Chen et al., 1999; Infante et al., 1999). Infante et al. (1999) incubated Golgi membranes with recombinant fragments of GMAP-210 fused to GST. They concluded that the main interaction with membranes is mediated by the NH2-terminal 375 amino acids, and reported that a fusion between GFP and this portion of the protein was targeted to the Golgi in vivo. In addition, the COOH-terminal 202 residues of GMAP-210, which includes the GRAB domain, was reported to be targeted to centrosomes in vivo, and to bind γ-tubulin in vitro (Infante et al., 1999; Rios et al., 2004). In contrast, Chen et al. (1999) used fluorescence microscopy to examine in vivo the location of a series of GMAP-210 deletions. They reported that the COOH-terminal 226 residues of GMAP-210 are targeted to the Golgi. To investigate this further, we expressed in COS cells GFP fusions to the NH2-terminal 372 residues or the COOH-terminal 223 residues of GMAP-210. Protein blotting showed that the two fusion proteins were intact and accumulated to comparable levels (unpublished data). Fig. 7 A shows that the NH2-terminal fragment was not detectably associated with the Golgi, and instead fluorescence was observed throughout the cytoplasm. In contrast, the COOH-terminal domain localized to a juxtanuclear structure, which colocalized with the Golgi protein GM130, but did not colocalize with the centrosomal marker γ-tubulin (Fig. 7 A). It has recently been reported that overexpression of full-length GMAP-210 in COS cells results in accumulation of γ-tubulin on Golgi membranes and that this depends on the COOH-terminal 202 residues of the protein. However, in COS cells expressing either the COOH-terminal 223 residues or full-length GMAP-210, we did not observe γ-tubulin colocalizing with the protein on the Golgi (Fig. 7 A), even when the level of the full-length protein was so high that it induced Golgi fragmentation as previously reported (Pernet-Gallay et al., 2002). Therefore, our results indicate that the COOH terminus of GMAP-210 is responsible for Golgi rather than centrosomal targeting, in agreement with Chen et al. (1999).
Figure 7.
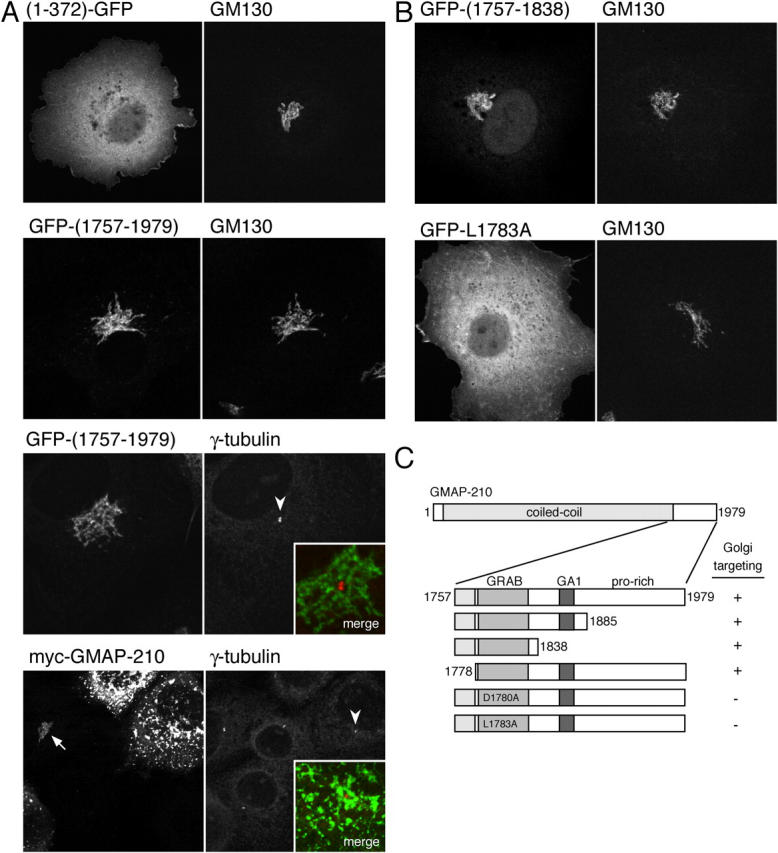
The COOH-terminal region of human GMAP-210 mediates targeting to the Golgi rather than centrosomes. (A) Confocal micrographs of COS cells expressing the NH2-terminal 372 amino acids of GMAP-210 COOH-terminally tagged with GFP, or the COOH-terminal 223 amino acids of GMAP-210 NH2-terminally tagged with GFP, or full-length GMAP-210 with an NH2-terminal myc tag. Cells were also labeled with antibodies against the endogenous Golgi protein GM130 or γ-tubulin. At very high levels of GMAP-210, normal Golgi morphology (arrow) is lost and the Golgi becomes fragmented as described previously (Pernet-Gallay et al., 2002), and yet γ-tubulin is still only on the centrosome (arrowheads indicate the centrosomes shown in merged inset). (B and C) Illustrative confocal images of COS cells expressing portions of GMAP-210 fused to GFP and costained with GM130 as in A, along with a summary of the results from all GMAP-210 portions examined.
Transfection of GFP fusions to parts of the GMAP-210 COOH-terminal region showed that residues 1757 to 1838 are sufficient for Golgi targeting (Fig. 7, B and C). This region spans the GRAB domain, but does not include the GA1 motif. Moreover, mutation of conserved residues in the GRAB domain, D1780A and L1783A (equivalent to D407 and L410 in yeast), resulted in a diffuse cytosolic distribution of the tagged protein (Fig. 7 B). Together, these results indicate that the COOH-terminal GRAB domain in the mammalian protein GMAP-210 is not only related to that in yeast Rud3p but shares an in vivo role of mediating Golgi targeting.
Discussion
Here, we have shown that the coiled-coil protein Rud3p is recruited to the Golgi via a conserved COOH-terminal GRAB domain and that this recruitment is mediated by the combined action of the GTP-binding protein Arf1p and the membrane protein Erv14p. The GRAB domain binds directly to Arf1p-GTP, and appears to be structurally related to the GRIP domain that binds to the Arf-like GTPase Arl1. Because the GRAB and GRIP domain are present in both trypanosomes and humans, it is tempting to speculate that the two systems arose by a gene duplication early in eukaryotic evolution, perhaps associated with an increase in the complexity of a primitive Golgi stack.
Arf1 has previously been shown to play several roles in Golgi function, of which the best characterized is the direct binding to coatomer to form COPI-coated vesicles (Serafini et al., 1991; Donaldson et al., 1992). In addition, Arf1 (and the closely related class II Arfs present in metazoans) has been implicated in recruiting several other coat proteins to Golgi membranes including the AP1, AP3, AP4, and GGA clathrin adaptors that form coated vesicles on the trans-Golgi network (Nie et al., 2003). Finally, yeast two-hybrid screens have revealed interactions between Arfs and effectors of unknown function, including arfaptin, a Golgi protein with a BAR domain, and arfophilin/FIP3, which is found on endosomes and also interacts with Rab11 (Kanoh et al., 1997; Shin et al., 1999).
Although it seems clear that a number of peripheral membrane proteins share a dependency on Arf1 for their Golgi association, they nonetheless have different distributions within the Golgi stack. Thus, Rud3p is recruited only to the cis-Golgi, whereas AP1 and GGAs are on the trans-Golgi, and COPI is throughout the Golgi stack with a concentration on the cis-side. This broad distribution of Arf1 effectors in the Golgi is consistent with Arf1 GEFs being found on both cis and trans cisternae (Kawamoto et al., 2002; Zhao et al., 2002). However, this raises the question of how Rud3p and other Arf1 effectors are specifically recruited to only a subset of Golgi membranes that contain active Arf1. In most cases, the basis for this specificity is not certain, although it presumably reflects the combinatorial recognition of Arf1 in conjunction with additional determinants with a more restricted distribution on Golgi membranes. For coat proteins, the recognition of the cytoplasmic tails of cargo proteins may play a role. GGAs are known to bind to ubiquitin that is added to specific transmembrane proteins as they pass through the trans-Golgi (Pelham, 2004). Likewise, COPI recognizes KKXX and other recycling signals on membrane proteins that are removed from the cis-Golgi cisternae before they can move to later compartments (Kreis et al., 1995). In contrast, the trans-Golgi recruitment of AP1, and several Golgi-specific PH domains, has been suggested to be dependent on both Arf1 and PtdIns(4)P (Levine and Munro, 2002; Wang et al., 2003). In all of these cases, the determinant that would act with Arf1 is also present on non-Golgi membranes, and hence Arf1 would be critical for ensuring a Golgi-restricted distribution.
Rud3p appears to have a distinct distribution from other Arf effectors on Golgi membranes in that it is restricted to just the cis-Golgi. We have found that this recruitment to a subset of Arf-containing membranes is dependent on Erv14p. This integral membrane protein was originally identified as a component of purified COPII vesicles and found to cycle between ER and Golgi (Powers and Barlowe, 1998, 2002). Moreover, Erv14p is related to the D. melanogaster protein Cornichon, one of two Erv14p relatives in metazoans. Mutations in Cornichion cause defects in signaling by the transforming growth factor-α like molecule Gurken (Roth et al., 1995). Gurken is synthesized with a COOH-terminal transmembrane domain and is secreted from oocytes after cleavage by a Rhomboid protease in the Golgi apparatus (Urban et al., 2002). However, in Cornichon mutants Gurken accumulates in the internal membranes of the oocyte (Roth et al., 1995). Similarly, deletion of ERV14 from yeast causes the plasma membrane protein Axl2p, but not other secretory cargo, to accumulate in the ER (Powers and Barlowe, 1998). This finding suggests that Erv14p and Cornichon act as cargo receptors to facilitate the efficient ER exit of specific transmembrane proteins. It may also be that Erv14p has additional roles, as its overexpression suppresses temperature-sensitive mutations in Dsl1p, a protein required for Golgi to ER traffic (VanRheenen et al., 2001). In addition, Erv14p is required for efficient ER-associated degradation of misfolded soluble but not transmembrane substrates (Caldwell et al., 2001).
Although the specification of the cis-Golgi by the combinatorial action of a GTPase found throughout the Golgi stack and a membrane protein that is found in the ER and cis-Golgi appears logical, the precise molecular role of Erv14p in this system remains unclear. We have been unable to detect an interaction between Erv14p and either Arf1p or Rud3p by cross-linking followed by immunoprecipitation (unpublished data). Thus, either there are no reactive residues in suitable positions to be cross-linked, or instead Erv14p is required for the localization or activity of a further protein that plays a more direct role. Experiments in which ER to Golgi transport is blocked suggest that Rud3p does not recycle through the ER, implying that any association between Erv14p and Rud3p is not stable (Kim, 2003). Interestingly, in mammalian cells, although we have shown that the GRAB domain of GMAP-210 mediates Golgi targeting, we have not been able to detect direct binding between this COOH-terminal region of GMAP-210 and Arf1, suggesting that additional factors make an even greater contribution to membrane recruitment in this system (unpublished data).
It has recently been suggested that the COOH-terminal region of GMAP-210 binds to γ-tubulin (Rios et al., 2004). This suggestion followed on from a report that the COOH terminus is targeted to centrosomes and that the NH2 terminus is targeted to the Golgi apparatus (Infante et al., 1999), and it has lead to the proposal that GMAP-210 acts to organize the Golgi ribbon around centrosomes (Rios et al., 2004). However, our in vivo targeting experiments could not reproduce these findings, but instead showed clearly that the COOH terminus rather than the NH2 terminus of GMAP-210 has Golgi-targeting activity. Moreover, we do not find evidence for GMAP-210 recruiting γ-tubulin to Golgi membranes. The bases of these discrepancies is unclear, but it should be noted that our data are in agreement with those of Chen et al. (1999) who found that the COOH-terminal 226 residues of GMAP-210 are sufficient for Golgi targeting. Moreover, previous localization studies on γ-tubulin have not reported detectable Golgi staining, including those using GFP-fusions that might be expected to obviate concerns about antibody accessibility (Khodjakov and Rieder, 1999; Piel et al., 2000; Yvon et al., 2002). Certainly, our results are hard to reconcile with the proposal that GMAP-210 anchors the Golgi to the pericentriolar region by binding centrosomes and γ-tubulin via its COOH terminus (Rios et al., 2004).
Although our data do not bear on an alternative function of GMAP-210, it seems likely that its role, and that of Rud3p, requires that the proteins are accurately targeted to the cis-Golgi. For at least Rud3p, the Golgi glycosylation defects in yeast lacking the protein imply a role in the retrograde traffic within the Golgi, a role that apparently becomes essential when other recycling processes that depend on Ypt6p are absent. We have also found that both GMAP-210 and Rud3p have a conserved GA1-motif COOH-terminal to the GRAB domain. This motif is important for Rud3p function, but not targeting, suggesting that it could be responsible for bringing a protein on the cis-Golgi membrane into close association with those interacting with the NH2-terminal coiled-coil regions of the GRAB domain proteins. Like most other golgins, the set of interaction partners for Rud3p remain to be elucidated, but consistent with their proposed role in the organization of the Golgi it is now becoming clear that the targeting of golgins is tightly regulated, and understanding the basis of this regulation seems likely to reveal how the architecture of the Golgi is established and maintained.
Materials and methods
Yeast strains and plasmids
Unless otherwise stated, yeast strains were based on BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) or deletion strains made within this background from the EUROSCARF consortium (Table I). Assays of the functionality of tagged and mutant forms of Rud3p were performed in AGY24, a ric1Δ strain based on IAY11 with the covering plasmid pEH (ADE3, RIC1; Whyte and Munro, 2001). Tagging and truncation of the RUD3 ORF at the NH2 terminus or tagging of the USO1 ORF at the COOH terminus were performed using PCR-based homologous recombination (Wach et al., 1997). Other tagged proteins were expressed from pRS series CEN plasmids under the control of a constitutive PHO5 promoter unless otherwise stated, with pE1 being GFP-Rud3p in CEN URA3. Rud3p was tagged at the NH2 terminus with GFP and RFP (DsRed (tdimer2(12)), with an intervening GAGA linker, and point mutants were generated with Quikchange (Stratagene). Arf1p was expressed from a CEN plasmid tagged at the COOH terminus with GFP. EGFP-tagged NH2- and COOH-terminal fragments of human GMAP-210 were expressed in a vector containing a cytomegalovirus promoter, an SV40 origin of replication (CLONTECH Laboratories, Inc.), and myc-GMAP-210 in pcDNA3.1 (provided by F. Barr and J. Egerer, Max Planck Institute for Biochemistry, Martinsried, Germany).
Table I. S. cerevisiae strains generated in the course of this work.
| Name | Genotype |
|---|---|
| AGY09 | SEY6210 GFP-RUD3::S.p. his5+ |
| AGY10 | C13-ABYS-86 (MATa pra1-1 prb1-1 prc1-1 cps1-3 ura3-52 leu2-3 112 his3), HA-RUD3::S.p. his5+ |
| AGY14 | BY4741 erv14Δ::kanMX4, GFP-RUD3::S.p. his5+ |
| AGY18 | SEY6210 GFP-rud3ΔN104::S.p. his5+ |
| AGY19 | SEY6210 GFP-rud3ΔN194::S.p. his5+ |
| AGY20 | SEY6210 GFP-rud3ΔN294::S.p. his5+ |
| AGY21 | BY4741, GFP-RUD3::S.p. his5+ |
| AGY22 | BY4741 USO1-GFP::S.p. his5+ |
| AGY23 | BY4741 erv14Δ::kanMX4 USO1-GFP::S.p. his5+ |
| AGY24 | IAY11 ([MATα ade2-1 trp1-1can 1-1000 leu2-3 112 his3-11,15 ura3-52 ade3-Δ853] ric1Δ::his3 rud3Δ::kanMX4
+ pEH [RIC1 in pRS316]). |
| AGY25 | SEY6210 GFP-rud3ΔN405::S.p. his5+ |
| AGY26 | BY4741 rud3Δ::kanMX4 ARF1-GFP::S.p. his5+ |
Preparation of recombinant proteins
Arf1p, Arf3p, or Arp1p lacking the NH2-terminal 14 residues were expressed with GST fused to their NH2 termini by using plasmid pGEX-6p-2 (Amersham Biosciences). Ypt proteins with the COOH-terminal 4 (Ypt1p) or 5 (Ypt6p, Ypt31p, Ypt32p) residues replaced by GST were expressed with the expression vector pOPT. A Quikchange mutagenesis kit was used to introduce the following mutations: Arf1p (Q71L), Arf3p (Q71L), Ypt1p (Q67L), Ypt6p (Q67L), Ypt31p (Q72L), and Ypt32p (Q72L). Arf and Ypt GST fusion proteins were expressed in the E. coli BL21-GOLD (DE3) strain (Stratagene). Cells were induced at OD 600 ≈ 0.7 for 3–4 h with 0.2 mM of IPTG at 22°C, and lysates were prepared by sonication in 10 ml of lysis buffer (PBS, 1% [vol/vol] Triton X-100, 200 μM GDP, 5 mM MgCl2, and 5 mM β-mercaptoethanol) containing protease inhibitors. The lysates were clarified by centrifugation at 12,000 g for 10 min. For coexpression experiments, Arf1p (T31N) and Arf1p (Q71L) were coexpressed with the COOH-terminal 126 amino acids of Rud3p in the polycistronic vector pOPCG and also expressed in the E. coli BL21-GOLD (DE3) strain (Stratagene).
Affinity chromatography with immobilized GTPases
GST-Arf1p, GST-Arf3p, or GST-Arl1p were purified, and then loaded with either GDP or GTP-γ-S as described previously (Panic et al., 2003b). In brief, bacterial lysates were incubated with glutathione Sepharose beads (Amersham Biosciences) at 4°C for 30 min. The beads were washed in lysis buffer without detergent, followed by NS buffer (20 mM Tris-HCl, pH 8.0, 110 mM KCl, 5 mM MgCl2, and 1 mM DTT) containing 200 μM GDP, and then NE buffer (NS buffer with 10 mM EDTA) with either 10 μM GDP or GTP-γ-S. This was followed by 3 × 30-min incubations in NE buffer containing 1 mM GDP or GTP-γ-S and 1 × 20-min incubation in NS buffer containing the same nucleotides. All incubations were at RT.
Yeast lysates were prepared from 2 g of spheroplasts lysed by dounce homogenization in 8 ml of NS buffer containing protease inhibitors. Lysates were clarified by centrifugation at 100,000 g for 30 min at 4°C, followed by filtration through 0.45-μm filters, and then were applied to 100 μl GST-Arf1p:GDP or GST-Arf1p:GTP-γ-S (or the equivalent GST-Arf3p or GST-Arl1p beads) and incubated for 2 h at 4°C in the presence of 100 μM GDP or GTP-γ-S, respectively. The beads were washed in NS buffer and eluted with 100 μl of SDS sample buffer. In the case of the GTP-locked GTPase mutants, the nucleotide loading step was omitted and, after binding of the GST fusions to beads and washing in NS buffer, lysates were added and incubated in the presence of 100 μM GTP, and washed and eluted as for the nucleotide-loaded forms.
For coexpression experiments, E. coli cells expressing Arf1p (T31N or Q71L) and the Rud3p COOH-terminal domain were lysed in lysis buffer (50 mM Tris-HCl, pH 7.5, 100 mM KCl, and 5 mM MgCl2) containing protease inhibitors and either 200 μm GDP or GTP. After clarification by centrifugation at 12,000 g for 10 min at 4°C, the supernatant was added to 100 μl of glutathione Sepharose beads. The beads were rotated at 4°C for 30 min and washed four times with lysis buffer, and bound proteins were eluted by the addition of 100 μl of SDS sample buffer.
Immunoblotting and fluorescence microscopy
Yeast cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and probed with primary antibodies in PBS, 5% (wt/vol) dried milk, and 0.1% (vol/vol) Tween 20 for 1 h. Species-specific HRP secondary antibodies were detected by ECL (Amersham Biosciences). Live yeast expressing GFP fusions were photographed at RT under coverslips using a microscope (model Axioscop 2; Carl Zeiss MicroImaging, Inc.), a CCD camera (Princeton Instruments) using 1–2-s exposures, and IPLabs acquisition software. For cells expressing both RFP and GFP fusion proteins, images were obtained on a Radiance confocal microscope (Bio-Rad Laboratories).
Mammalian tissue culture
COS cells were transfected using FuGene (Roche), split onto glass slides, and fixed 30–48 h after transfection with 4% (wt/vol) PFA (or methanol/acetone for localization of γ-tubulin). Cells were permeabilized with 0.5% (vol/vol) Triton X-100 in PBS, blocked with 20% (vol/vol) FCS/0.25% (vol/vol) Tween 20 in PBS, and stained in the same solution. Mouse mAbs to GM130 (BD Biosciences) and γ-tubulin (GTU-88; Sigma-Aldrich) were detected with Alexa546 secondary antibodies (Molecular Probes), the cells mounted in Fluoromount-G (Southern Biotechnology Associates, Inc.), and viewed with a Radiance confocal microscope (Bio-Rad Laboratories).
Online supplemental material
Table S1 lists all of the genes whose deletion showed synthetic lethality with RIC1 and for which deletion strains were examined for perturbation of GFP-Rud3p. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200407088/DC1.
Acknowledgments
We thank James Whyte for help in the initial stages of the Rud3p localization screen, Hugh Pelham and Katja Röper for comments on the manuscript, and Francis Barr and Johannes Egerer for reagents and discussions.
This work was supported by the UK Medical Research Council (S. Munro) and the Canadian Institute of Health Research, Genome Canada, and Genome Ontario (C. Boone).
Abbreviations used in this paper: GA1, GRAB-associated 1; GRAB, GRIP-related Arf-binding.
References
- Abe, A., N. Emi, M. Tanimoto, H. Terasaki, T. Marunouchi, and H. Saito. 1997. Fusion of the platelet-derived growth factor receptor β to a novel gene CEV14 in acute myelogenous leukemia after clonal evolution. Blood. 90:4271–4277. [PubMed] [Google Scholar]
- Barr, F.A., and B. Short. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405–413. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. [DOI] [PubMed] [Google Scholar]
- Behnia, R., B. Panic, J.R. Whyte, and S. Munro. 2004. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat. Cell Biol. 6:405–413. [DOI] [PubMed] [Google Scholar]
- Caldwell, S.R., K.J. Hill, and A.A. Cooper. 2001. Degradation of endoplasmic reticulum (ER) quality control substrates requires transport between the ER and Golgi. J. Biol. Chem. 276:23296–23303. [DOI] [PubMed] [Google Scholar]
- Campbell, R.E., O. Tour, A.E. Palmer, P.A. Steinbach, G.S. Baird, D.A. Zacharias, and R.Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA. 99:7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., N. Ballew, and C. Barlowe. 1998. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17:2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, K.H., Y. Chen, T.T. Chen, W.H. Chou, P.L. Chen, Y.Y. Ma, T.L. Yang-Feng, X. Leng, M.J. Tsai, B.W. O'Malley, et al. 1997. A thyroid hormone receptor coactivator negatively regulated by the retinoblastoma protein. Proc. Natl. Acad. Sci. USA. 94:9040–9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., P.L. Chen, C.F. Chen, Z.D. Sharp, and W.H. Lee. 1999. Thyroid hormone, T3-dependent phosphorylation and translocation of Trip230 from the Golgi complex to the nucleus. Proc. Natl. Acad. Sci. USA. 96:4443–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, A., D. Rahman, D.J. Pappin, J. Lucocq, and M. Lowe. 2003. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J. Cell Biol. 160:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, J.G., D. Cassel, R.A. Kahn, and R.D. Klausner. 1992. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc. Natl. Acad. Sci. USA. 89:6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangi Setty, S.R., M.E. Shin, A. Yoshino, M.S. Marks, and C.G. Burd. 2003. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 (Arl1p) is regulated by the Arf-like GTPase 3 (Arl3p). Curr. Biol. 13:401–404. [DOI] [PubMed] [Google Scholar]
- Gillingham, A.K., and S. Munro. 2003. Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta. 1641:71–85. [DOI] [PubMed] [Google Scholar]
- Huang, C.F., Y.W. Liu, L. Tung, C.H. Lin, and F.J. Lee. 2003. Role for Arf3p in development of polarity, but not endocytosis, in Saccharomyces cerevisiae. Mol. Biol. Cell. 14:3834–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante, C., F. Ramos-Morales, C. Fedriani, M. Bornens, and R.M. Rios. 1999. GMAP-210, A cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J. Cell Biol. 145:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh, H., B.T. Williger, and J.H. Exton. 1997. Arfaptin 1, a putative cytosolic target protein of ADP-ribosylation factor, is recruited to Golgi membranes. J. Biol. Chem. 272:5421–5429. [DOI] [PubMed] [Google Scholar]
- Kawamoto, K., Y. Yoshida, H. Tamaki, S. Torii, C. Shinotsuka, S. Yamashina, and K. Nakayama. 2002. GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic. 3:483–495. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., and C.L. Rieder. 1999. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.W. 2003. Characterization of Grp1p, a novel cis-Golgi matrix protein. Biochem. Biophys. Res. Commun. 303:370–378. [DOI] [PubMed] [Google Scholar]
- Kim, D.W., M. Sacher, A. Scarpa, A.M. Quinn, and S. Ferro-Novick. 1999. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol. Biol. Cell. 10:3317–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis, T.E., M. Lowe, and R. Pepperkok. 1995. COPs regulating membrane traffic. Annu. Rev. Cell Dev. Biol. 11:677–706. [DOI] [PubMed] [Google Scholar]
- Lee, J.W., H.S. Choi, J. Gyuris, R. Brent, and D.D. Moore. 1995. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol. Endocrinol. 9:243–254. [DOI] [PubMed] [Google Scholar]
- Levine, T.P., and S. Munro. 2002. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12:695–704. [DOI] [PubMed] [Google Scholar]
- Lu, L., and W. Hong. 2003. Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol. Biol. Cell. 14:3767–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266:513–525. [DOI] [PubMed] [Google Scholar]
- McConville, M.J., S.C. Ilgoutz, R.D. Teasdale, B.J. Foth, A. Matthews, K.A. Mullin, and P.A. Gleeson. 2002. Targeting of the GRIP domain to the trans-Golgi network is conserved from protists to animals. Eur. J. Cell Biol. 81:485–495. [DOI] [PubMed] [Google Scholar]
- Munro, S. 2004. Organelle identity and the organization of membrane traffic. Nat. Cell Biol. 6:469–472. [DOI] [PubMed] [Google Scholar]
- Munro, S., and B.J. Nichols. 1999. The GRIP domain—a novel Golgi-targeting domain found in several coiled-coil proteins. Curr. Biol. 9:377–380. [DOI] [PubMed] [Google Scholar]
- Nie, Z., D.S. Hirsch, and P.A. Randazzo. 2003. Arf and its many interactors. Curr. Opin. Cell Biol. 15:396–404. [DOI] [PubMed] [Google Scholar]
- Panic, B., O. Perisic, D.B. Veprintsev, R.L. Williams, and S. Munro. 2003. a. Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol. Cell. 12:863–874. [DOI] [PubMed] [Google Scholar]
- Panic, B., J.R. Whyte, and S. Munro. 2003. b. The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr. Biol. 13:405–410. [DOI] [PubMed] [Google Scholar]
- Pelham, H.R. 2004. Membrane traffic: GGAs sort ubiquitin. Curr. Biol. 14:R357–R359. [DOI] [PubMed] [Google Scholar]
- Pernet-Gallay, K., C. Antony, L. Johannes, M. Bornens, B. Goud, and R.M. Rios. 2002. The overexpression of GMAP-210 blocks anterograde and retrograde transport between the ER and the Golgi apparatus. Traffic. 3:822–832. [DOI] [PubMed] [Google Scholar]
- Piel, M., P. Meyer, A. Khodjakov, C.L. Rieder, and M. Bornens. 2000. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, J., and C. Barlowe. 1998. Transport of Axl2p depends on Erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J. Cell Biol. 142:1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, J., and C. Barlowe. 2002. Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol. Biol. Cell. 13:880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger, C., B. Short, V. De Corte, E. Bruyneel, A. Haas, R. Kopajtich, J. Gettemans, and F.A. Barr. 2004. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3ζ. J. Cell Biol. 164:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, R.M., A.M. Tassin, C. Celati, C. Antony, M.C. Boissier, J.C. Homberg, and M. Bornens. 1994. A peripheral protein associated with the cis-Golgi network redistributes in the intermediate compartment upon brefeldin A treatment. J. Cell Biol. 125:997–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, R.M., A. Sanchis, A.M. Tassin, C. Fedriani, and M. Bornens. 2004. GMAP-210 recruits γ-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell. 118:323–335. [DOI] [PubMed] [Google Scholar]
- Robinson, J.S., D.J. Klionsky, L.M. Banta, and S.D. Emr. 1988. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8:4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, S., F.S. Neuman-Silberberg, G. Barcelo, and T. Schupbach. 1995. Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 81:967–978. [DOI] [PubMed] [Google Scholar]
- Serafini, T., L. Orci, M. Amherdt, M. Brunner, R.A. Kahn, and J.E. Rothman. 1991. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 67:239–253. [DOI] [PubMed] [Google Scholar]
- Setty, S.R., T.I. Strochlic, A.H. Tong, C. Boone, and C.G. Burd. 2004. Golgi targeting of ARF-like GTPase Arl3p requires its Nα-acetylation and the integral membrane protein Sys1p. Nat. Cell Biol. 6:414–419. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., O.N. Jensen, A.V. Podtelejnikov, F. Sagliocco, M. Wilm, O. Vorm, P. Mortensen, A. Shevchenko, H. Boucherie, and M. Mann. 1996. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two-dimensional gels. Proc. Natl. Acad. Sci. USA. 93:14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, O.H., A.H. Ross, I. Mihai, and J.H. Exton. 1999. Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors. J. Biol. Chem. 274:36609–36615. [DOI] [PubMed] [Google Scholar]
- Siniossoglou, S., S.Y. Peak-Chew, and H.R. Pelham. 2000. Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J. 19:4885–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns, T., R.A. Kahn, D. Botstein, and M.A. Hoyt. 1990. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol. Cell. Biol. 10:6690–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, A.H., G. Lesage, G.D. Bader, H. Ding, H. Xu, X. Xin, J. Young, G.F. Berriz, R.L. Brost, M. Chang, et al. 2004. Global mapping of the yeast genetic interaction network. Science. 303:808–813. [DOI] [PubMed] [Google Scholar]
- Tsukada, M., E. Will, and D. Gallwitz. 1999. Structural and functional analysis of a novel coiled-coil protein involved in Ypt6 GTPase-regulated protein transport in yeast. Mol. Biol. Cell. 10:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, S., J.R. Lee, and M. Freeman. 2002. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 21:4277–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen, S.M., X. Cao, S.K. Sapperstein, E.C. Chiang, V.V. Lupashin, C. Barlowe, and M.G. Waters. 1999. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J. Cell Biol. 147:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen, S.M., B.A. Reilly, S.J. Chamberlain, and M.G. Waters. 2001. Dsl1p, an essential protein required for membrane traffic at the endoplasmic reticulum/Golgi interface in yeast. Traffic. 2:212–231. [DOI] [PubMed] [Google Scholar]
- Wach, A., A. Brachat, C. Alberti-Segui, C. Rebischung, and P. Philippsen. 1997. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 13:1065–1075. [DOI] [PubMed] [Google Scholar]
- Wang, Y.J., J. Wang, H.Q. Sun, M. Martinez, Y.X. Sun, E. Macia, T. Kirchhausen, J.P. Albanesi, M.G. Roth, and H.L. Yin. 2003. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 114:299–310. [DOI] [PubMed] [Google Scholar]
- Weide, T., M. Bayer, M. Koster, J.P. Siebrasse, R. Peters, and A. Barnekow. 2001. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte, J.R., and S. Munro. 2001. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell. 1:527–537. [DOI] [PubMed] [Google Scholar]
- Wu, M., L. Lu, W. Hong, and H. Song. 2004. Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat. Struct. Mol. Biol. 11:86–94. [DOI] [PubMed] [Google Scholar]
- Yvon, A.M., J.W. Walker, B. Danowski, C. Fagerstrom, A. Khodjakov, and P. Wadsworth. 2002. Centrosome reorientation in wound-edge cells is cell type specific. Mol. Biol. Cell. 13:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., T.K. Lasell, and P. Melancon. 2002. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol. Biol. Cell. 13:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]