Abstract
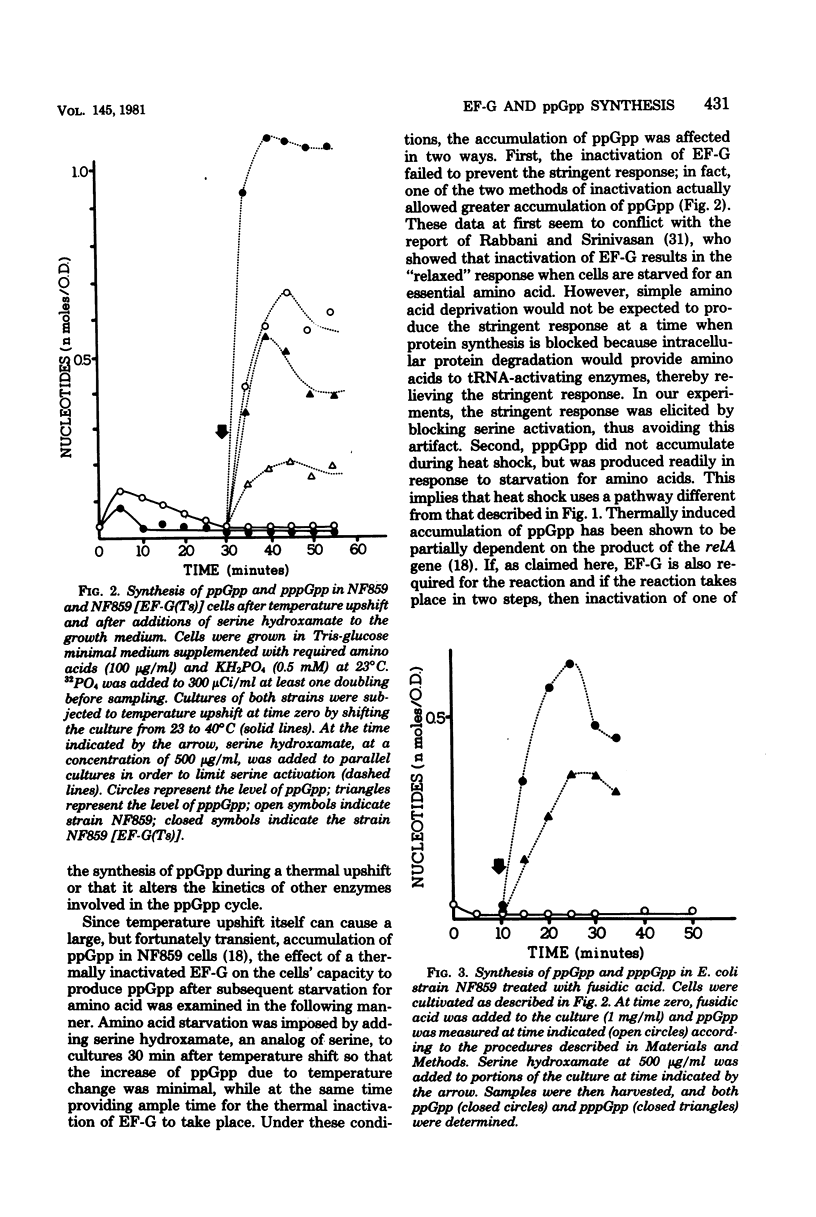
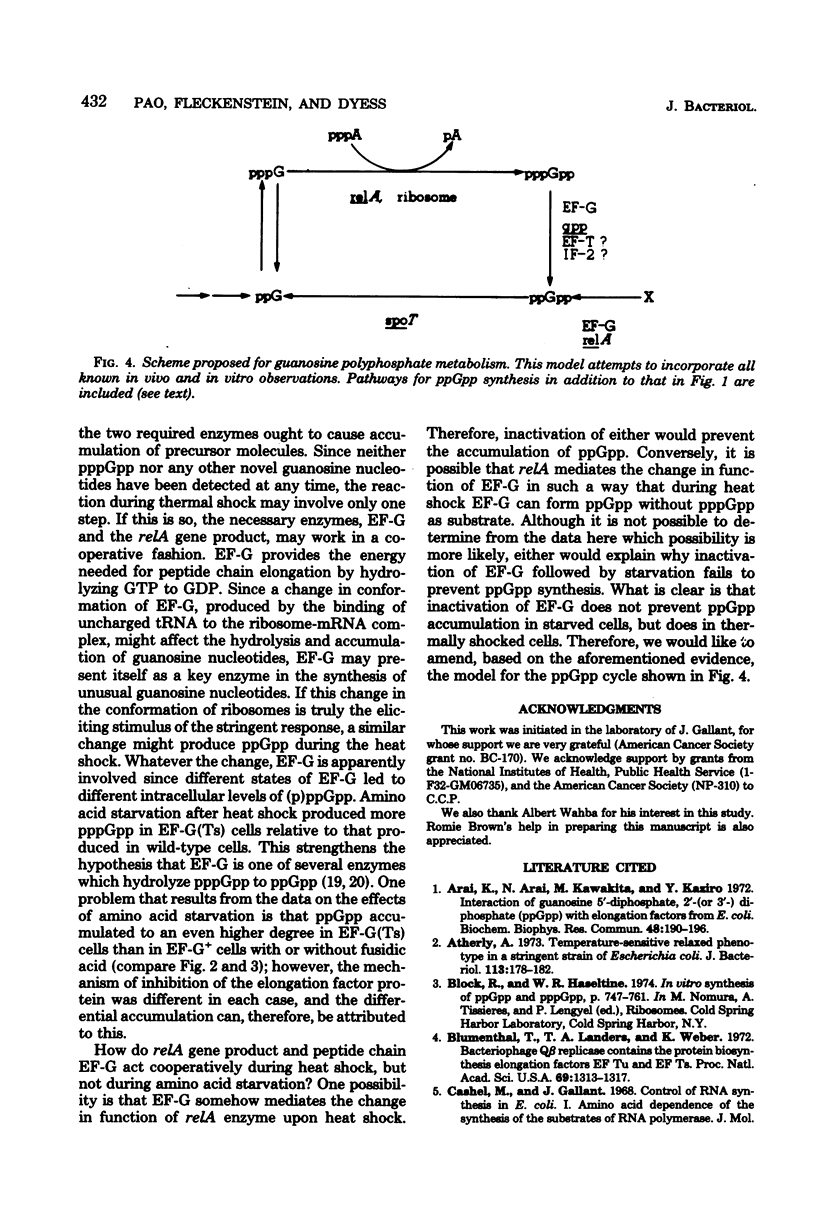
In a wild-type strain (relA+) of Escherichia coli, starvation of amino acid led to an immediate cessation of the synthesis of stable ribonucleic acids, together with the accumulation of an unusual nucleotide, guanosine 5'-diphosphate 3'-diphosphate, commonly known as ppGpp. This compound also accumulated during heat shock. When temperature-sensitive protein synthesis elongation factor G (EF-G) was introduced into E. coli NF859, a relA+ strain, the synthesis of ppGpp was reduced to approximately one-half that of wild-type EF-G+ cells at a nonpermissive temperature of 40 degrees C. Furthermore, fusidic acid, an inhibitor of protein synthesis which specifically inactivates EF-G, prevented any accumulation of ppGpp during the heat shock. We suggest that a functional EF-G protein is necessary for ppGpp accumulation under temperature shift conditions, possibly by mediating changes in the function of another protein, the relA gene product. However, EF-G is probably not required for the synthesis of ppGpp during the stringent response, since its inactivation did not prevent ppGpp accumulation during amino acid starvation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Arai N., Kawakita M., Kaziro Y. Interaction of guanosine 5'-diphosphate, 2'-(or 3'-) diphosphate(ppGpp) with elongation factors from E. coli. Biochem Biophys Res Commun. 1972 Jul 11;48(1):191–196. doi: 10.1016/0006-291x(72)90361-0. [DOI] [PubMed] [Google Scholar]
- Atherly A. G. Temperature-sensitive relaxed Phenotype in a stringent strain of Escherichia coli. J Bacteriol. 1973 Jan;113(1):178–182. doi: 10.1128/jb.113.1.178-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Chu F., Miller D. L., Schulz T., Weissbach H., Brot N. DNA-directed in vitro synthesis of elongation factor Tu. Biochem Biophys Res Commun. 1976 Dec 20;73(4):917–927. doi: 10.1016/0006-291x(76)90209-6. [DOI] [PubMed] [Google Scholar]
- Cochran J. W., Byrne R. W. Isolation and properties of a ribosome-bound factor required for ppGpp and ppGpp synthesis in Escherichia coli. J Biol Chem. 1974 Jan 25;249(2):353–360. [PubMed] [Google Scholar]
- Dennis P. P. Influence of the stringent control system on the transcription of ribosomal ribonucleic acid and ribosomal protein genes in Escherichia coli. J Bacteriol. 1977 Feb;129(2):580–588. doi: 10.1128/jb.129.2.580-588.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of the transcriptional activities of ribosomal protein genes in E. coli. Nature. 1975 Jun 5;255(5508):460–465. doi: 10.1038/255460a0. [DOI] [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- Fast R., Sköld O. Biochemical mechanism of uracil uptake regulation in Escherichia coli B. Allosteric effects on uracil phosphoribosyltransferase under stringent conditions. J Biol Chem. 1977 Nov 10;252(21):7620–7624. [PubMed] [Google Scholar]
- Fiil N. P., Willumsen B. M., Friesen J. D., von Meyenburg K. Interaction of alleles of the relA, relC and spoT genes in Escherichia coli: analysis of the interconversion of GTP, ppGpp and pppGpp. Mol Gen Genet. 1977 Jan 7;150(1):87–101. doi: 10.1007/BF02425329. [DOI] [PubMed] [Google Scholar]
- Furano A. V., Wittel F. P. Syntheses of elongation factors Tu and G are under stringent control in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):898–901. [PubMed] [Google Scholar]
- Gallant J., Irr J., Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971 Sep 25;246(18):5812–5816. [PubMed] [Google Scholar]
- Gallant J., Palmer L., Pao C. C. Anomalous synthesis of ppGpp in growing cells. Cell. 1977 May;11(1):181–185. doi: 10.1016/0092-8674(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Hamel E., Cashel M. Role of guanine nucleotides in protein synthesis. Elongation factor G and guanosine 5'-triphosphate,3'-diphosphate. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3250–3254. doi: 10.1073/pnas.70.11.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Interactions of guanosine triphosphate analogues with elongation factor G of Escherichia coli. Eur J Biochem. 1976 Apr 1;63(2):431–440. doi: 10.1111/j.1432-1033.1976.tb10245.x. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Cashel M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J Biol Chem. 1972 Nov 10;247(21):7067–7072. [PubMed] [Google Scholar]
- Kaplan S., Atherly A. G., Barrett A. Synthesis of stable RNA in stringent Escherichia coli cells in the absence of charged transfer RNA. Proc Natl Acad Sci U S A. 1973 Mar;70(3):689–692. doi: 10.1073/pnas.70.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Post L., Nomura M. DNA-dependent in vitro synthesis of fibosomal proteins, protein elongation factors, and RNA polymerase subunit alpha: inhibition by ppGpp. Cell. 1976 Nov;9(3):439–448. doi: 10.1016/0092-8674(76)90089-1. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Goldfine H. The involvement of guanosine 5-diphosphate-3-diphosphate in the regulation of phospholipid biosynthesis in Escherichia coli. Lack of ppGpp inhibition of acyltransfer from acyl-ACP to sn-glycerol 3-phosphate. J Biol Chem. 1975 Jul 10;250(13):4911–4917. [PubMed] [Google Scholar]
- Maher D. L., Dennis P. P. In vivo transcription of E. coli genes coding for rRNA, ribosomal proteins and subunits of RNA polymerase: influence of the stringent control system. Mol Gen Genet. 1977 Oct 20;155(2):203–211. doi: 10.1007/BF00393161. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Cashel M., Weissbach H. The interaction of guanosine 5'-diphosphate, 2' (3')-diphosphate with the bacterial elongation factor Tu. Arch Biochem Biophys. 1973 Feb;154(2):675–682. doi: 10.1016/0003-9861(73)90022-2. [DOI] [PubMed] [Google Scholar]
- Morrissey J. J., Cupp L. E., Weissbach H., Brot N. Synthesis of ribosomal proteins L7L12 in relaxed and stringent strains of Escherichia coli. J Biol Chem. 1976 Sep 25;251(18):5516–5521. [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Evidence for a direct effect on fatty acid synthesis in rela gene control of membrane phospholipid synthesis. J Mol Biol. 1976 Mar 25;102(1):167–172. doi: 10.1016/0022-2836(76)90080-2. [DOI] [PubMed] [Google Scholar]
- Rabbani E., Srinivasan P. R. Role of the translocation factor G in the regulation of ribonucleic acid synthesis. J Bacteriol. 1973 Mar;113(3):1177–1183. doi: 10.1128/jb.113.3.1177-1183.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDS M. K., ROBERTS R. B. The effects of a tryptophan-histidine deficiency in a mutant of Escherichia coli. J Bacteriol. 1952 Apr;63(4):505–511. doi: 10.1128/jb.63.4.505-511.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Kaziro Y. Amino acid-dependent control of the transport of alpha-methyl glucoside in E. coli. Biochem Biophys Res Commun. 1969 Jan 6;34(1):99–103. doi: 10.1016/0006-291x(69)90534-8. [DOI] [PubMed] [Google Scholar]
- Somerville C. R., Ahmed A. Mutants of Escherichia coli defective in the degradation of guanosine 5'-triphosphate, 3'-diphosphate (pppGpp). Mol Gen Genet. 1979 Feb 1;169(3):315–323. doi: 10.1007/BF00382277. [DOI] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Travers A., Clark B. F. Inhibition of translation initiation complex formation by MS1. FEBS Lett. 1972 Jun 15;23(2):163–166. doi: 10.1016/0014-5793(72)80331-4. [DOI] [PubMed] [Google Scholar]


