Abstract
Rho1p, an essential Rho-type GTPase in Saccharomyces cerevisiae, activates its effectors in the GTP-bound form. Here, we show that Rho1p in secretory vesicles cannot activate 1,3-β-glucan synthase, a cell wall synthesizing enzyme, during vesicular transport to the plasma membrane. Analyses with an antibody preferentially reacting with the GTP-bound form of Rho1p revealed that Rho1p remains in the inactive form in secretory vesicles. Rom2p, the GDP/GTP exchange factor of Rho1p, is preferentially localized on the plasma membrane even when vesicular transport is blocked. Overexpression of Rom2p results in delocalization of Rom2p and accumulation of 1,3-β-glucan in secretory vesicles. Based on these results, we propose that Rho1p is kept inactive in intracellular secretory organelles, resulting in repression of the activity of the cell wall–synthesizing enzyme within cells.
Keywords: RHO1; ROM2; budding yeast; 1,3-β-glucan; antibody
Introduction
Eukaryotic cells consist of several organelles containing distinct sets of cellular materials, of which there are many. The compositions of macromolecules such as proteins, lipids, and carbohydrates are different in different organelles. Cellular materials in organelles are localized either by selective transport from other organelles or by specific synthesis after transport of requisite biosynthetic enzymes. This theory adequately explains the mechanisms for the incorporation of cell wall materials in the budding yeast Saccharomyces cerevisiae. During morphogenesis of growing yeast cells, cell wall materials are exclusively incorporated into the surface of buds (Pruyne and Bretscher, 2000). Among the structural components of the yeast cell wall (Cabib et al., 2001), the biosyntheses of mannoproteins and chitin have been well understood. Mannoproteins are synthesized intracellularly and transported to the periplasmic space, whereas chitin is synthesized by chitin synthetase, which is transported to the plasma membrane through chitosome. In contrast, the biosynthetic process of 1,3-β-glucan polymer and the mechanism of its incorporation into the cell wall remain largely unknown, although 1,3-β-glucan is the most abundant filamentous component of the yeast cell wall.
Many enzymes activated at specific organelles use characteristic mechanisms to repress the activity before transported to the proper destinations. After synthesis and transport to the specific organelles, such enzymes are activated by irreversible processes like posttranslational modifications and protein folding. Peptide cleavages or chemical modifications of residues are required during biosynthesis for activation of many proteases in the lysosome or the vacuole (Wolff et al., 1996; Turk et al., 2001). Cleavage of the signal peptides is required for the translocation and activation of mitochondrial proteins (Du et al., 2000). Like ATPases (Leng et al., 1999), folding of many enzymes is accomplished during transport with the assistance of assembly factors that are required to bring about their activity in the final destinations.
Small GTPases have been known to activate many cellular regulatory processes. Rho-type GTPases regulate their effectors involved in cell morphogenesis, membrane trafficking, cell contraction, cell adhesion, cell motility, gene expression, and many other cellular processes (Hall, 1998; Kaibuchi et al., 1999). The activity of Rho-type GTPase is generally regulated by switching between a GDP-bound inactive state and a GTP-bound active state with conformational changes (Wei et al., 1997; Ihara et al., 1998). The budding yeast contains the essential Rho-type GTPase, Rho1p (Madaule et al., 1987), like other eukaryotic cells, and is known to have five proteins as its effector molecules, involved in many cellular processes (Guo et al., 2001; Saka et al., 2001). Although Rho1p has been found colocalized with its effectors on the cell surface in many reported cases (Yamochi et al., 1994; Qadota et al., 1996; Ayscough et al., 1999), the mechanism for the activation of Rho1p during transport to the plasma membrane has remained unclear.
Among the effectors of Rho1p, Fks1p/2p, a putative catalytic subunit of 1,3-β-glucan synthase (GS),* is an essential target of upstream signals toward cell wall remodeling and cell morphogenesis. Rho1p regulates Fks1p/2p through direct interaction (Drgonová et al., 1996; Qadota et al., 1996). Evidence is accumulating that GS is precisely regulated in response to temporally preceding upstream signals (Pruyne and Bretscher, 2000); First, various factors are involved in the regulation of GS activity in yeast cells. Both Rom2p, the GDP/GTP exchange factor (GEF) of Rho1p (Ozaki et al., 1996), and Wsc family proteins, putative surface sensors on the cell wall (Gray et al., 1997; Verna et al., 1997; Philip and Levin 2001), play crucial roles in GS activation through Rho1p (Sekiya-Kawasaki et al., 2002). Lrg1p, the GTPase-activating protein of Rho1p, is involved in GS activity on the plasma membrane (Watanabe et al., 2001). Second, a posttranslational modification of Rho1p by the geranylgeranyl group is required for both binding with and activation of Fks1p (Inoue et al., 1999). Third, FKS2 expression is induced by signals of cell integrity impairment, calcineurin, and carbon source depletion (Zhao et al., 1998). Finally, movement of Fks1p on the plasma membrane is required for proper cell wall remodeling (Utsugi et al., 2002).
Here, we investigated the transport and activation mechanisms of nascent Rho1p in yeast cells. For this purpose, we analyzed the biosynthetic process of nascent GS exploiting the methods to assay GS activity both in vivo and in vitro and to monitor the localization of the product in yeast cells. Moreover, in order to determine the state of Rho1p directly, we have produced an antibody that preferentially reacts with activated Rho1p. Several lines of evidence indicated that Rho1p remains in the inactive form even in secretory vesicles in the final transport step to the plasma membrane. The repression of Rho1p activity in secretory vesicles was attributable to shortage of Rom2p in vesicles. Our results indicated that Rho1p is kept inactive in secretory organelles and is activated on its arrival at the plasma membrane, where Rom2p is localized.
Results
GS is transported to the plasma membrane through the secretory pathway
We analyzed the biosynthetic and transport processes of nascent GS after synthesis of the subunit proteins Rho1p and Fks1p/2p. To examine how Rho1p and Fks1p/2p are transported to the plasma membrane, we observed their localization when vesicular transport was blocked by sec mutations (Kaiser et al., 1997). Consistent with previous reports (Yamochi et al., 1994; Qadota et al., 1996; Ayscough et al., 1999), immunofluorescent microscopic observations revealed that Rho1p and Fks1p/2p were localized at the site of growth in wild-type cells incubated at 25°C or shifted to 37°C and incubated for 2 h (Fig. 1 and unpublished data). Rho1p and Fks1p/2p were also localized at the site of growth in sec mutant cells incubated at 25°C (unpublished data). The localization of Rho1p and Fks1p/2p in sec mutant cells did not alter by a shift to 37°C and a subsequent incubation for 10 min (unpublished data). However, after incubation of sec mutant cells at the restrictive temperature for 2 h, Rho1p and Fks1p/2p were detected not at the site of growth, but in intracellular organelles (Fig. 1 and unpublished data). In sec12 and sec16 cells, both of which are defective in transport from the ER to the Golgi, Rho1p and Fks1p/2p were mislocalized to the cytoplasm and had a punctate appearance. In sec1 and sec6 cells with defects in transport from secretory vesicles to the plasma membrane, Rho1p and Fks1p/2p were ubiquitously present. Introduction of the additional mutation of end4, which leads to defects in endocytosis (Raths et al., 1993), did not alter the Rho1p and Fks1p/2p localization in sec1 mutant cells (Fig. 1). These results implied that Rho1p and Fks1p/2p localized in sec mutant cells before the temperature shift were degraded, and that the intracellular proteins observed after the temperature shift were newly synthesized proteins in the exocytic pathway. On the basis of these results, Rho1p and Fks1p/2p may well be transported to the plasma membrane through the secretory pathway after their synthesis on the ER.
Figure 1.
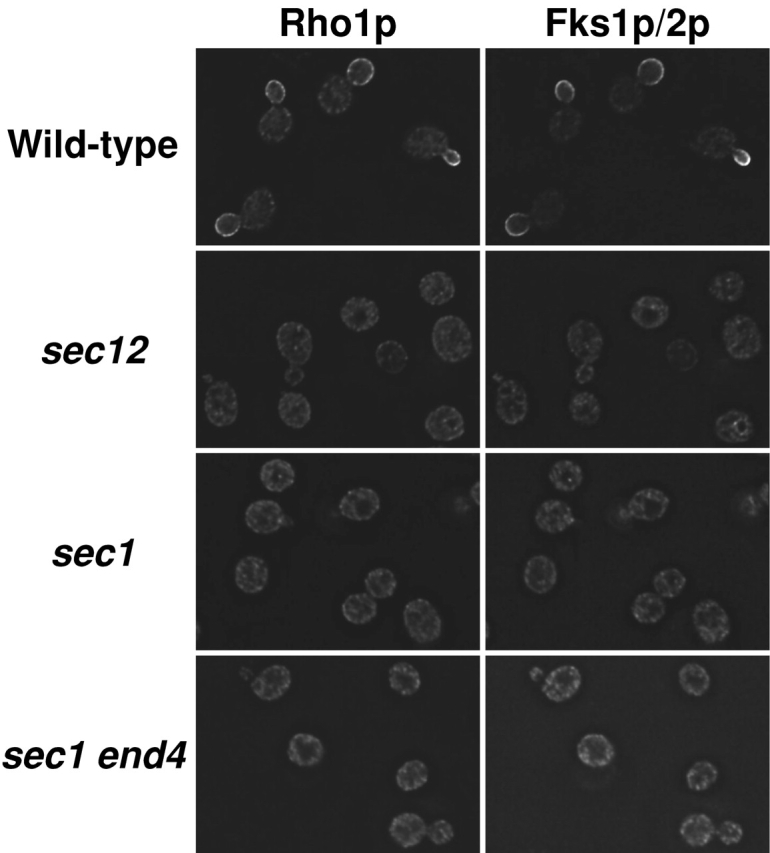
Localization of Rho1p and Fks1p/2p in cells shifted to 37°C. Cells were cultured in YPD at 25°C, shifted to 37°C and cultured for 2 h. Cultured cells were fixed with formaldehyde and then stained for immunofluorescence microscopy with the anti-Rho1p antibody (left) or the anti-Fks1p/2p antibody (right). Strains used were as follows: wild-type (YPH500), sec12, sec1, and sec1 end4.
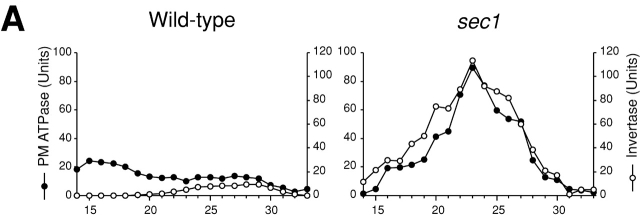
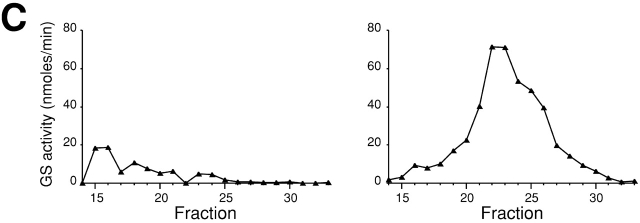
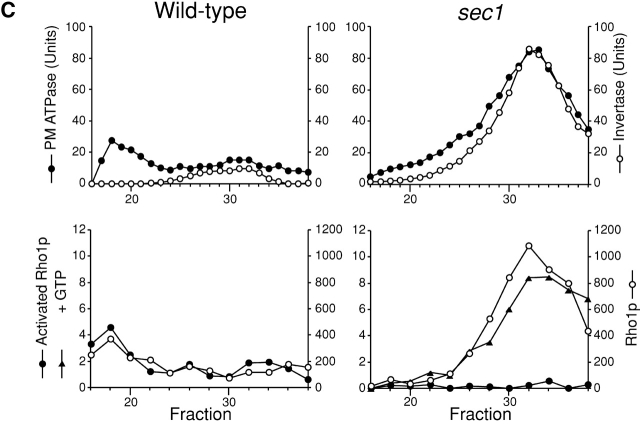
We confirmed by cell fractionations that movement of nascent GS is attributable to vesicular transport. Secretory vesicles were isolated from sec1 cells cultured at the restrictive temperature for 2 h after growth at the permissive temperature and were used to examine whether Rho1p and Fks1p/2p are detected in secretory vesicle fractions. As described previously (Walworth and Novick, 1987; McCaffrey et al., 1991), sec1 cell lysate was subjected to differential centrifugations, and the high-speed pellet obtained was fractionated further on the basis of vesicular size by gel exclusion chromatography. First, we examined the distribution of marker enzymes in the final fractions. Invertase, a marker enzyme of secretory vesicles, was eluted from the column as a single peak with its maximum at fraction 23 (Fig. 2 A, right). Plasma membrane ATPase accumulated in secretory vesicles by sec1 mutation was co-eluted with invertase. Next, we examined the distribution of Rho1p and Fks1p/2p by immunoblotting analysis and found that the distribution of Fks1p/2p was indistinguishable from in the elution profile of invertase (Fig. 2 B, right). In sec1 cells, Rho1p was also found in the secretory vesicle fractions (Fig. 2 B, right), consistent with a preceding report (McCaffrey et al., 1991). By contrast, Rho1p and Fks1p/2p were not distributed to the secretory vesicle fractions in wild-type cells, but were detected in fractions centering at 15 (Fig. 2 B, left), which coincided with the those of plasma membrane based on plasma membrane ATPase activity measurements (Fig. 2 A, left). Thus, Rho1p and Fks1p/2p are indeed localized in secretory vesicles when vesicular transport is blocked by the sec1 mutation.
Figure 2.
Secretory vesicle fractions of sec1 mutant and wild-type cells. Wild-type (left) or sec1 (right) cells were incubated at 37°C for 2 h, lysed, and subjected to differential centrifugations. The high-spin pellet was applied to a Sephacryl™ S-1000 column, and 4-ml fractions were collected. (A) Distributions of plasma membrane ATPase (closed circles) and invertase activity (open circles). (B) Immunoblotting analysis of GS-containing fractions. The amounts of Rho1p and Fks1p/2p were estimated with the guinea pig antiserum against Rho1p and the mouse mAb against Fks1p/2p, respectively. (C) Distributions of GS activity in the presence of GTP-γS.
Nascent GS is kept inactive in secretory vesicles
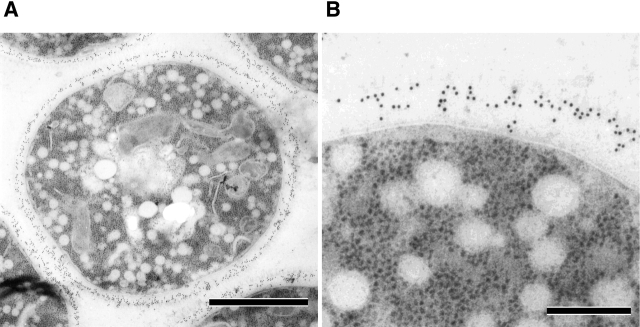
To identify where in vesicular transport steps nascent GS becomes activated, we examined whether 1,3-β-glucan is detected in intracellular organelles. An immunoelectron microscopic observation with an anti-1,3-β-glucan antibody revealed that 1,3-β-glucan is not detected in any organelles other than cell wall layers in wild-type cells (unpublished data). In addition, after shift to the restrictive temperature, 1,3-β-glucan was not detected in secretory vesicles accumulated in sec1 cells, but it was observed in cell wall layers (Fig. 3, A and B ; Table I). These results suggested that 1,3-β-glucan is not synthesized even in secretory vesicles, the final step of vesicular transport.
Figure 3.
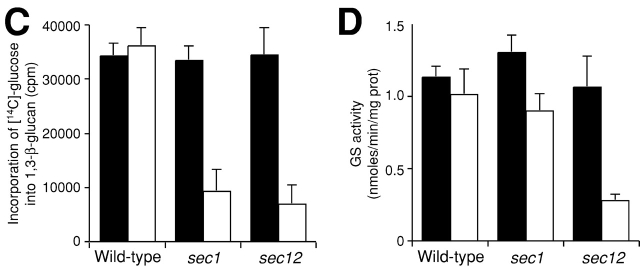
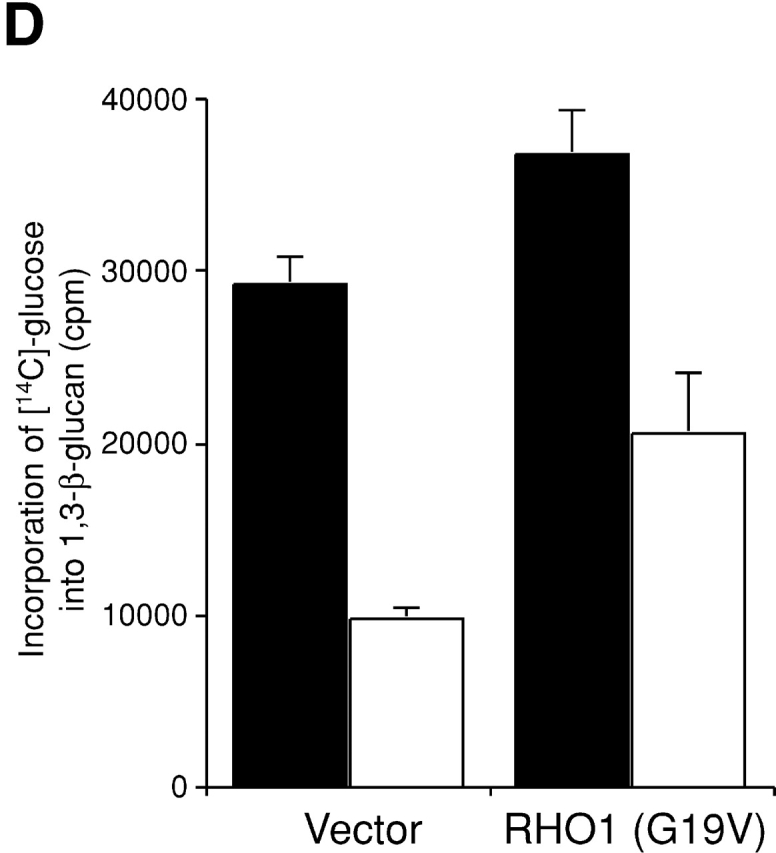
1,3-β-Glucan is not synthesized in intracellular organelles. (A) Immunogold labeling with the anti-1,3-β-glucan antibody in sec1 cells shifted to 37°C and cultured for 2 h. Bar, 1 μm. (B) The magnification image. Bar, 200 nm. (C) Reduced incorporation of glucose into 1,3-β-glucan in sec cells. Cells were cultured in YPD in the presence of [14C]glucose either at 25°C (black bars) or at 34°C for 2 h (white bars). The data represent the means and SDs of at least three experiments. (D) GS activity of the membrane fractions isolated from sec mutant cells. Cells were cultured either at 25°C (black bars) or at 37°C for 2 h (white bars), from which membrane fractions were isolated and assayed for GS activity in the presence of UDP-[14C]glucose and GTP-γS. The data represent the means and SDs of at least four experiments.
Table I.
Localization of immunogold labeling with the anti-1,3-β-glucan antibody
| Strain [plasmid] | Number of gold particles in intracellular structure per cell | Number of gold particles in cell wall per cell |
|---|---|---|
| sec1 | 20 ± 20 | 688 ± 404 |
| sec1 [RHO1 (G19V)] | 233 ± 75 | 687 ± 321 |
| sec1 [ROM2] | 221 ± 77 | 542 ± 249 |
| Wild-type [RHO1 (G19V)] |
52 ± 27 | 768 ± 183 |
| Wild-type [ROM2] | 45 ± 16 | 792 ± 250 |
Data represent the means and SDs of at least 20 cells.
To confirm this, we next measured in vivo 1,3-β-glucan synthesis when vesicular transport was blocked. We labeled cells with [14C]glucose for 2 h at 25 or 34°C and isolated fractions containing 1,3-β-glucan from them to estimate the rate of incorporation into 1,3-β-glucan. We found that incorporation is reduced in sec1 and sec12 mutant cells after shift to 34°C (Fig. 3 C). In wild-type cells, significant levels of incorporation were detected both at 25 and 34°C and comparable incorporation rates were observed in the sec mutants at the permissive temperature. These results further support the idea that GS remains inactive in intracellular organelles until it is transported to the plasma membrane.
Rho1p is not activated in secretory vesicles
When we measure in vitro GS activity of the membrane fraction, we assay in the presence of an excess amount of guanosine 5′-[γ-thio]triphosphate (GTP-γS), a nonhydrolyzable analogue of GTP (Abe et al., 2001), and thus assume that virtually all of Rho1p is in its active form. The membrane fraction isolated from rom2Δ cells exhibited normal in vitro GS activity (unpublished data), suggesting that Rho1p binds GTP-γS without GEF under this condition. Therefore, if the absence of 1,3-β-glucan synthesis activity in intracellular organelles is caused by a lack of the GTP-bound form of Rho1p, GS activity of the membrane fraction must be detectable in the presence of GTP-γS. In contrast, if the deficiency in enzymatic activity to produce 1,3-β-glucan results from some other mechanism(s), such as the absence of an essential GS modification and scarcity of other activators, GS activity will remain undetectable in the membrane fraction even in the presence of GTP-γS. To distinguish these two possibilities, we measured the GS activity in the membrane fraction isolated from sec mutant cells that were incubated at 25°C or shifted to 37°C and kept at this temperature for 2 h. The in vitro GS activity in the membrane fraction of wild-type cells stayed essentially the same after the temperature shift-up (Fig. 3 D). sec mutants cells incubated at the permissive temperature also exhibited significant GS activity. GS activity remained significant in the membrane fraction isolated from sec1 cells (Fig. 3 D) and sec6 cells (unpublished data) even after the 2-h incubation at 37°C. Fractionation analysis revealed that GS activity was largely localized to accumulated secretory vesicles in the sec1 mutant (Fig. 2 C and unpublished data). These results showed that the inability to synthesize 1,3-β-glucan in secretory vesicles is due to the scarcity of the GTP-bound form of Rho1p. In contrast, the membrane fraction of sec12 cells shifted to 37°C and cultured for 2 h exhibited reduced GS activity. As transport from the ER to the Golgi is defective in sec12, these results suggest that GS in the ER is kept inactive by mechanism(s) other than deficiency in the GTP-bound form of Rho1p.
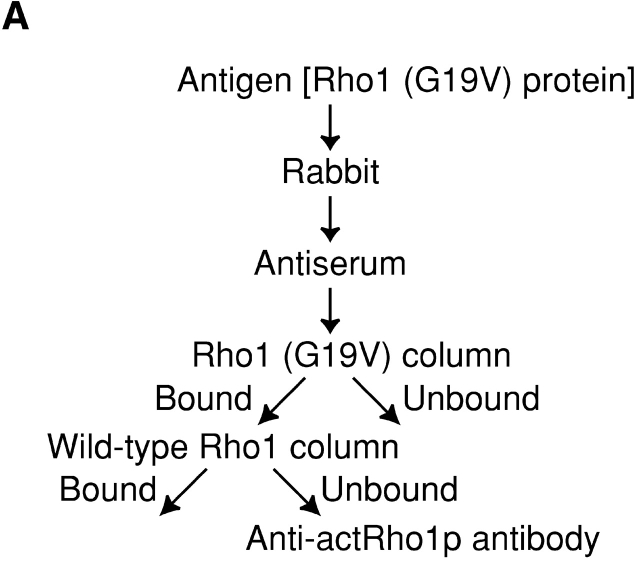
To directly examine the dearth of the GTP-bound form of Rho1p in secretory vesicles, we produced an antibody that preferentially reacts with the GTP-bound form of Rho1p (Fig. 4 A). As an antigen, we used the recombinant Rho1 (G19V) protein (constitutively active mutant protein) purified from Trichoplusia ni insect cells (Inoue et al., 1999). With the successive use of two columns, we purified an antibody that reacts with activated Rho1p with no reactivity with wild-type Rho1p. To determine the specificity of the antibody, we immunoprecipitated Rho1p in each state with the purified antibody. The resultant antibody, anti-actRho1p, successfully immunoprecipitated Rho1p in the presence of GTP-γS, while failing to significantly immunoprecipitate Rho1p in the absence of GTP-γS, even when GDP is added (Fig. 4 B, right). A control antibody reacting with wild-type Rho1p immunoprecipitated Rho1p irrespective of the presence of the nucleotides (Fig. 4 B, left). These results demonstrated that the anti-actRho1p antibody reacts specifically with the activated form of Rho1p.
Figure 4.
Localization of activated Rho1p detected with the purified anti-actRho1p antibody. (A) Affinity purification of the anti-actRho1p antibody. Antibody was raised against purified recombinant Rho1 (G19V) protein. Antiserum was loaded on an affinity column to which Rho1 (G19V) protein had been bound. The bound antibody was eluted, applied to another column charged with wild-type Rho1 protein, and the flow-through fractions were collected. (B) Immunoprecipitation of Rho1p with the anti-Rho1p (left), or the anti-actRho1p (right) antibody in the presence (+) or absence (−) of GDP and GTP-γS. Rho1p was detected by immunoblotting analysis with the guinea pig antiserum against Rho1p. (C) Immunoprecipitation of activated Rho1p. Wild-type (left) or sec1 mutant (right) cells were incubated at 37°C for 2 h. The cells were lysed in the presence or absence of 4 μM GTP, and fractionated by differential centrifugations. The high-spin pellet was applied to a Sephacryl™ S-1000 column without GTP, and 3-ml fractions were collected. Top panels, distribution of plasma membrane ATPase (closed circles) and invertase (open circles). Bottom panels, the distributions of activated Rho1p (closed circles and triangles) and total Rho1p (open circles). Aliquots of each fraction were assayed by immunoprecipitation with the anti-actRho1p, and detected by immunoblotting analysis with the guinea pig antiserum against Rho1p. The relative amount was quantified with a cooled CCD camera (LAS-1000plus; Fuji Photo Film).
Equipped with the anti-actRho1p, we next examined the localization of activated Rho1p in wild-type and sec1 cells. We incubated wild-type and sec1 cells at 37°C for 2 h, performed fractionation, and immunoprecipitated activated Rho1p in resultant fractions with the anti-actRho1p antibody. Plasma membrane ATPase activity was distributed in the vicinity of fraction 18 obtained from wild-type cells (Fig. 4 C, top). In contrast, invertase activity was distributed at around fraction 32 in the case of the sec1 cells shifted to 37°C. Activated Rho1p was immunoprecipitated in fractions containing the plasma membrane separated from wild-type cells (Fig. 4 C, bottom). On the other hand, activated Rho1p was not detected in secretory vesicle fractions isolated from the sec1 cells. In the fractionation experiments, the secretory vesicle fractions contained a larger amount of Rho1p than the plasma membrane fraction. When we lysed sec1 cells in the presence of an excess amount of GTP and fractionated by gel chromatography in the absence of GTP, activated Rho1p was detected in the secretory vesicle fractions, suggesting the stability of Rho1p throughout the experiments. These analyses with the antibody indicated that Rho1p is in the inactive form in secretory vesicles.
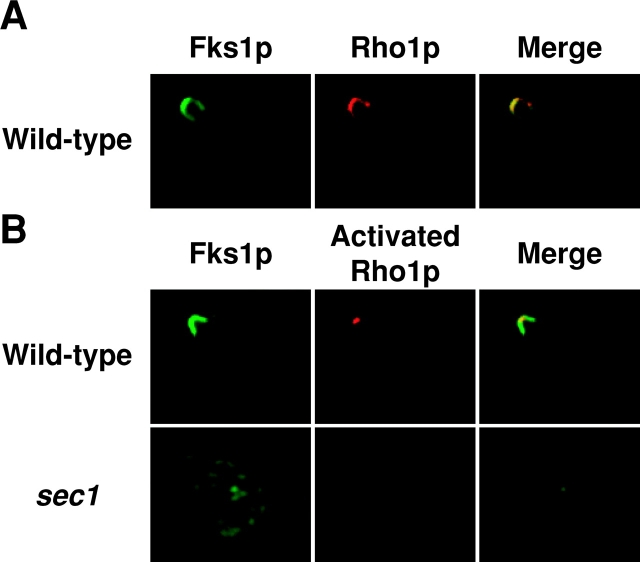
To verify this, we examined the localization of activated Rho1p with the antibody by immunofluorescence microscopy. We incubated wild-type and sec1 cells for 2 h at 25 and 37°C, respectively, and subsequently observed the localization of activated Rho1p. In wild-type cells, Rho1p was detected either in a large region in a small or large bud or at the site of bud emergence in an unbudded cell, and in either case, mostly colocalized with Fks1p/2p (Fig. 5 A). In contrast, the anti-actRho1p antibody stained a restricted region in the small bud in wild-type cells (Fig. 5 B). These results indicated that Rho1p is activated exclusively at the tip of small buds, and that wild-type Rho1p in other regions is in the inactive form. In sec1 cells, activated Rho1p was apparently absent in the intracellular region and the plasma membrane (Fig. 5 B). Together, the results suggested that Rho1p remains inactive in secretory vesicles and is activated in a restricted region of the plasma membrane.
Figure 5.
Distribution of wild-type Rho1p and activated Rho1p. (A) Colocalization of wild-type Rho1p and Fks1p/2p. Wild-type cells were cultured at 25°C, fixed, and stained for immunofluorescence microscopy with the anti-Fks1p/2p antibody (green) or the anti-Rho1p antibody (red). (B) Localization of activated Rho1p to a restricted region on the plasma membrane in wild-type cells. Wild-type cells were cultured at 25°C (top panels), whereas sec1 mutant cells were incubated at 37°C for 2 h (bottom panels), and stained for immunofluorescence microscopy with the anti-Fks1p/2p antibody (green) or the anti-actRho1p antibody (red).
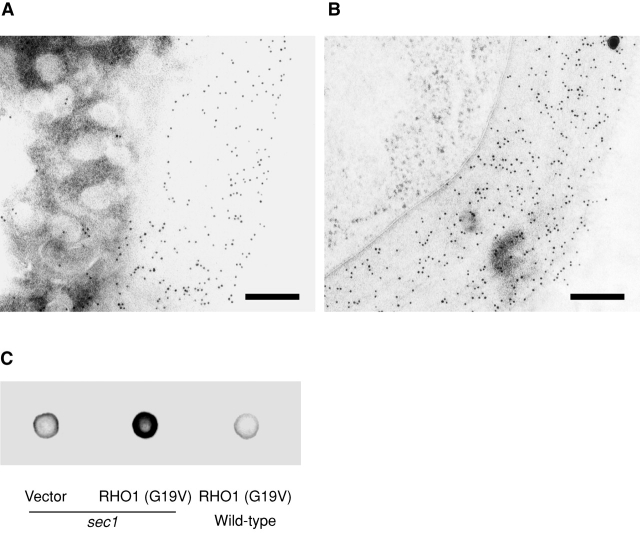
If accumulation of the inactive form of Rho1p results in impairment of 1,3-β-glucan synthesis in secretory vesicles, expression of active RHO1 must lead to synthesis of 1,3-β-glucan in secretory vesicles. Immunoelectron microscopic observations with the anti-1,3-β-glucan antibody revealed that 1,3-β-glucan accumulated in intracellular compartments after expression of the activated form of Rho1p (Fig. 6 A and Table I). In wild-type cells, expression of the activated form of Rho1p did not result in accumulation of 1,3-β-glucan in intracellular compartments (Fig. 6 B and Table I). To confirm these, we detected 1,3-β-glucan by dot blot analysis. Expression of active RHO1 increased 1,3-β-glucan in the high-speed pellet of sec1 cells (Fig. 6 C). Further fractionation analysis with gel chromatography revealed that 1,3-β-glucan detected in sec1 cells expressing active RHO1 was largely localized in secretory vesicles (unpublished data). We also measured in vivo 1,3-β-glucan synthesis in sec1 cells expressing the activated form of Rho1p (Fig. 6 D). The incorporation of [14C]glucose was reduced in sec1 cells at the restrictive temperature. Expression of active RHO1 in sec1 cells partially restored the incorporation. These results demonstrated that expression of active RHO1 in sec1 mutant cells leads to 1,3-β-glucan synthesis in secretory vesicles.
Figure 6.
Artificial synthesis of 1,3-β-glucan by expression of activated RHO1 in secretory vesicles. (A) sec1 cells expressing the active form of RHO1. sec1 cells transformed with YCpU-RHO1 (G19V) were incubated at 37°C for 2 h, fixed by the freeze-substituted fixation method, and 1,3-β-glucan was detected by the mouse mAb to 1,3-β-glucan. Bar, 200 nm. (B) Wild-type cells expressing the active form of RHO1. Bar, 200 nm. (C) 1,3-β-Glucan in high-speed pellets. Cells were incubated at 37°C for 2 h. The high-spin pellet was resuspended and 10 μg protein was blotted. 1,3-β-Glucan was detected by the mouse mAb against 1,3-β-glucan. (D) Incorporation of [14C]glucose into 1,3-β-glucan in sec1 cells. Cells were cultured in the presence of [14C]glucose at 25°C (black bars) or at 34°C for 2 h (white bars).
Rom2p, GEF of Rho1p, is localized to the plasma membrane when vesicular transport is blocked
One possible mechanism for inactivation of Rho1p in secretory vesicles is through the unavailability of Rho1p upstream regulatory factors in secretory vesicles. To identify upstream factors of 1,3-β-glucan synthesis, we isolated multicopy suppressors of a temperature-sensitive GS mutation (Sekiya-Kawasaki et al., 2002). Among the multicopy suppressor genes obtained, WSC1 and ROM2 were found to be essential for activating GS upstream of Rho1p. Rom2p is the GEF of Rho1p (Ozaki et al., 1996), whereas Wsc1p was proposed to be a transmembrane protein localized on the plasma membrane and is an upstream regulator of Rom2p (Philip and Levin, 2001).
To test the possibility that Wsc1p or Rom2p is involved in the inactivation of Rho1p in secretory vesicles, we checked the localization of Wsc1p and Rom2p in sec1 cells at the restrictive temperature. We constructed Wsc1p and Rom2p with an HA or a GFP tag at the COOH terminus. All the fusion proteins were judged to be functional because the temperature-sensitive growth defect as well as the cell lysis phenotype in wsc1Δ and rom2Δ cells was suppressed by expression of Wsc1p and Rom2p fusion proteins, respectively (unpublished data). Next, we examined the subcellular localization of Wsc1-GFP and Rom2-GFP by direct microscopic observations. As described previously (Verna et al., 1997), Wsc1-GFP was localized at the site of growth in wild-type and sec1 cells at 25°C, whereas Wsc1-GFP was distributed all around cells of the sec1 mutant at the restrictive temperature, (unpublished data). These results suggested that Wsc1-GFP accumulates in secretory vesicles when vesicular transport is blocked. As formerly reported (Manning et al., 1997; Audhya and Emr, 2002), Rom2-GFP was localized at the site of growth in wild-type and sec1 cells at 25°C (unpublished data). Interestingly, culturing at 37°C for 2 h did not alter the localization of Rom2-GFP both in wild-type (Fig. 7 A) and sec1 cells (Fig. 7 B). In sec1 cells, even after a 4-h incubation at the restrictive temperature, Rom2-GFP remained localized at bud tips (unpublished data). To examine whether the Rom2-GFP localized to the site of growth at the restrictive temperature in sec1 cells is a product of synthesis that took place after blockage of vesicular transport, we observed the Rom2-GFP localization in sec1 cells in the presence of cycloheximide, a protein synthesis inhibitor. In these cells, Rom2-GFP was no longer localized at the site of growth (Fig. 7 C), suggesting that Rom2-GFP localized before vesicular transport is blocked got degraded. Therefore, we judge that Rom2-GFP shown in Fig. 7 B was synthesized after the block on vesicular transport.
Figure 7.
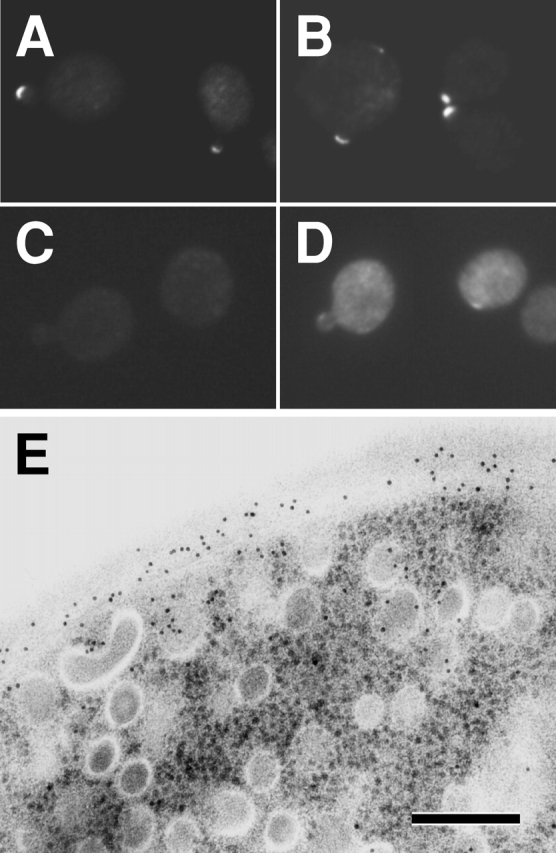
Rom2p is localized on the plasma membrane even when vesicular transport is blocked. (A–D) Localization of Rom2-GFP fusion protein. rom2Δ cells transformed with YCpU-ROM2-GFP were incubated at 37°C for 2 h (A). sec1 rom2Δ cells transformed with YCpU-ROM2-GFP were incubated at 37°C for 2 h in the absence (B) or presence (C) of cycloheximide. sec1 rom2Δ cells transformed with YEpU-ROM2-GFP were incubated at 37°C for 2 h (D). Cultured cells were all fixed in methanol, and were subjected to observation. (E) Immunogold labeling with the anti-1,3-β-glucan antibody in sec1 cells overexpressing ROM2. sec1 cells were transformed with YEpU-ROM2. Transformants were incubated at 37°C for 2 h, and 1,3-β-glucan was detected. Bar, 200 nm.
To examine whether Rom2p is localized on the plasma membrane or secretory vesicles close to the plasma membrane, we isolated the plasma membrane from wild-type cells expressing ROM2-HA, and analyzed the distribution of the fusion protein. Immunoblotting analysis revealed that Rom2-HA is enriched in plasma membrane fractions (Fig. 8 A). Next, we isolated secretory vesicles from sec1 cells expressing ROM2-HA, and examined whether Rom2-HA is enriched in secretory vesicle fractions. Immunoblotting analysis indicated that Rom2-HA is enriched in fractions before the peak of invertase activity (Fig. 8 B), which were found to be those of the plasma membrane (Fig. 2 and Fig. 4). Together with these facts, we conclude that Rom2p is absent in secretory vesicles because the localization of Rom2p is independent of vesicular transport.
Figure 8.
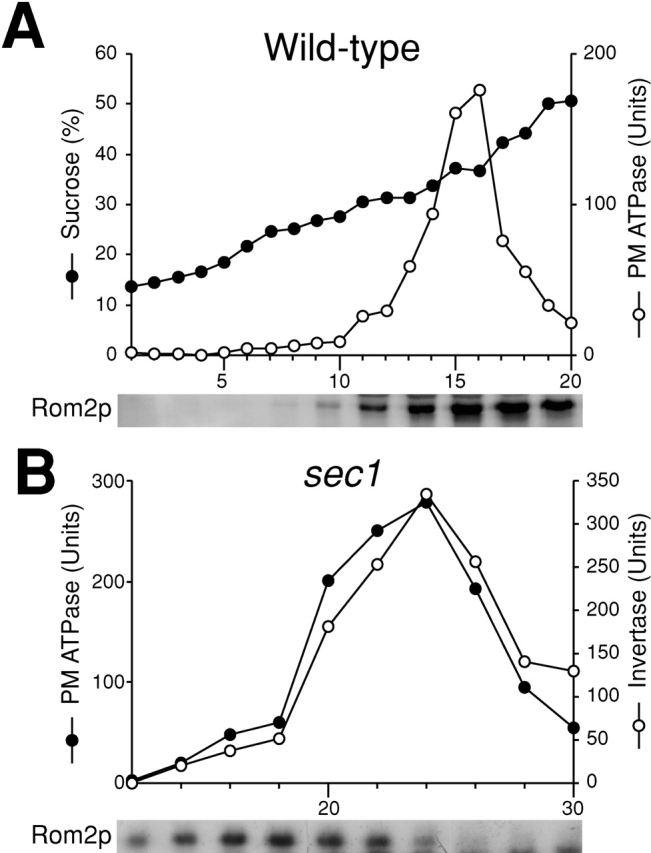
Subcellular fractionation analysis of Rom2p when vesicular transport is blocked. (A) Distribution of Rom2p on the plasma membrane in wild-type cells. Wild-type cells were incubated in YPD at 37°C for 2 h. Cell lysate was fractionated on a 20–60% sucrose density gradient. Top, distribution of plasma membrane ATPase (open circles) and sucrose density (closed circles). Bottom, immunoblotting analysis of Rom2-HA with an anti-HA antibody. (B) Distribution of Rom2p in Sephacryl™ S-1000 column fractions of sec1 cells. Top, distribution of plasma membrane ATPase (closed circles) and invertase (open circles). Bottom, immunoblotting analysis of Rom2-HA.
The results raised the possibility that the scarcity of Rom2p in secretory vesicles causes Rho1p to remain inactive in secretory vesicles. To confirm this, we examined whether 1,3-β-glucan is synthesized in secretory vesicles in sec1 cells when ROM2 is overexpressed. Overexpressed Rom2-GFP in sec1 cells was localized over entire cells (Fig. 7 D) and was also ubiquitously observed in wild-type cells (unpublished data), suggesting the cytoplasmic localization of overexpressed Rom2-GFP. In sec1 cells overexpressing ROM2, 1,3-β-glucan was detected in secretory vesicles by immunoelectron microscopy with the anti-1,3-β-glucan antibody (Fig. 7 E and Table I). In wild-type cells overexpressing ROM2, 1,3-β-glucan was not detected in intracellular organelles (Table I). These results further support the theory that Rho1p is held inactive in secretory vesicles due to Rom2p deficiency in the organelles.
Synthesis of 1,3-β-glucan causes toxic effects
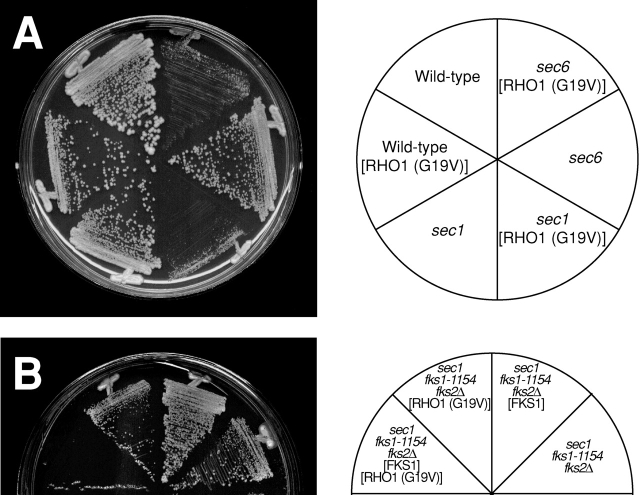
To test the possibility that the activation of Rho1p in secretory vesicles affects cell proliferation, we examined the growth phenotypes of sec1 and sec6 cells overexpressing the activated form of RHO1. Overexpression of the activated form of RHO1 led to growth inhibition of sec1 and sec6 cells at 31°C, a semipermissive temperature at which the temperature-sensitive sec mutants can grow (Fig. 9 A). In wild-type and sec12 cells, only a slight inhibitory effect of overexpressing the activated form of RHO1 on growth was observed (Fig. 9 A and unpublished data), suggesting that the effect is specific to late-acting sec mutants.
Figure 9.
Effect of activated RHO1 overexpression on the growth of wild-type and sec mutant cells. (A) Inhibition of cell proliferation in sec1 and sec6 mutant cells. Wild-type (YPH500), sec1, and sec6 cells were transformed with a control vector, or YEpU-RHO1 (G19V), and incubated for 3 d at 31°C. [RHO1 (G19V)] indicates the transformants with YEpU-RHO1 (G19V). (B) Suppression of inhibition by disturbing 1,3-β-glucan synthesis. sec1 fks1–1154 fks2Δ cells were transformed with a control vector, YEpT-RHO1 (G19V), or YCpU-FKS1, and incubated for 3 d at 31°C. [RHO1 (G19V)] and [FKS1] indicate the transformants with YEpU-RHO1 (G19V) and YCpU-FKS1, respectively.
The growth-inhibitory effect by overexpression of the activated form of RHO1 depends on the synthesis of 1,3-β-glucan. As GS-defective mutants, we used the temperature-sensitive fks1–1154 fks2Δ mutant with reduced catalytic activity of GS both at the permissive and restrictive temperatures (Sekiya-Kawasaki et al., 2002). In contrast to sec1 cells overexpressing activated RHO1, the growth inhibition of sec1 cells overexpressing activated RHO1 was suppressed by the introduction of the fks1–1154 and fks2Δ mutations at 31°C (Fig. 9 B). Further expression of FKS1 in this strain complemented the fks1–1154 fks2Δ defect and thereby suppressed growth inhibition (Fig. 9 B), leading to accumulation of 1,3-β-glucan in intracellular organelles (unpublished data). Taking these results together, we conclude that among the effectors of Rho1p, the activation of Fks1p/2p in secretory vesicles causes toxic effects on cells.
Discussion
To examine the state of Rho1p in yeast cells, we have produced an antibody preferentially reacting with activated Rho1p. This is the first antibody specifically reacting with the active state of any small GTPase. Rho1p is homologous to mammalian RhoA, which assumes distinct conformations in the GDP- and GTP-bound states (Wei et al., 1997; Ihara et al., 1998). Presumably, the antibody recognizes the conformation of GTP-bound Rho1p. Although it had been very difficult to monitor the states of small GTPase in cells, it recently became possible to observe the states of small GTPase in mammalian cells by fluorescent resonance energy transfer analysis (Mochizuki et al., 2001; Del Pozo et al., 2002), which directly monitors the activation and localization of small GTPase in living cells simultaneously. However, this analytical technique may produce artifactual results because cells need to be injected with photo-labeled proteins. In fact, the GFP-Rho1 fusion protein exhibits a different localization from authentic Rho1p (unpublished data). Other small GTPases in the budding yeast are supposed to have their own activation mechanisms at the organelles of their residence (Hoyt, 2000; Nern and Arkowitz, 2000; Schmidt and Hall, 2002). However, one crucial problem of these models is that the states of these small GTPases remain undetermined in yeast cells. This kind of antibody will be useful in definitively identifying the state of small GTPases in cells of yeast to mammalian cells because it produces no known artifacts.
We found that overexpression of the activated form of RHO1 in sec1 or sec6 cells inhibits cell proliferation, which is dependent on the activity of Fks1p. This result suggested that artificial synthesis of 1,3-β-glucan in secretory vesicles is unsuitable for cell proliferation. GS inactivation in intracellular organelles can be explained in several ways. One possibility is that the activation of GS on the plasma membrane is required for morphological changes, whereas 1,3-β-glucan synthesis in intracellular organelles is undesirable for cell morphology. Alternatively, 1,3-β-glucan synthesis in intracellular organelles may impair cell wall integrity or uniform cell wall structure.
At the restrictive temperature, sec mutations affecting late secretory steps cause accumulation of at least two types of vesicles, whose sizes are indistinguishable by gel chromatography (Harsay and Bretscher, 1995). Our data suggested that Fks1p and Rho1p exist in the same vesicles; GS activity measured in the presence of GTP-γS was detected in secretory vesicle fractions of sec1 mutant cells. Furthermore, by immunoelectron microscopy and dot blot analyses, 1,3-β-glucan was detected in vesicles that accumulated in sec1 mutant cells expressing activated Rho1p. These results suggest that both Fks1p and Rho1p are transported from the Golgi to the plasma membrane in particular secretory vesicles.
The membrane fraction of sec12 and sec16 mutant cells transferred to the restrictive temperature exhibited reduced GS activity even in the presence of GTP-γS. The reduced activity was not recovered by addition of the activated form of Rho1p (unpublished data). Only a minor effect of overexpressing activated RHO1 on growth of sec12 mutant cells was observed. These results suggested that GS is also kept inactive in the ER by mechanism(s) other than Rho1p inactivation. One conceivable mechanism for the inactivation of GS in this organelle is through its inhibition by phytosphingosine, an intermediate of sphingolipid, as phytosphingosine is a potent inhibitor of GS and is localized to the ER (Abe et al., 2001). Thus, there may be two mechanisms, inhibition by phytosphingosine and inactivation of Rho1p, to prevent GS from being activated in the ER. These mechanisms may doubly repress the activity of the cell wall–synthetic enzyme within the ER.
After synthesis in the ER, Rho1p is geranylgeranylated by geranylgeranyl transferase, a modification required for transport and attachment of Rho1p to the membrane (Ohya et al., 1996; Inoue et al., 1999). That Rho1p is transported to the plasma membrane through vesicular transport after geranylgeranylation is indicated by the following lines of evidence. First, like many membrane-bound proteins transported through the secretory pathway, Rho1p is not localized on the plasma membrane in sec mutants. Second, Rho1p accumulates in secretory vesicles in sec1 cells at the restrictive temperature. Third, Rho1p is largely found in the membrane fraction in sec12 cells at the restrictive temperature (unpublished data), whereas it is located in the soluble fraction in a mutant with a defect in geranylgeranyl transferase (Ohya et al., 1996). Together, Rho1p is probably modified with the geranylgeranyl group in the ER and transported to the plasma membrane through the secretory pathway. However, this mechanism may not apply to all prenylated proteins because transport of another prenylated protein, a-factor, was unaffected by blocks on the secretory pathway (Kuchler and Thorner, 1992).
In this investigation we showed the independence of Rom2p localization on normal vesicular transport. Like other GEFs, Rom2p contains a Dbl homologous domain and a pleckstrin homology domain (Ozaki et al., 1996; Lorberg et al., 2001). As it was shown that the pleckstrin homology domain of Rom2p interacts with PI4,5P2 and a defect in PI4,5P2 synthesis leads to delocalization of Rom2p (Audhya and Emr, 2002), the localization of Rom2p depends on that of PI4,5P2. One possible mechanism for the transport of Rom2p to the plasma membrane is Rom2p recruitment to the plasma membrane by PI4,5P2, which is transported to the plasma membrane through a pathway distinct from normal vesicular transport. Further study is needed to resolve this question.
Materials and methods
Strains and growth conditions
YPH499 and YPH500 were used as the wild-type strains. SEY5016 (sec1–1), ANS6–2D (sec6–4), ANY8–5A (sec12–1), and ANS16–1B (sec16–2) strains were provided by A. Nakano (RIKEN, Japan) and R. Schekman (University of California, Berkeley, Berkeley, CA). YOC2573 (sec1–1 wsc1Δ), YOC2576 (sec1–1 rom2Δ), and YOC3123 (sec1–1 end4Δ) were constructed by PCR-mediated gene disruptions described previously (Sekiya-Kawasaki et al., 2002). Strain YOC3018 (sec1 fks1–1154 fks2Δ) was produced by a successive cross between SEY5016 and YOC1087 (fks1–1154 fks2Δ). Yeast cells were grown in rich medium (YPD; 1% bacto yeast extract [Difco], 2% bacto peptone [Difco], and 2% glucose [Wako Chemicals]), or in appropriately supplemented synthetic growth medium (SD; 0.67% yeast nitrogen base [Difco] and 2% glucose). For -Ura and -Trp selections, 0.5% casamino acid (Difco) was added to SD.
Plasmids
Plasmids pYO821 (YEpU-RHO1 (G19V)), pYO964 (YCpU-RHO1 (G19V)), pYO965 (YCpU-RHO1 (Q68L)), pYO2326 (YCpL-FKS1), pYO2366 (YEpU-ROM2), and pYO821 (YEpU-RHO1 (G19V)) are as described previously (Sekiya-Kawasaki et al., 2002). pYO2515 (YCpU-WSC1-HA) and pYO2516 (YCpU-ROM2-HA) were produced by introducing a PCR-amplified fragment containing the corresponding ORF and the 0.5-kb upstream region into pTS903CU, a gift from Y. Kikuchi (University of Tokyo, Tokyo, Japan). Introduction of a PCR-amplified fragment containing the ORF of WSC1 and ROM2 and the 0.5-kb upstream region into pRS316-GFP, which was constructed for the expression of fusion genes with EGFP (CLONTECH Laboratories, Inc.), resulted in pYO2517 (YCpU-WSC1-GFP) and pYO2518 (YCpU-ROM2-GFP), respectively. pYO2519 (YEpU-ROM2-GFP) was constructed by introducing a fragment containing both the 0.5-kb upstream region and ROM2-GFP from pYO2518 into a 2-μm origin vector, pYO326 (Sekiya-Kawasaki et al., 2002).
Morphological observations
Procedures for immunofluorescence microscopy were as described previously (Pringle et al., 1989). Anti-Fks1p/2p (2D8, a gift from S.B. Inoue and T. Watanabe; Nippon Roche, Kamakura, Japan), and anti-Rho1p antibodies were used as primary antibodies, whereas Alexa Fluor® 488 goat anti–mouse and Alexa Fluor® 546 goat anti–rabbit antibodies (Molecular Probes, Inc.) were used as secondary antibodies. Cells were viewed under an imaging microscope (Axioplan 2; Carl Zeiss MicroImaging, Inc.). Deconvolution was performed using AutoDeblur software (AutoQuant Imaging, Inc). GFP fusion proteins were observed as formerly described (Guo et al., 2001). In brief, cells were collected, fixed in methanol at −20°C for 10 min, washed with acetone at −20°C, washed with PBS three times, resuspended in PBS, and subjected to observation. Cells were viewed under a microscope (Leitz DMR; Leica). Images were captured using a CCD camera (CoolSNAP HQ; Nippon Roper) and MetaMorph® imaging software (Universal Imaging Corp.). All the images presented were processed using Adobe Photoshop® software.
Incorporation of [14C]glucose into 1,3-β-glucan
Cells were grown to early log phase at 25°C and were then cultured at either the permissive or the restrictive temperature for 2 h. The cultured cells were diluted to OD600 of 0.5 with 1 ml 0.5% glucose medium containing 10 μCi [14C]glucose, and were labeled for 2 h. The labeled cells were harvested and extracted with 1 N NaOH. The insoluble pellets were resuspended in 10 mM Tris/HCl, pH 7.5, containing 5 mg/ml zymolyase 100T (Seikagaku) and incubated for 20 h at 37°C. After digestion, the zymolyase-resistant material was removed by centrifugation (15,000 g for 20 min) and the supernatant was filtered through a microcon-10 membrane (Amicon Bioseparations). The flow-through fraction was dried by a vacuum evaporator and applied to Unifilter-GF/C (Packard Instrument Co.). The differences in the incorporation rates in strains were normalized by ΔOD600, measured before and after the labeling period. Because a significant value of ΔOD600 was required for the normalization, we preferred mild condition (34°C) as the restrictive temperature. The small incorporation observed in sec mutant cells at the restrictive temperature may be due to this mild condition.
Preparation of the membrane fraction and measurement of in vitro GS activity
Cells were grown at 25°C in 1 l medium in a 2-l flask until the OD600 of the culture reached one. All the following procedures were performed at 4°C. The cells were harvested, washed with 1 mM EDTA, and disrupted by Multi-beads shocker (Yasui Kikai) with 10-ml glass beads in 20 ml of the breaking solution consisting of 0.5 M NaCl and 1 mM EDTA with 1 mM phenylmethylsulfonyl fluoride. The crude lysate was centrifuged at 1,500 g for 5 min so that cell debris and unbroken cells are sedimented. The supernatant was centrifuged at 100,000 g for 30 min in a rotor (model RP70T; Hitachi) with Himac CP 65 (Hitachi). The resultant pellet was suspended (the membrane fraction) with the membrane buffer (50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 1 mM β-mercaptoethanol, and 33% glycerol) and homogenized with a Dounce homogenizer. GS activity of the membrane fraction was measured after an excess amount of GTP-γS was added and incubated for at least 30 min on ice.
Purification of secretory vesicles
Secretory vesicles were purified as specified before (Walworth and Novick, 1987) with some modifications. Cells were grown at 25°C, harvested, resuspended in low glucose medium, and after incubation for 2 h at 37°C, were harvested and washed with ice-cold 10 mM NaN3. The washed cells were resuspended in spheroplasting buffer (1.4 M sorbitol, 50 mM KPi, pH 7.5, 10 mM, 40 mM β-mercaptoethanol, and zymolyase-100T), and incubated at 37°C for 45 min. The spheroplasts were resuspended in lysis buffer (0.8 M sorbitol, 10 mM triethanolamine, and 1 mM EDTA, adjusted to pH 7.2 with acetic acid and protease inhibitors), and lysed by a Dounce homogenizer. After cell debris was removed, the supernatant was centrifuged at 13,000 g for 20 min in a rotor (model RP70T; Hitachi). The supernatant was centrifuged at 100,000 g for 1 h in the same rotor. The resultant pellet was resuspended in the lysis buffer, and loaded on a 100 × 1.6 cm Sephacryl™ S-1000 column (Amersham Biosciences). The column was eluted, and 4-ml (Fig. 2 and Fig. 8 B) or 3-ml (Fig. 4 C) fractions were collected. For GS activity measurements (Fig. 2), 3 ml of each fraction was centrifuged at 100,000 g for 1 h at 4°C in a rotor (S100AT5; Hitachi), and the pellet was resuspended with 200 μl of the aforementioned membrane buffer. The plasma membrane ATPase activity (Serrano, 1988) and invertase activity (Harsay and Bretscher, 1995) in the fractions were assayed as described previously.
Fractionation on sucrose density gradients
Cell organelles were fractionated on equilibrium density gradients according to previously published procedures (Roberg et al., 1999) with several modifications. Cultures were grown in 1 l medium until the OD600 of the cultures reached one. Cells were resuspended in 5 ml STE 10 (10% sucrose in the breaking solution with protease inhibitors), and lysed with glass beads by Multi-beads shocker. The lysate was cleared of debris by centrifugation at 300 g for 3 min, and the supernatant was layered on top of 20-ml 20–60% linear sucrose gradient in the breaking solution. Samples were centrifuged at 100,000 g for 36 h at 4°C in a rotor (P28S; Hitachi), and fractions of 1 ml were collected from the top of the gradient.
Antibody production and affinity purification
Anti-Rho1p and anti-actRho1p antibodies were raised against recombinant Rho1 and Rho1 (G19V) proteins, respectively. Approximately 0.1 mg of either protein (a gift from S.B. Inoue and T. Watanabe) was injected into rabbits, and antiserum was obtained after the ninth injection of the antigen. The anti-Rho1p antibody raised was affinity-purified by a HiTrap NHS-activated column (Amersham Biosciences) with 1 mg of recombinant GST-Rho1 protein bound. The column was eluted with 3 ml of 100 mM glycine at pH 2.5, and the eluted fraction was concentrated with Centriprep (Amicon Bioseparations) after addition of 0.3 ml 1 M Tris/HCl, pH 8.0. On the other hand, the anti-actRho1p antibody collected was affinity purified by a HiTrap NHS-activated column with 1 mg recombinant Rho1 (G19V) protein bound. The column was eluted with 10 ml of 100 mM glycine, pH 2.5. The eluted fraction was mixed with 1 ml of 1 M Tris/HCl, pH 8.0, and was loaded on an NHS-activated column with 1 mg recombinant GST-Rho1 protein bound. The flow-through fraction was concentrated with Centriprep.
Immunoprecipitation of Rho1p
Recombinant wild-type Rho1 protein was suspended in IP buffer (20 mM Tris/HCl, pH 8.0, 5 mM MgCl2, 1 mM EDTA, 0.6% CHAPS, and 2 mM phenylmethylsulfonyl fluoride), and was incubated for 1 h at 4°C without nucleotides or with either 10 μM GTP-γS or GDP. Either the anti-Rho1p or the anti-actRho1p antibody was used at 1:500 dilution. After a 2-h incubation at 4°C, protein A Sepharose beads (Amersham Biosciences) were added and the samples were further incubated for 2 h at 4°C. The beads were then washed with IP buffer, from which proteins were eluted by boiling in SDS sample buffer for 10 min. Protein samples were loaded on SDS-polyacrylamide gels, transferred to PVDF membranes (Amersham Biosciences), and blotted with a guinea pig antiserum against Rho1p (Ohya et al., 1996). After reaction with ECL-Plus (Amersham Biosciences), Rho1p was detected and quantified with a cooled CCD camera (LAS-1000plus; Fuji Photo Film).
For immunoprecipitation of activated Rho1p from fractions from gel chromatography, 0.1 M NaCl and 0.6% CHAPS were added to 1 ml of the collected fractions. Subsequently, the suspension was left at 4°C for 2 h and the activated Rho1p was immunoprecipitated from the fractions and detected as described above.
Immunoelectron microscopic analysis
Thin sections of yeast cells were prepared by the freeze-substituted fixation method as described previously (Sun et al., 1992), except that HPM010 (BAL-TEC AG) or EMCPS (Leica) for cell freezing and EMAFS (Leica) for warming were used. For 1,3-β-glucan immunolabeling, a mouse mAb against 1,3-β-glucan (Biosupplies) and a secondary antibody conjugated with 10-nm gold particles were used. The labeled thin sections were viewed under an electron microscope (model H7600; Hitachi) at 100 kV.
Acknowledgments
We thank A. Nakano and R. Schekman for the yeast strains; Y. Kikuchi for the plasmid; S.B. Inoue, and T. Watanabe for the recombinant Rho1p and valuable discussions; and K. Homma for a critical reading of the manuscript. Thanks also go to the members of the Laboratory of Signal Transduction for helpful discussions.
This work was supported in part by a grant (#14380325) for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan to Y. Ohya. This research was also assisted by research fellowships of the Japan Society for the Promotion of Science for Young Scientists to M. Abe.
Footnotes
Abbreviations used in this paper: GEF, GDP/GTP exchange factor; GTP-γS, guanosine 5′-[γ-thio]triphosphate; GS, 1,3-β-glucan synthase.
References
- Abe, M., I. Nishida, M. Minemura, H. Qadota, Y. Seyama, T. Watanabe, and Y. Ohya. 2001. Yeast 1,3-beta-glucan synthase activity is inhibited by phytosphingosine localized to the endoplasmic reticulum. J. Biol. Chem. 276:26923–26930. [DOI] [PubMed] [Google Scholar]
- Audhya, A., and S.D. Emr. 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2:593–605. [DOI] [PubMed] [Google Scholar]
- Ayscough, K.R., J.J. Eby, T. Lila, H. Dewar, K.G. Kozminski, and D.G. Drubin. 1999. Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol. Biol. Cell. 10:1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib, E., D.H. Roh, M. Schmidt, L.B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679–19682. [DOI] [PubMed] [Google Scholar]
- Del Pozo, M.A., W.B. Kiosses, N.B. Alderson, N. Meller, K.M. Hahn, and M.A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232–239. [DOI] [PubMed] [Google Scholar]
- Drgonová, J., T. Drgon, K. Tanaka, R. Kollár, G.-C. Chen, R.A. Ford, C.S.M. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 272:277–279. [DOI] [PubMed] [Google Scholar]
- Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 102:33–42. [DOI] [PubMed] [Google Scholar]
- Gray, J.V., J.P. Ogas, Y. Kamada, M. Stone, D.E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., F. Tamanoi, and P. Novick. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353–360. [DOI] [PubMed] [Google Scholar]
- Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science. 279:509–514. [DOI] [PubMed] [Google Scholar]
- Harsay, E., and A. Bretscher. 1995. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, M.A. 2000. Exit from mitosis: spindle pole power. Cell. 102:267–270. [DOI] [PubMed] [Google Scholar]
- Ihara, K., S. Muraguchi, M. Kato, T. Shimizu, M. Shirakawa, S. Kuroda, K. Kaibuchi, and T. Hakoshima. 1998. Crystal structure of human RhoA in a dominantly active form complexed with a GTP analogue. J. Biol. Chem. 273:9656–9666. [DOI] [PubMed] [Google Scholar]
- Inoue, S.B., H. Qadota, M. Arisawa, T. Watanabe, and Y. Ohya. 1999. Prenylation of Rho1p is required for activation of yeast 1,3-β-glucan synthase. J. Biol. Chem. 274:38119–38124. [DOI] [PubMed] [Google Scholar]
- Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459–486. [DOI] [PubMed] [Google Scholar]
- Kaiser, C.A., R.E. Gimeno, and D.A. Shaywitz. 1997. Protein secretion, membrane biogenesis, and endocytosis. The Molecular and Cellular Biology of Yeast Saccharomyces cerevisiae. J.R. Pringle, J.R. Broach, and E.W. Jones, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 91–227.
- Kuchler, K., and J. Thorner. 1992. Secretion of peptides and proteins lacking hydrophobic signal sequences: the role of adenosine triphosphate-driven membrane translocators. Endocr. Rev. 13:499–514. [DOI] [PubMed] [Google Scholar]
- Leng, X.H., T. Nishi, and M. Forgac. 1999. Transmembrane topography of the 100-kDa a subunit (Vph1p) of the yeast vacuolar proton-translocating ATPase. J. Biol. Chem. 274:14655–14661. [DOI] [PubMed] [Google Scholar]
- Lorberg, A., J.J. Jacoby, H.P. Schmitz, and J.J. Heinisch. 2001. The PH domain of the yeast GEF Rom2p serves an essential function in vivo. Mol. Genet. Genomics. 266:505–513. [DOI] [PubMed] [Google Scholar]
- Madaule, P., R. Axel, and A.M. Myers. 1987. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 84:779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, B.D., R. Padmanabha, and M. Snyder. 1997. The Rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell. 8:1829–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey, M., J.S. Johnson, B. Goud, A.M. Myers, J. Rossier, M.R. Popoff, P. Madaule, and P. Boquet. 1991. The small GTP-binding protein Rho1p is localized on the Golgi apparatus and post-Golgi vesicles in Saccharomyces cerevisiae. J. Cell Biol. 115:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, N., S. Yamashita, K. Kurokawa, Y. Ohba, T. Nagai, A. Miyawaki, and M. Matsuda. 2001. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 411:1065–1068. [DOI] [PubMed] [Google Scholar]
- Nern, A., and R.A. Arkowitz. 2000. Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J. Cell Biol. 148:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya, Y., B.E. Caplin, H. Qadota, M.F. Tibbetts, Y. Anraku, J.R. Pringle, and M.S. Marshall. 1996. Mutational analysis of the beta-subunit of yeast geranylgeranyl transferase I. Mol. Gen. Genet. 252:1–10. [PubMed] [Google Scholar]
- Ozaki, K., K. Tanaka, H. Imamura, T. Hihara, T. Kameyama, H. Nonaka, H. Hirano, Y. Matsuura, and Y. Takai. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196–2207. [PMC free article] [PubMed] [Google Scholar]
- Philip, B., and D.E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, J.R., R.A. Peterson, A.E. Adams, T. Sterns, D.G. Drubin, B.K. Haarer, and E.W. Jones. 1989. Fluorescence microscopy methods in yeast. Methods Cell Biol. 31:357–436. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. II. The role of the cortical actin organization. J. Cell Sci. 113:571–585. [DOI] [PubMed] [Google Scholar]
- Qadota, H., C.P. Python, S.B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D.E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 272:279–281. [DOI] [PubMed] [Google Scholar]
- Raths, S., J. Rohrer, F. Crausaz, and H. Riezman. 1993. end3 and end4: Two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol. 120:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg, K.J., M. Crotwell, P. Espenshade, R. Gimeno, and C.A. Kaiser. 1999. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J. Cell Biol. 145:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka, A., M. Abe, H. Okano, M. Minemura, H. Qadota, T. Utsugi, A. Mino, K. Tanaka, Y. Takai, and Y. Ohya. 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276:46165–46171. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., and A. Hall. 2002. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16:1587–1609. [DOI] [PubMed] [Google Scholar]
- Sekiya-Kawasaki, M., M. Abe, A. Saka, D. Watanabe, K. Kono, M. Minemura-Asakawa, S. Ishihara, T. Watanabe, and Y. Ohya. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics. 162:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, R. 1988. H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 157:533–544. [DOI] [PubMed] [Google Scholar]
- Sun, G.H., A. Hirata, Y. Ohya, and Y. Anraku. 1992. Mutations in yeast calmodulin cause defects in spindle pole body functions and nuclear integrity. J. Cell Biol. 119:1625–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk, V., B. Turk, and D. Turk. 2001. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 20:4629–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi, T., M. Minemura, A. Hirata, M. Abe, D. Watanabe, and Y. Ohya. 2002. Movement of yeast 1,3-β-glucan synthase is essential for uniform cell wall synthesis. Genes Cells. 7:1–9. [DOI] [PubMed] [Google Scholar]
- Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 94:13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth, N.C., and P.J. Novick. 1987. Purification and characterization of constitutive secretory vesicles from yeast. J. Cell Biol. 105:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, D., M. Abe, and Y. Ohya. 2001. Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-β-glucan synthesis. Yeast. 18:943–951. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Y. Zhang, U. Derewenda, X. Liu, W. Minor, R.K. Nakamoto, A.V. Somlyo, A.P. Somlyo, and Z.S. Derewenda. 1997. Crystal structure of RhoA-GDP and its functional implications. Nat. Struct. Biol. 4:699–703. [DOI] [PubMed] [Google Scholar]
- Wolff, A.M., N. Din, and J.G. Petersen. 1996. Vacuolar and extracellular maturation of Saccharomyces cerevisiae proteinase A. Yeast. 12:823–832. [DOI] [PubMed] [Google Scholar]
- Yamochi, W., K. Tanaka, H. Nonaka, A. Maeda, T. Musha, and Y. Takai. 1994. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125:1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C., U.S. Jung, P. Garrett-Engele, T. Roe, M.S. Cyert, and D.E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]