Abstract
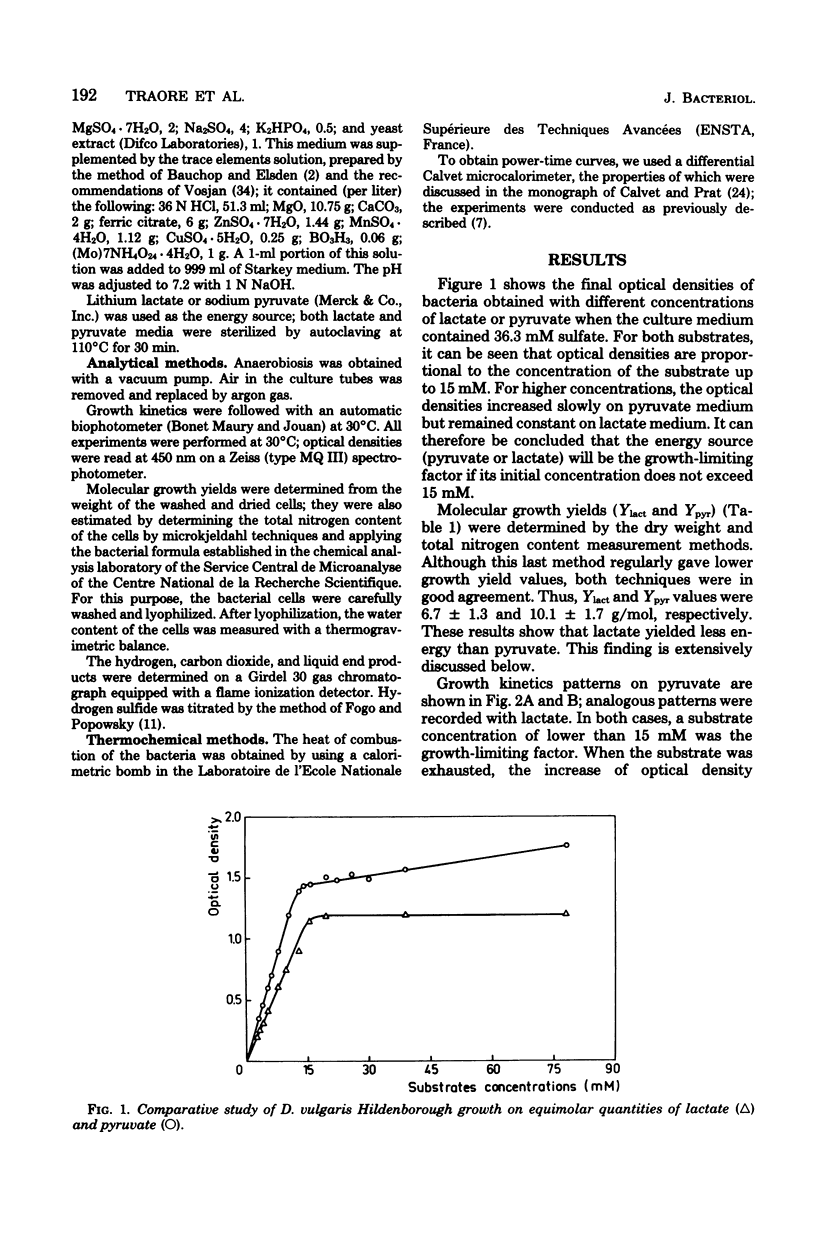
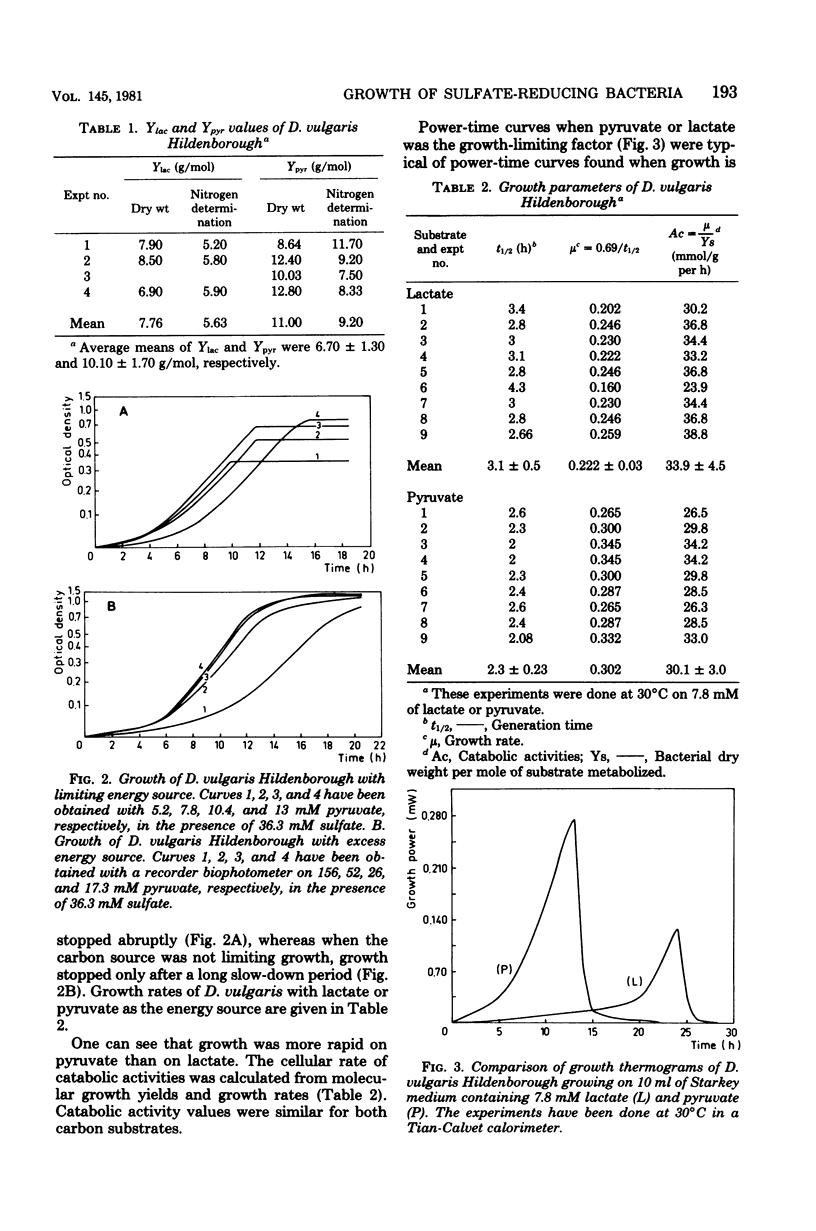
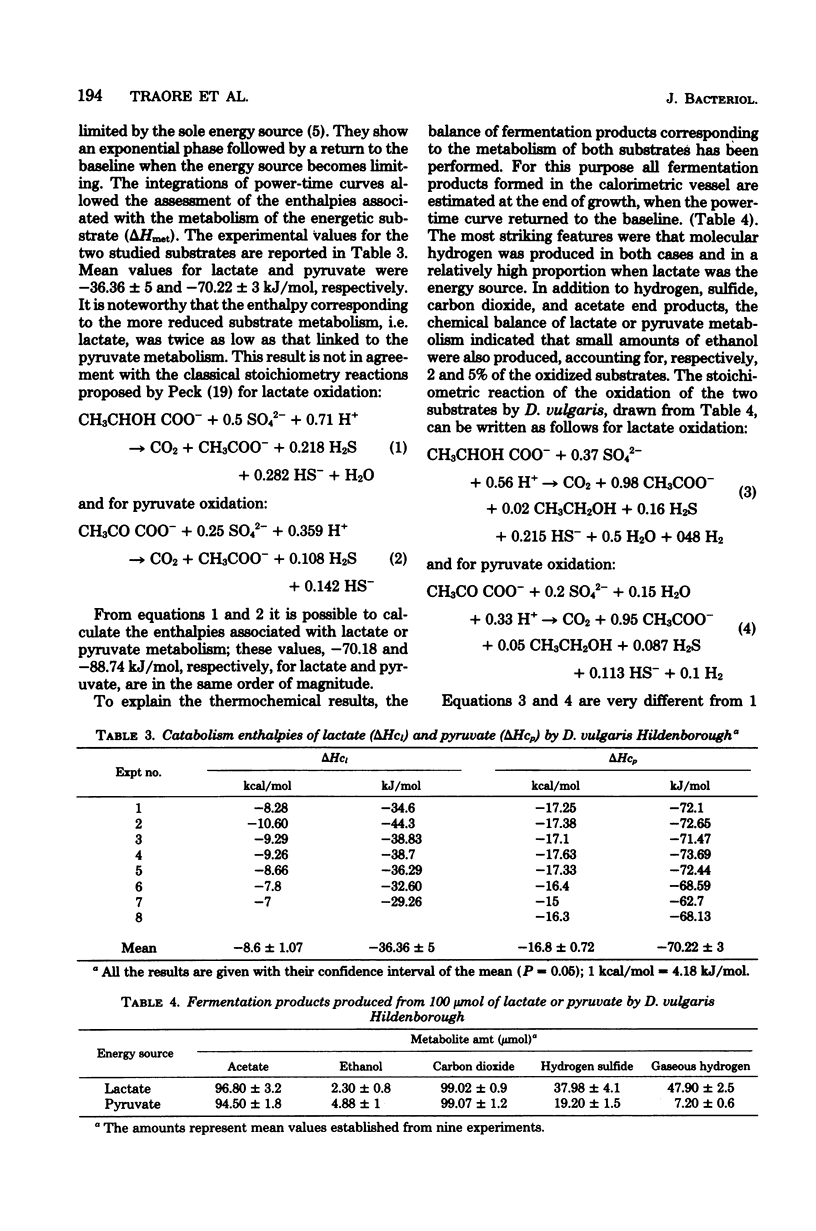
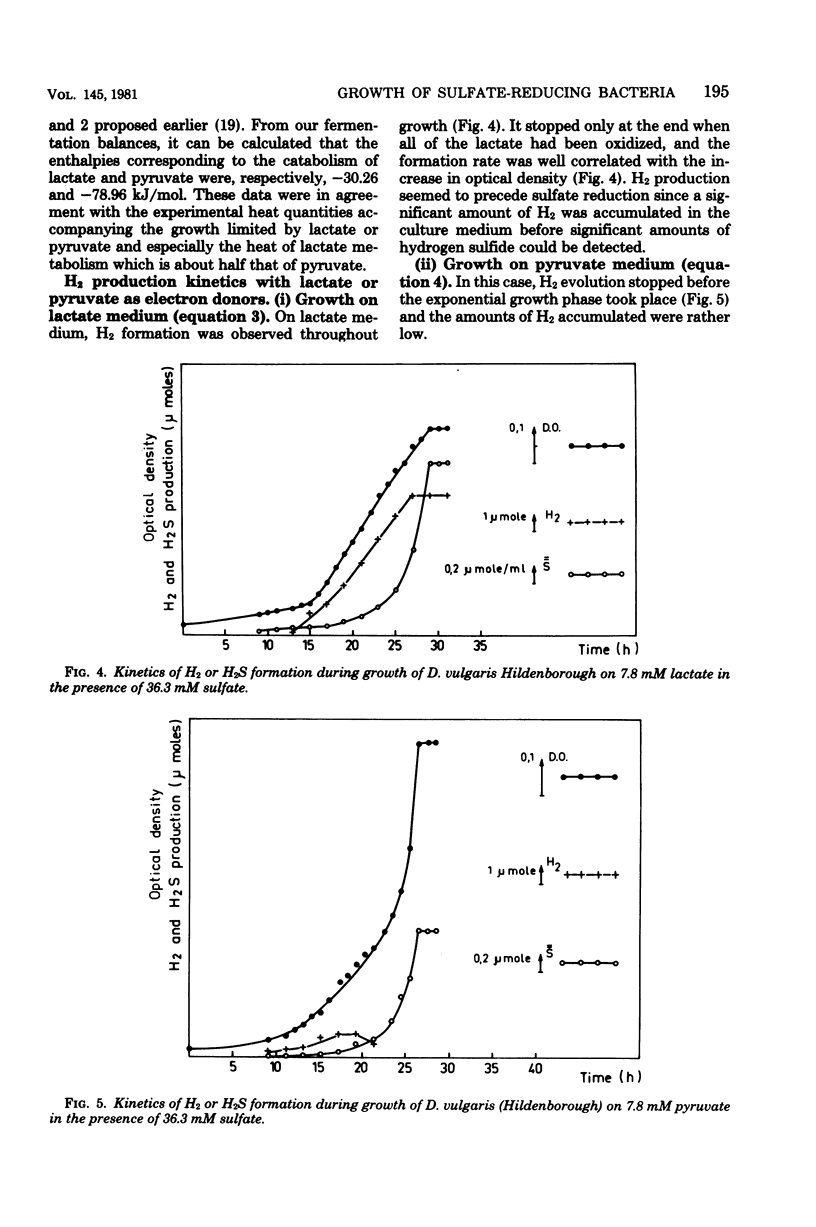
The metabolism of Desulfovibrio vulgaris Hildenborough grown on medium containing lactate or pyruvate plus a high concentration of sulfate (36 mM) was studied. Molecular growth yields were 6.7 +/- 1.3 and 10.1 +/- 1.7 g/mol for lactate and pyruvate, respectively. Under conditions in which the energy source was the sole growth-limiting factor, we observed the formation of 0.5 mol of hydrogen per mol of lactate and 0.1 mol of hydrogen per mol of pyruvate. The determination of metabolic end products revealed that D. vulgaris produced, in addition to normal end products (acetic acid, carbon dioxide, hydrogen sulfide) and molecular hydrogen, 2 and 5% of ethanol per mol of lactate and pyruvate, respectively. Power-time curves of growth of D. vulgaris on lactate and pyruvate were obtained, by the microcalorimetric Tian-Calvet apparatus. The enthalpies (delta Hmet) associated with the oxidation of these substrates and calculated from growth thermograms were -36.36 +/- 5 and -70.22 +/- 3 kJ/mol of lactate and pyruvate, respectively. These experimental values were in agreement with the homologous values assessed from the theoretical equations of D. vulgaris metabolism of both lactate and pyruvate. The hydrogen production by this sulfate reducer constitutes an efficient regulatory system of electrons, from energy source through the pathway of sulfate reduction. This hydrogen value may thus facilitate interactions between this strain and other environmental microflora, especially metagenic bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BELAICH J. P., PRAT H. [Glucose-limiting thermogenesis and growth of Pseudomonas lindneri]. C R Seances Soc Biol Fil. 1963 Jun 10;157:316–322. [PubMed] [Google Scholar]
- Badziong W., Thauer R. K. Growth yields and growth rates of Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate and hydrogen plus thiosulfate as the sole energy sources. Arch Microbiol. 1978 May 30;117(2):209–214. doi: 10.1007/BF00402310. [DOI] [PubMed] [Google Scholar]
- Belaich A., Belaich J. P. Microcalorimetric study of the anaerobic growth of Escherichia coli: growth thermograms in a synthetic medium. J Bacteriol. 1976 Jan;125(1):14–18. doi: 10.1128/jb.125.1.14-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich A., Belaich J. P. Microcalorimetric study of the anaerobic growth of Escherichia coli: measurements of the affinity of whole cells for various energy substrates. J Bacteriol. 1976 Jan;125(1):19–24. doi: 10.1128/jb.125.1.19-24.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich J. P., Senez J. C., Murgier M. Microcalorimetric study of glucose permeation in microbial cells. J Bacteriol. 1968 May;95(5):1750–1757. doi: 10.1128/jb.95.5.1750-1757.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. R., LeGall L., Peck H. D. Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J Bacteriol. 1974 Nov;120(2):994–997. doi: 10.1128/jb.120.2.994-997.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauque G., Herve D., Le Gall J. Structure-function relationship in hemoproteins: the role of cytochrome c3 in the reduction of colloidal sulfur by sulfate-reducing bacteria. Arch Microbiol. 1979 Jun;121(3):261–264. doi: 10.1007/BF00425065. [DOI] [PubMed] [Google Scholar]
- MECHALAS B. J., RITTENBERG S. C. Energy coupling in Desulfovibrio desulfuricans. J Bacteriol. 1960 Oct;80:501–507. doi: 10.1128/jb.80.4.501-507.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R. Iron and the synthesis of cytochrome c3. J Gen Microbiol. 1956 Aug;15(1):186–193. doi: 10.1099/00221287-15-1-186. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr Phosphorylation coupled with electron transfer in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Jan 4;22(1):112–118. doi: 10.1016/0006-291x(66)90611-5. [DOI] [PubMed] [Google Scholar]
- Postgate J. R., Campbell L. L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev. 1966 Dec;30(4):732–738. doi: 10.1128/br.30.4.732-738.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENEZ J. C. Some considerations on the energetics of bacterial growth. Bacteriol Rev. 1962 Jun;26:95–107. [PMC free article] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]


