Abstract
The proper segregation of sister chromatids in mitosis depends on bipolar attachment of all chromosomes to the mitotic spindle. We have identified the small molecule Hesperadin as an inhibitor of chromosome alignment and segregation. Our data imply that Hesperadin causes this phenotype by inhibiting the function of the mitotic kinase Aurora B. Mammalian cells treated with Hesperadin enter anaphase in the presence of numerous monooriented chromosomes, many of which may have both sister kinetochores attached to one spindle pole (syntelic attachment). Hesperadin also causes cells arrested by taxol or monastrol to enter anaphase within <1 h, whereas cells in nocodazole stay arrested for 3–5 h. Together, our data suggest that Aurora B is required to generate unattached kinetochores on monooriented chromosomes, which in turn could promote bipolar attachment as well as maintain checkpoint signaling.
Keywords: mitosis; chromosome segregation; kinetochores; spindle assembly checkpoint; chemical biology
Introduction
The proper segregation of replicated chromatids during mitosis requires that the two sister kinetochores on each chromosome become attached to the opposing spindle poles. During mitosis in higher animal cells, this bipolar, or amphitelic, attachment is often established through a transient intermediate step in which the chromosome first becomes attached to a single spindle pole (Mitchison and Kirschner, 1984; Rieder and Salmon, 1998). Usually, such monopolar chromosomes are attached with only one of their kinetochores (monotelic attachment), but in some cases, both sister kinetochores become attached to the same pole. This syntelic attachment is unstable, and, with rare exceptions, it is corrected before anaphase onset.
Anaphase is initiated by the ubiquitin ligase anaphase-promoting complex (APC)* and the protease separase (Nasmyth, 2001). In most eukaryotic cells, APC activation is actively suppressed by the spindle assembly checkpoint as long as at least one unattached kinetochore is present (Rieder et al., 1995). Unattached kinetochores recruit and activate Mad2 and other checkpoint proteins that inhibit the APC (Shah and Cleveland, 2000). It has also been reported that the checkpoint can respond to the mere absence of tension at kinetochores in both meiotic and mitotic cells (Li and Nicklas, 1995; Nicklas et al., 2001; Skoufias et al., 2001; Stern and Murray, 2001; Taylor et al., 2001; Shannon et al., 2002). However, because the absence of tension might be converted into a decreased microtubule occupancy of kinetochores (King and Nicklas, 2000), it is possible that the checkpoint does not directly sense the absence of tension but rather the lack of full attachment (Waters et al., 1998; Nicklas et al., 2001).
How tension stabilizes the binding of microtubules to kinetochores, and how the checkpoint signal is abrogated when a chromosome finally acquires stable bipolar attachment, is unknown. Recent genetic work in budding yeast suggests that the Aurora kinase Ipl1 is required both for proper chromosome segregation by destabilizing incorrect kinetochore–microtubule interactions, and for maintaining the spindle assembly checkpoint in the absence of tension (Biggins et al., 1999; Biggins and Murray, 2001; Tanaka et al., 2002). Aurora kinases are a family of evolutionarily conserved mitotic kinases. Metazoans contain up to three members of the Aurora kinase family (Aurora A, B, and C), whereas budding and fission yeast only possess one family member (Ipl1 and Ark1, respectively) (Nigg, 2001). Aurora A is enriched at centrosomes, whereas Aurora B and its binding partners INCENP (inner centromere protein) and Survivin are “chromosomal passenger” proteins (Adams et al., 2001a). Aurora B appears to be required for several mitotic processes, including chromosome congression to the spindle equator, chromosome segregation, and cytokinesis (Kaitna et al., 2000; Adams et al., 2001b; Giet and Glover, 2001; Kaitna et al., 2002; Kallio et al., 2002; Murata-Hori and Wang, 2002; Murata-Hori et al., 2002).
We describe here the identification of a small molecule inhibitor perturbing mitosis based on its ability to induce polyploidy in mammalian cells. Our data indicate that the mitotic effects of this compound, which we call Hesperadin, are due to inhibition of Aurora B function. Hesperadin causes mammalian cells to enter anaphase in the presence of monooriented chromosomes, which may be syntelically attached. Our data are consistent with the hypothesis that Aurora B activity is required to correct syntelic attachments, and that this correction function may indirectly maintain spindle checkpoint signaling by generating unattached kinetochores.
Results
An indolinone that causes polyploidy
In the course of a synthesis program for novel indolinones, we tested the effect of several compounds on cell proliferation. One of these, Hesperadin (Fig. 1 A; Walter et al., 2002), had dramatic effects; HeLa cells treated with 50 nM of Hesperadin stopped proliferating but did not stop growing, and over a 6-d period, the cell diameter increased more than sevenfold (from ∼20 to >150 μm; Fig. 1 B). During this time, the cells acquired enlarged lobed nuclei (see Fig. 4 i). FACS® analysis revealed that the increase in nuclear size correlated with polyploidization, reaching a 32C DNA content on day 3 (Fig. 1 B; unpublished data). At later stages, FACS® could not be used to analyze these cells, presumably because they had grown too big to enter the measuring capillary.
Figure 1.

Hesperadin causes polyploidy in HeLa cells. (A) Chemical structure of Hesperadin. (B) Hesperadin (50 nM) was added to logarithmically growing HeLa cells (day 0). At the indicated time points, the DNA content was determined by flow cytometry, and phase contrast micrographs were taken. From day 4 on, the DNA content could not be measured by flow cytometry (n.a., not applicable). (C) HeLa cells were synchronized by double thymidine treatment and released either into 100 nM Hesperadin (right) or a corresponding concentration (0.01%) of the solvent DMSO (left). Cells were harvested at the indicated time points after release, and the DNA content was analyzed by flow cytometry. 330 nM nocodazole was added to one sample of each series, and these cells were harvested at 14.5 h after release from thymidine. log, logarithmically growing untreated HeLa cells. (D) Samples from the same experiment as in C were analyzed by SDS-PAGE and immunoblotting.
Figure 4.
Hesperadin-treated HeLa cells show alignment and segregation defects, but sister chromatid separation is intact. HeLa cells were left untreated or were treated with 50 nM Hesperadin for different periods of time before harvesting by mitotic shake off, followed by chromosome spreading and Giemsa staining. (a) Normal metaphase plate. Inset, normal degree of sister chromatid resolution. Compare the gap between sisters (arrow) in a and c. (b) Normal onset of anaphase. (c) Typical appearance of a late prometaphase in Hesperadin-treated cultures. Defects in alignment and sister chromatid resolution (arrow) are apparent. Some chromosomes are bent at the centromeric region (inset, examples marked by squares), implying microtubule attachment. (d–f) Typical appearance of early anaphases in Hesperadin-treated cells. Chromosomes often seem to be segregated to opposite poles, but many sister chromatids stay in close proximity (double arrows). One example of sister chromatids being pulled to opposite poles is marked by arrowheads. (g and h) Typical appearance of chromatin decondensation in Hesperadin-treated cells. (i) Typical interphase in Hesperadin-treated cultures.
Hesperadin causes defects in mitosis and cytokinesis
To analyze how Hesperadin causes polyploidy, we filmed HeLa cells expressing a GFP-tagged version of histone H2B (Kanda et al., 1998) in the presence of 100 nM Hesperadin (see Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). During mitosis, the cells rounded normally, condensed their chromosomes, and aligned some, but not all, chromosomes on a metaphase-like plate. The cells then exited mitosis in the presence of nonaligned chromosomes. During this time, the chromatin mass appeared to be stretched toward opposite poles, but the chromosomes were not segregated into two distinct masses. The cells attempted cytokinesis, which was accompanied by intense blebbing of the plasma membrane. However, the cleavage furrow ultimately regressed to produce a single cell in which the stretched chromosomal masses decondensed to form an irregularly shaped restitution nucleus.
Hesperadin does not inhibit APC and separase activation
To understand how Hesperadin causes mitotic defects, we arrested HeLa cells by double-thymidine treatment in S phase, released them into 100 nM Hesperadin, and analyzed their progression through mitosis (Fig. 1, C and D). Immunoblot analysis revealed that the APC subunit Cdc27 underwent an electrophoretic mobility shift and that the APC substrates cyclin A, cyclin B, securin, and Cdc20 were degraded in mitosis, indicating that APC phosphorylation and activation were not inhibited by Hesperadin (Fig. 1 D; unpublished data). Also, the activation of separase appeared unaffected, as we observed cleavage of separase and the cohesin subunit Scc1 (unpublished data). All of these events were delayed by 60–90 min relative to control cells. Phase contrast microscopy of living cells showed that Hesperadin-treated cells began to round up about 1 h later than control cells (unpublished data), and immunoblotting revealed that mitotic dephosphorylation of Cdk1 also occurred ∼60–90 min later in Hesperadin-treated cells (unpublished data), suggesting that the observed delay in APC and separase activation may indirectly be caused by a slight delay in mitotic entry.
Hesperadin inhibits Aurora B function
As a mitotic marker, we also analyzed phosphorylation of serine 10 on histone H3 in the same experiment. We found that immunoblotting yielded a phospho-histone H3 signal in mitotic Hesperadin-treated cells that was greatly reduced relative to controls (Fig. 2 A). Immunofluorescence microscopy confirmed this result (Fig. 2 B). Because mitotic H3-Ser10 phosphorylation depends on Aurora B (Adams et al., 2001a), Hesperadin appears to inhibit Aurora B function.
Figure 2.
Hesperadin inhibits histone H3 phosphorylation and causes midspindle defects. (A) Samples from the same experiment as in Fig. 1 (C and D) were analyzed by SDS-PAGE and immunoblotting with anti–phospho-histone H3 antibody. (B) HeLa cells were treated with Hesperadin or DMSO (control) for 16 h and immunostained with anti–phospho-histone H3. DNA was stained with DAPI. Chromosomes that have not aligned at the metaphase plate are indicated by arrowheads. (C) HeLa cells were treated as in B. Cells were costained with α-tubulin and either CYK-4 or MKLP-1 antibodies. DNA was stained with DAPI. In Hesperadin-treated cells, chromatin is not segregated properly in anaphase, indicated by arrows. (D) Cdk1 or human Aurora B were immunoprecipitated from mitotic HeLa cell extracts, and in vitro kinase activity was determined using histone H1 or histone H3, respectively, as a substrate. Hesperadin was added to the kinase reactions at the indicated concentrations. As a control, 0.1% DMSO was added (corresponding to the DMSO concentration in the sample containing 5,000 nM Hesperadin). Further controls included a kinase reaction without substrate as well as mock immunoprecipitates by unspecific antibodies.
In Caenorhabditis elegans, Aurora B is required for the formation of a central spindle during anaphase and for stable association of the centralspindlin complex with this structure (for review see Adams et al., 2001a). Immunofluorescence microscopy revealed that Hesperadin-treated cells in which chromosomes were stretched toward opposite poles, i.e., which had entered anaphase, failed to assemble a central spindle and to properly localize the human centralspindlin subunits CYK-4 and MKLP1 (Fig. 2 C). These findings further indicate that Hesperadin inhibits Aurora B and imply that Aurora B function is also required for central spindle assembly in human cells.
Approximately 250 nM Hesperadin was required to half-maximally inhibit the ability of immunoprecipitated Aurora B to phosphorylate histone H3 (Fig. 2 D). In contrast, dose response experiments on HeLa cells revealed that polyploidization and loss of mitotic histone H3-Ser10 phosphorylation were induced by only 20–100 nM Hesperadin (unpublished data). Currently, we do not know if the different doses that are required to inhibit Aurora B function in cells and in vitro are due to technical differences in the assays used, or due to an intracellular accumulation of Hesperadin. It also remains possible that Aurora B function is inhibited indirectly, e.g., by inhibiting an enzyme required for Aurora B activation. We conclude that Hesperadin inhibits Aurora B function in living cells either directly or indirectly.
Specificity of Hesperadin
When we measured the activity of 25 kinases in the presence of 1 μM Hesperadin, we found that Hesperadin markedly reduced the activity of six kinases (AMPK, Lck, MKK1, MAPKAP-K1, CHK1, and PHK) (see Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). In the same assay, in vitro inhibition of multiple kinases has also been found for other presumably specific inhibitors (Davies et al., 2000). Inhibition of the MAP kinase kinase (MEK1/MKK1) has been reported to block cells in G2 (Wright et al., 1999; Hayne et al., 2000) and could therefore account for the delay in mitotic entry that we observe. However, immunostaining of Hesperadin- and control-treated cells with a phosphospecific antibody for the MKK1 substrates Erk1 and Erk2 did not reveal any difference in staining (unpublished data), suggesting that at the used concentration, Hesperadin does not inhibit MKK1 activity in vivo.
We cannot formally exclude that Hesperadin influences AMPK, CHK1, Lck, MAPKAP-K1/p90Rsk, or PHK activity in vivo. However, based on their known cellular functions, their inhibition is unlikely to cause the phenotype that we describe here. Furthermore, Cdk1/cyclin B was half-maximally inhibited at 2.8 μM, and Cdk2/cyclinE and Cdk4/cyclinD1 at >10 μM (Fig. 2 D; unpublished data), suggesting that Cdks are not inhibited in vivo.
Aurora B RNA interference (RNAi) phenocopies Hesperadin treatment
To further test if the phenotype induced by Hesperadin is due to the inhibition of Aurora B function, we used RNA interference (RNAi). In HeLa cells treated with small inhibitory RNAs (siRNAs) directed against Aurora B, the level of Aurora B protein was substantially reduced, as judged by both immunoblotting (Fig. 3 A) and immunofluorescence (Fig. 3 B), whereas Aurora A levels were affected only to a minor extent (Fig. 3 A). Immunofluorescence microscopy revealed that mitotic cells without detectable Aurora B staining frequently contained chromosomes that were not aligned on the spindle equator (Fig. 3 B) and phospho-histone H3 staining was often reduced or absent (Fig. 3 B). We also observed elongated cells, presumably undergoing anaphase, with some chromosomes that were located at opposite poles but others that were lagging (Fig. 3 C). α-Tubulin staining revealed that these cells did not form a midspindle structure (Fig. 3 C). Instead of being highly localized, as in control anaphase cells, Survivin was diffusely localized in the region between the poles (Fig. 3 C). siRNA cultures also contained enlarged cells with multiple nuclei and micronuclei (Fig. 3 D) that were likely the consequence of cytokinesis defects. Together, these observations reveal that human Aurora B is required for mitotic phosphorylation of histone H3, chromosome alignment, chromosome segregation, formation of a midspindle, and cytokinesis, consistent with earlier observations in C. elegans embryos and Drosophila melanogaster (for review see Adams et al., 2001a). Hesperadin-treated cells exhibit a very similar phenotype (Figs. 1 and 2), which is further evidence that Hesperadin inhibits Aurora B function in vivo. We therefore named our inhibitor Hesperadin in reference to the antique goddess of dusk, Hespera, who is the counterpart of Aurora, the goddess of dawn.
Figure 3.
Aurora B RNAi in human cells induces a similar phenotype as Hesperadin. (A) HeLa cells were transfected with Aurora B–targeting siRNA duplex (RNAi AurB) or with H2O replacing the siRNA (control). After 50 h, cells were lysed in sample buffer. Samples were resolved by SDS-PAGE and immunoblotted. (B) Cells treated as in A were processed for immunofluorescence 48 h after transfection. Cells were costained for Aurora B (in green) and phospho-histone H3 (in red). DNA was stained with DAPI (left panel, right panel in blue, also in C and D). Chromosomes that have not aligned to the metaphase plate are indicated by arrowheads. (C and D) Cells treated as in A were processed for immunofluorescence 72 h after transfection. Cells were stained for α-tubulin (in green) and Survivin (C, in red). Cells treated with Aurora B siRNA duplex did not form a proper midspindle and showed segregation defects, indicated by an arrow.
Aurora B function is not required for chromosome condensation or sister chromatid separation
To investigate the role of Aurora B in chromosome segregation, we performed chromosome spreading of mitotic HeLa cells treated with Hesperadin. Although normal metaphase and anaphase figures (Fig. 4 , a and b) were absent, prometaphase cells showed some degree of chromosome alignment, and chromosomes were frequently bent in the centromeric region (Fig. 4 c, inset, black squares). Such bendings are infrequent in nocodazole-treated cells (compare with Fig. 8 A) and therefore are likely to arise from microtubule attachments. This, and other observations (see below), suggests that most chromosomes become attached to the mitotic spindle when Aurora B is inhibited.
Figure 8.

Hesperadin quickly overrides the mitotic arrest induced by taxol or monastrol. (A, B, and C) HeLa cells were arrested in nocodazole (330 nM), taxol (10 μM), or monastrol (100 μM). Hesperadin (100 nM) or the solvent DMSO was added, and cells were followed by chromosome spreading (A and B) or immunofluorescence microscopy (C). For immunofluorescence microscopy (C), cells were stained with α-tubulin (left), DNA was counterstained with DAPI (right). (D) Percentage of cells arrested in mitosis in the presence of the different drugs is shown as a function of time. Numbers were obtained from the samples shown in A–C. (E) PtK1 cells were treated with monastrol, and mitotic entry was followed by differential interference contrast microscopy. Selected stills of a time-lapse movie are shown, elapsed time in h:min is shown in the lower right. 500 nM Hesperadin was added at time point 1:10 when the typical monoastral spindle had formed, and mitotic exit was followed. All sister chromatids move into the single polar region.
The overall compaction of chromatin in prometaphase and the association of condensin with chromosomes did not seem to be affected (Fig. 4, c–f; unpublished data), suggesting that Aurora B function is not required for chromosome condensation in human cells. Notably, however, in preanaphase chromosomes, sister chromatids were often less resolved in Hesperadin-treated cells in that the interchromatid distance was smaller than in chromosomes from untreated cells (Fig. 4, compare c with a, insets, arrow). Strikingly, this resolution defect in prometaphase did not preclude the separation of sister chromatids during anaphase (Fig. 4, d–f).
All anaphase figures appeared highly aberrant in chromosome spreads from Hesperadin-treated cells. Although the sister chromatids clearly disjoined, many were found next to each other (Fig. 4, d–f, arrows) and were also in close proximity at the time of chromosome decondensation (Fig. 4, g and h). It seems likely that spindle forces were present in Hesperadin-treated cells during anaphase, because some sister chromatids had been pulled to opposite poles (Fig. 4 d, arrowheads). Separated sister chromatids in close proximity, therefore, could have arisen either from unattached chromosomes or from chromosomes whose two sister chromatids were attached to the same spindle pole. Together, these observations suggest that the chromosome segregation defect seen in Hesperadin-treated cells does not result from defects in chromosome condensation or sister chromatid separation.
Aurora B function is required during chromosome attachment to the mitotic spindle
To test if Hesperadin's inhibitory effect on sister chromatid resolution in prometaphase could be responsible for the observed chromosome alignment defect, e.g., by preventing complete resolution of sister kinetochores, we analyzed when in mitosis Hesperadin addition causes chromosome segregation defects. Cells were arrested by nocodazole, and Hesperadin was only added shortly before releasing cells from nocodazole so that cells progressed through prometaphase in the absence of Hesperadin (Fig. 5 A). In this case, the resolution of sister chromatids was not affected, but the cells nevertheless exited mitosis with the same chromosome alignment and segregation defects as described above (Fig. 5 C; see Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). In contrast, cells exited mitosis without abnormalities in anaphase or telophase (Fig. 5 D) and with similar kinetics as controls (Fig. S2) when Hesperadin was washed out before the cells were released from nocodazole (Fig. 5 B), i.e., when spindles assembled in the absence of Hesperadin. These observations indicate that the defect in chromosome segregation induced by Hesperadin can be specifically ascribed to the inhibition of Aurora B function during the process of chromosome attachment.
Figure 5.
Aurora B function is required at the time of microtubule attachment to kinetochores. (A and C) HeLa cells were arrested in nocodazole. Hesperadin or the solvent DMSO were added shortly before release from nocodazole (outlined in A). Chromosome spreads were performed with the arrested cells after addition of Hesperadin or DMSO (noc-arrest), directly after washing out nocodazole (0'), and at different time points after release from nocodazole (15–150'). Representative images are shown in C. For a quantification, see Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). (B and D) HeLa cells were arrested in nocodazole in the presence of Hesperadin or the solvent DMSO. Hesperadin and DMSO were washed out before releasing cells from nocodazole (outlined in B). Chromosome spreads were performed with the arrested cells after Hesperadin or DMSO washout (noc-arrest), directly after washing out nocodazole (0'), and at different time points after release from nocodazole (15–150'). Representative images are shown in D. For a quantification, see Fig. S2.
Aurora B function is required to ensure bipolar attachments before anaphase
To understand the nature of the observed chromosome segregation defect, we filmed Hesperadin-treated living PtK1 cells by differential interference contrast microscopy. At nuclear envelope breakdown (NEB; Fig. 6 , time 0:00), some chromosomes were usually positioned at an equal distance between the two spindle poles and appeared to rapidly acquire a normal bipolar attachment (Fig. 6, time 0:06). However, other chromosomes that were positioned closer to one of the poles at NEB rapidly acquired a monopolar attachment to that pole. Like monooriented chromosomes in untreated cells (Rieder and Salmon, 1998), these chromosomes exhibited pronounced oscillatory motions toward and away from the pole they were attached to (Fig. 6; see Video 2, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1).
Figure 6.

Monoorientation is not corrected in Hesperadin-treated PtK1 cells before anaphase. Live cell imaging of a PtK1 cell treated with 500 nM Hesperadin. Selected stills of a time-lapse movie (Video 2, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1), elapsed time from NEB in h:min is shown in the upper left. The approximate positions of the spindle poles are marked by yellow stars. Red dots indicate the centromeric region of two different chromosomes. After NEB (0:00), some chromosomes align to the metaphase plate, but the majority of chromosomes are monooriented (0:17–0:44). Monooriented chromosomes move on the spindle (compare distance between red dots and yellow stars), but they fail to congress. Upon the onset of anaphase, sister chromatids of bioriented chromosomes are pulled to opposite poles (example marked by red arrowheads), whereas both sisters of the monooriented chromosomes move to the same pole.
The number of bioriented versus monooriented chromosomes varied between different cells (unpublished data), but one striking feature that all cells had in common was that initially monooriented chromosomes did not achieve biorientation until the onset of anaphase. Time-lapse studies revealed that Hesperadin also causes chromosome biorientation defects in human CF-PAC and RPE-1 cells, indicating that this effect is not specific for PtK1 cells (unpublished data). Because cells normally do not enter anaphase in the presence of monooriented chromosomes, Hesperadin could cause a spindle assembly checkpoint defect. The failure to congress all chromosomes might therefore be a secondary consequence of a premature onset of anaphase. To evaluate this possibility, we compared the duration from NEB to the onset of anaphase in Hesperadin-treated or control cells filmed in a 37°C warm room (Table I). Although, on average, Hesperadin-treated cells enter anaphase earlier after NEB than controls (after 16 and 23 min, respectively), most remain in prometaphase longer (16 min on average) than the time it usually takes for chromosomes in untreated cells to become attached in a bipolar fashion (13 min on average). The failure to achieve biorientation therefore cannot be fully attributed to a precocious exit from mitosis.
Table I. Time spent in prometaphase by PtK1 cells treated with Hesperadin.
| PtK1 cells | NEB to last chromosome congressing | NEB to anaphase onset | No. of monooriented chromosomes at anaphase onset | n |
|---|---|---|---|---|
| min:sec | min:sec | |||
| Controla | 13:19 (08:30–21:14) | 23:12 (14:00–50:45) | 0 | 16 |
| Hesperadina | NA | 16:27 (10:30–23:46) | 3.6 (1–6) | 23 |
PtK1 cells were left untreated or were treated with 500 nM Hesperadin, and followed by video microscopy in a 37°C warm room.
Sister chromatids of bioriented chromosomes moved to opposite poles after they disjoined at anaphase onset (Fig. 6, 0:58, arrowheads), demonstrating that they were indeed attached in an amphitelic fashion. At the same time, both sister chromatids of monooriented chromosomes moved to the same pole (Fig. 6, 0:58), suggesting the possibility that they were attached in a syntelic manner. Note that when a monotelic chromosome undergoes anaphase, only the attached chromatid moves poleward whereas the unattached chromatid initially shows no motion (Rieder et al., 1986; Ault and Rieder, 1992).
The chromosome movements observed during prometaphase and anaphase in Hesperadin-treated cells demonstrate that spindle forces are present and acting on the chromosomes. We often found, however, that the sister chromatids of bioriented chromosomes did not move as far apart during anaphase as they do in untreated cells (Fig. 6, 0:58). It is possible that anaphase B spindle elongation does not occur in Hesperadin-treated cells because a spindle midzone is not assembled (Fig. 2 C), or the force-producing mechanism for anaphase A is impaired, or chromosome decondensation occurs prematurely.
Syntelic attachment is more frequent in Hesperadin-treated cells than in control-treated cells
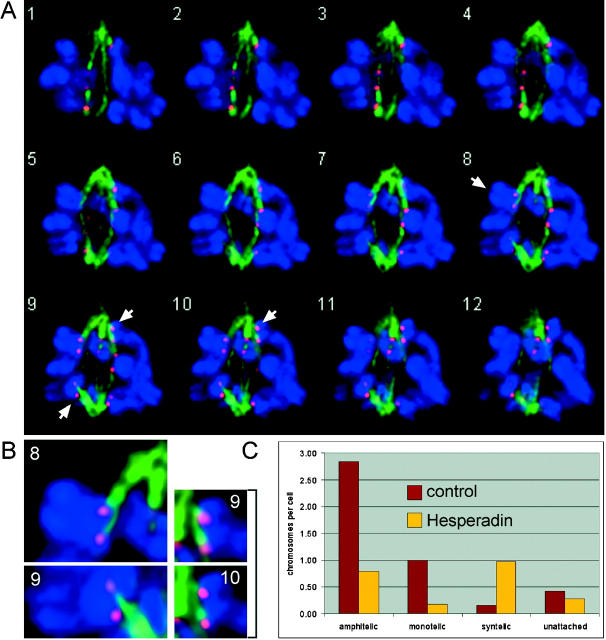
To determine the type of attachment of monooriented chromosomes in Hesperadin-treated cells, we performed deconvolution microscopy on PtK1 or PtK2 cells that were fixed and stained with anti-tubulin antibodies and CREST serum (Fig. 7 ; see Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). We were not able to determine the type of attachment for each chromosome of a prometaphase cell. However, we found, on average, one clearly syntelic chromosome in Hesperadin-treated cells, whereas only one in six control cells contained a clearly syntelic chromosome. Conversely, control cells contained, on average, one chromosome that could clearly be identified as monotelic, whereas this number was reduced sixfold in Hesperadin-treated cells (Fig. 7 C; Fig. S3), indicating that Aurora B function might be required to convert syntelic into monotelic attachment.
Figure 7.
Hesperadin-treated PtK cells show a higher frequency of syntelic attachments during prometaphase than control-treated cells. (A and B) PtK1 cell treated with 500 nM Hesperadin for 3 h. Deconvolved images taken at 0.2-μm Z-interval. α-Tubulin (green), CREST (red), and DNA staining (blue). Chromosomes that are attached in a syntelic manner are marked by arrows and are enlarged in B. (C) PtK2 cells were treated with 500 nM Hesperadin or 0.1% DMSO (control) for 3 h. Deconvolution microscopy was performed as in A, and the type of attachment was determined for as many chromosomes as possible (control: 26 cells, 115 chromosomes; Hesperadin: 29 cells, 64 chromosomes; see Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). The average number of chromosomes per cell exhibiting a certain type of attachment is shown.
Hesperadin treatment rapidly overrides the spindle assembly checkpoint in taxol- and monastrol-treated cells but not in nocodazole-treated cells
To determine if Aurora B function is required for the spindle checkpoint, we treated HeLa cells with nocodazole, which induces a Mad2-dependent mitotic arrest because microtubules are depolymerized and kinetochores are unattached. We then added Hesperadin and followed the cells over time by either chromosome spreading (Fig. 8 A) or time-lapse video light microscopy (unpublished data). We found that nocodazole-treated cells remained arrested in mitosis for at least 3 h (Fig. 8, A and D; unpublished data) after addition of Hesperadin.
The spindle checkpoint is also activated by taxol (paclitaxel), which stabilizes microtubules. Surprisingly, when cells arrested with taxol were treated with Hesperadin, they exited mitosis within 1 h (Fig. 8, B and D). This observation, and our finding that Aurora B inhibition stabilizes syntelic attachments, raised the possibility that Hesperadin treatment turned off checkpoint signaling in taxol-treated cells because all kinetochores progressively accumulated stably attached microtubules.
To explore this hypothesis further, we created monopolar spindles in cells by treating them with the kinesin Eg5 inhibitor monastrol (Mayer et al., 1999). Under this condition, cells arrest in mitosis for at least 5 h with ∼70% of their chromosomes syntelically monooriented, whereas the remainder are monotelically monooriented (Kapoor et al., 2000). We found that cells arrested with monastrol exited mitosis within 1 h after addition of Hesperadin (Fig. 8, C and D). Moreover, when we added Hesperadin to monastrol-arrested Ptk1 cells, all chromatids moved toward the single pole of the monopolar spindles in anaphase (Fig. 8 E), consistent with the hypothesis that all monotelic chromosomes that are normally found in monastrol-treated cells were converted into syntelic states by Hesperadin treatment. To confirm this notion, we fixed monastrol-treated PtK1 cells 1 h after addition of Hesperadin, chose cells that still exhibited a monoastral spindle, and determined the number of kinetochores that were staining with Mad2 antibodies, e.g., that were presumably unattached. The number of Mad2-positive kinetochores decreased from 6.3 (range 3–9; n = 12 cells) in monastrol-treated control cells to 1.2 (range 0–5; n = 25 cells) after 1 h of Hesperadin treatment, indicating that inhibition of Aurora B function might indeed stabilize syntelic attachments.
BubR1 does not localize to kinetochores when cells are treated with Hesperadin and nocodazole
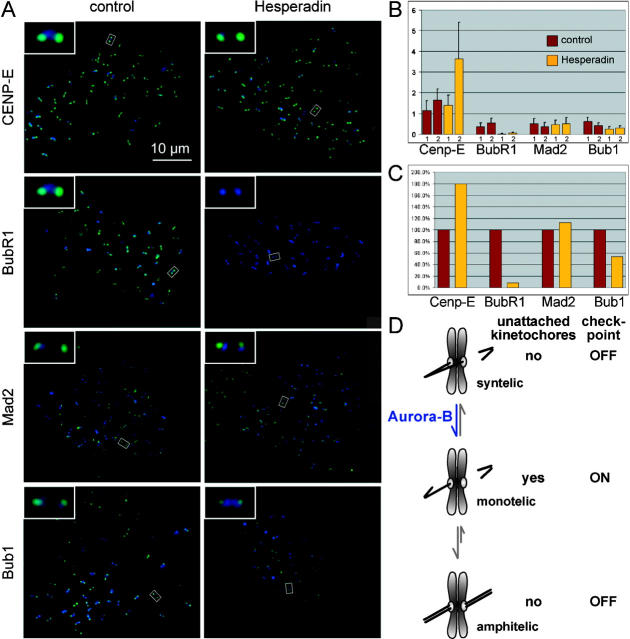
Hesperadin might induce mitotic exit in taxol- or monastrol-treated cells by stabilizing improper microtubule attachments. However, it is possible that Aurora B also has a direct role in the spindle assembly checkpoint, as even under conditions where none of the kinetochores are attached (Fig. 8, nocodazole), cells that are additionally treated with Hesperadin exit mitosis precociously (Fig. 8 D). Recruitment of checkpoint proteins to unattached kinetochores is thought to be necessary to maintain checkpoint signaling (Shah and Cleveland, 2000). We therefore tested whether inhibition of Aurora B function might impair this recruitment. We found that in the presence of nocodazole and Hesperadin, Mad2 and the motor protein CENP-E were still present at kinetochores. In contrast, kinetochore localization of BubR1 was abolished, and the intensity of Bub1 at kinetochores was diminished (Fig. 9 , A–C). Similar results were obtained in logarithmically growing HeLa cells (see Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1), where after addition of Hesperadin, Mad2 and CENP-E could be observed at kinetochores in early prometaphase, whereas BubR1 and Bub1 did not localize to kinetochores at any stage of mitosis. Together, these data suggest that Aurora B function is required for efficient kinetochore recruitment of BubR1 and Bub1, which in turn might be necessary for prolonged checkpoint signaling.
Figure 9.
BubR1 localization to kinetochores is abolished in HeLa cells treated with nocodazole and Hesperadin. (A) HeLa cells were arrested in mitosis with 10 μM nocodazole and then additionally treated with 100 nM Hesperadin or the solvent DMSO for 2 h, harvested by mitotic shake off, cytospun on slides, and processed for immunofluorescence with the indicated antibodies (in green). Kinetochores were labeled with CREST serum (in blue). The insets show magnifications of the kinetochore pairs marked by white rectangles. (B) Data, as in A, were quantified. For each cell, the average integrated intensity for the checkpoint protein was related to the average integrated intensity of the CREST signal. Bars show the average of the ratios obtained from 10 cells. The reduction of BubR1 signal intensity was significant in both independent experiments (1 and 2) (P < 0.0001, t test), whereas the reduction of Bub1 signal intensity was only significant in experiment 1 (experiment 1, P < 0.0001; experiment 2, P = 0.059). (C) Average of the two independent experiments shown in B, with the control data set to 100%. (D) Model for Aurora B function.
Discussion
In yeast and animal cells, Aurora B has important roles in regulating the segregation of sister chromatids. In budding yeast, the Aurora B homologue Ipl1 is required both for ensuring the correct biorientation of chromosomes (Tanaka et al., 2002) and for spindle checkpoint signaling (Biggins and Murray, 2001). Remarkably, Ipl1 is only required for checkpoint function in situations where kinetochores are attached to the spindle and is dispensable for checkpoint function when all kinetochores are unattached (Biggins and Murray, 2001). Aurora B is also required for checkpoint function and chromatid segregation in metazoans (for review see Shannon et al., 2002). However, the precise role(s) of Aurora B in these processes is poorly understood, and different experimental approaches have yielded different, and sometimes conflicting, results. For example, inhibiting Aurora B by RNAi in worms has been proposed to cause merotelic chromosome attachments (Kaitna et al., 2002), whereas monooriented chromosomes were observed in Xenopus cells injected with Aurora B antibodies (Kallio et al., 2002), and entirely unattached chromosomes were found in mammalian cells overexpressing a kinase-inactive mutant of Aurora B (Murata-Hori and Wang, 2002). A detailed analysis of Aurora B function in metazoans would therefore benefit from the availability of a rapid and reversible inhibitor of Aurora B function. Several observations indicate that Hesperadin represents such a compound: (a) Hesperadin inhibits phosphorylation of histone H3 on Ser10, which is catalyzed by Aurora B in mitosis (Fig. 2, A and B), (b) the phenotypes induced by knocking down Aurora B by RNAi and inhibiting its function by Hesperadin are highly similar (Figs. 1–3), (c) Aurora B is inhibited by low concentrations of Hesperadin in vitro (Fig. 2 D), (d) Aurora B in whole cell lysates specifically binds to Hesperadin, but not to a structurally related compound that does not show similar cellular effects (M. Steegmaier, personal communication), and (e) an independently identified compound (ZM447439) that targets Aurora kinases causes similar cellular effects (Ditchfield et al., 2003). To formally prove that inhibition of Aurora B is responsible for the mitotic phenotypes that are described here, it will be necessary in the future to identify mutations in Aurora B that render the kinase resistant to Hesperadin.
The function of Aurora B in promoting biorientation
The biorientation of chromosomes on the mitotic spindle is a stochastic process (Rieder and Salmon, 1998), and therefore chromosomes only rarely become connected to both spindle poles simultaneously. Instead, most chromosomes first capture microtubules from one pole, i.e., they are transiently monooriented, before they become attached to both poles. Our analysis revealed that inhibition of Aurora B dramatically affects this normal biorientation process. In Hesperadin-treated cells, some chromosomes become bipolar, but many others remain monooriented until cells enter anaphase and exit mitosis (Fig. 6). Our observations imply that the kinase activity of Aurora B is not required for either microtubule capture by kinetochores or the maintenance of the bioriented state once it has been achieved. Rather, Aurora B function is required to correct monoorientation, as was recently proposed for Ipl1 in budding yeast (Tanaka et al., 2002). Differing conclusions have been drawn from experiments in which Aurora B was inactivated by means other than specific inhibitors (see above). As those experimental conditions interfere not only with Aurora B's kinase activity, but also with its protein level or intracellular localization (Ditchfield et al., 2003), they could reveal additional functions for Aurora B that might not require its kinase activity.
Aurora B activity is required for correcting syntelic attachments
Why does inhibiting Aurora B prevent the normal biorientation process? Our immunofluorescence analysis indicates that Hesperadin-treated Ptk1 cells have more syntelic chromosomes than control-treated cells (Fig. 7). In untreated cells, syntelic attachments occur with low frequency (Fig. 7; Roos, 1976; Ault and Rieder, 1992; Nicklas, 1997). Nicklas (1997) argued that syntelic attachment is infrequent because kinetochores are embedded in a pit, and sister kinetochores would therefore not be accessible for microtubules from the same pole. Inhibition of Aurora B could thus cause syntelic attachments indirectly by changing kinetochore structure or the spatial resolution of sister kinetochores. Indeed, we observed in Hesperadin-treated cells that the resolution of sister chromatids in early mitosis is somewhat impaired (Fig. 4). However, when we temporally separated chromatid resolution and microtubule attachment by treating cells with nocodazole, we found that inhibition of Aurora B function at the time of microtubule attachment was sufficient to cause a chromosome segregation defect (Fig. 5), indicating that the sister resolution defect cannot be the sole cause for syntelically attached chromosomes.
Instead, the number of syntelic chromosomes in Hesperadin-treated cells could result from an abnormal stabilization of naturally occurring syntelic attachments. The correction of syntelic attachment in mitosis has not been studied, but it is assumed that such a mechanism must exist (Ault and Rieder, 1992; Nicklas, 1997). Our observation that monotelically attached chromosomes in monastrol-treated cells (Kapoor et al., 2000) may be converted into syntelically attached chromosomes upon Hesperadin treatment (Fig. 9) indicates that the correction mechanism depends on Aurora B activity.
The role of Aurora B in the spindle assembly checkpoint: direct, indirect, or both?
The spindle assembly checkpoint very faithfully ensures that anaphase is not initiated before all chromosomes have achieved bipolar attachment (Rieder et al., 1994). In striking contrast, cells treated with Hesperadin readily enter anaphase in the presence of monooriented chromosomes (Fig. 6). Likewise, Hesperadin is able to override the checkpoint arrest caused by taxol and monastrol (Fig. 8), but Aurora B function is not required for several hours to maintain a checkpoint arrest induced by nocodazole (Fig. 8). This situation is reminiscent of the role of Ipl1 in budding yeast (Biggins and Murray, 2001) in that Aurora B appears to be required for checkpoint signaling in the absence of tension but not in the absence of kinetochore attachments. It is possible that Aurora B is required to directly activate checkpoint proteins in the absence of tension, independent of its role in the correction of syntelic attachment. But because the correction function is thought to be activated by the lack of tension (Ault and Rieder, 1992; Nicklas, 1997; Tanaka et al., 2002), it is also conceivable that Aurora B is indirectly required for checkpoint signaling. According to this model, Aurora B would destabilize microtubule–kinetochore interactions at kinetochores that are not under proper tension or that impinge on the kinetochore at too acute an angle, and the resulting kinetochores that are either unattached or only occupied with low numbers of microtubules would then generate the primary signal for checkpoint signaling (Fig. 9 D). Hesperadin-treated cells would enter anaphase only once all kinetochores had been fully attached, which would then abolish checkpoint signaling. Because kinetochore attachment is a stochastic process, this model predicts that cells enter anaphase at different times after NEB. Indeed, we observed a high intercell variability between NEB and the onset of anaphase in Hesperadin-treated cells (20–50 min, n = 7 on a heated microscope stage; 11–24 min, n = 23 in a warm room).
This model also fits well with the observation that cells arrested with either taxol or monastrol always contain at least one kinetochore that appears to be unattached (Waters et al., 1998; Kapoor et al., 2000). Taxol- and monastrol-treated cells may therefore be arrested by the spindle checkpoint because Aurora B maintains a dynamic equilibrium between attached and unattached kinetochores. Inhibition of Aurora B would overcome this arrest because all kinetochores would eventually become fully attached. As predicted by this model, we observed that Hesperadin addition to monastrol-treated Ptk1 cells decreased the number of kinetochores staining with Mad2, suggesting that the monotelic chromosomes that existed in monastrol-arrested cells (Kapoor et al., 2000) had been converted into syntelic chromosomes once Aurora B was inhibited.
The stabilization of improper microtubule attachments is sufficient to explain the precocious exit from mitosis that Hesperadin induces in monastrol- and taxol-treated cells. However, we found that even under conditions where none of the kinetochores are attached, Aurora B function is required to maintain checkpoint signaling over prolonged periods of time (Fig. 8 D, >3 h), indicating that it might also have a direct role in the spindle assembly checkpoint. Consistent with this notion, we found that kinetochore localization of the checkpoint kinases BubR1 and Bub1 was impaired in Hesperadin-treated cells. It is conceivable that Mad2, which is still present at kinetochores in cells treated with nocodazole and Hesperadin, is sufficient to sustain the transient mitotic delay that we observe. In contrast, the low levels of Mad2 at kinetochores in taxol-arrested cells might not be sufficient to delay cells in mitosis when BubR1 is depleted from kinetochores by Hesperadin.
In summary, our data suggest that Aurora B has a dual role. It acts in the destabilization of improper microtubule attachments, which indirectly keeps checkpoint signaling active, but it also could have a more direct role in the spindle assembly checkpoint. This is consistent with data from budding yeast, where it was found that Ipl1 is required for the spindle assembly checkpoint in a kinetochore-dependent, but probably also in a kinetochore-independent, manner (Biggins and Murray, 2001). Likewise, Aurora B antibodies have been shown to overcome a nocodazole-induced arrest both in Xenopus egg extracts and cultured cells (Kallio et al., 2002), also suggesting a direct role of Aurora B in the spindle assembly checkpoint.
BubR1 and Bub1 could be Aurora B substrates that play a role in either of these pathways, or in both. It is conceivable that BubR1 and Bub1 themselves have dual roles. Both proteins have been shown to be required for checkpoint signaling in the presence of unattached kinetochores. Interestingly, in some experimental settings, their kinase activity is not required for checkpoint function (Sharp-Baker and Chen, 2001; Chen, 2002; Warren et al., 2002), but Bub1's kinase activity is essential for a genetically separable function that may be required for microtubule–kinetochore attachments (Abruzzi et al., 2002). It will therefore be interesting to test if Bub1's and BubR1's kinase activity and their Aurora B–dependent recruitment to kinetochores are required to regulate kinetochore attachments.
Materials and methods
Cell culture and synchronization
PtK1 cells were grown in Ham's F12, and PtK2 and HeLa cells were grown in DME, both supplemented with 10% FCS, 0.2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Thymidine arrest/release was performed as previously described (Waizenegger et al., 2000). Nocodazole was added to one sample when releasing from the second thymidine arrest. Nocodazole, taxol, and monastrol were used at 330 nM, 10 μM, and 100 μM, respectively, except for the experiment in Fig. 9 where nocodazole was used at 10 μM.
Hesperadin
Hesperadin was used at 50 or 100 nM on HeLa cells and at 100–500 nM on PtK1 cells. Phospho-histone H3 staining was reduced at 20 nM and absent at 100 nM in HeLa cells, and reduced at 50 nM and absent at 250 nM in PtK2 cells (not depicted).
Antibodies
The following antibodies were used: rabbit polyclonal against Cdc27, securin (Waizenegger et al., 2000), cyclin A (Upstate Biotechnology), phospho-histone H3 (Upstate Biotechnology), Survivin (Novus Biologicals), HsCYK-4, MKLP-1 (Mishima et al., 2002), Mad2 (BAbCO), mouse monoclonal against cyclin B1 (GNS1; Santa Cruz Biotechnology, Inc.), myc (9E10), HA (12CA5), α-tubulin (B-5-1-2; Sigma-Aldrich), Aurora A (IAK1), and Aurora B (AIM-1) (BD Biosciences). Rabbit polyclonal antibodies against CENP-E, BubR1, and Bub1 were from Tim Yen (Fox Chase Cancer Center, Philadelphia, PA), and CREST serum was from A. Kromminga (IMP, Hamburg, Germany).
RNAi
RNAi was performed using siRNA as previously described (Elbashir et al., 2001). The targeted region in the human Aurora B cDNA is 5′-AAGGTGATGGAGAATAGCAGT-3′. Synthetic sense and antisense oligonucleotides were obtained from Dharmacon. Annealing of siRNA oligos was performed according to Dharmacon's instructions. For control transfections, the same annealing reaction was set up using H2O instead of siRNA oligos. Experiments performed with a second duplex targeted to a different region in human Aurora B cDNA yielded similar results.
FACS® analysis
Methanol-fixed cell samples were washed with PBS and subsequently stained in PI buffer (50 μg/ml propidium iodide, 10 mM Tris, pH 7.5, 5 mM MgCl2, 200 μg/ml RNase A) for 20–40 min at 37°C.
Immunoblotting
HeLa cells were lysed as previously described (Waizenegger et al., 2000). Proteins were resolved by SDS-PAGE and transferred to Immobilon-P membrane (Millipore). Blocking and antibody incubations were in 4% low-fat milk in TBS-T (150 mM NaCl, 20 mM Tris, pH 8.0, 0.05% Tween 20), washings were in TBS-T. Blots were developed by ECL.
Immunofluorescence
Immunostaining of HeLa cells was performed as previously described (Waizenegger et al., 2000), except that for most experiments, the incubation step with 0.1% Triton X-100 before fixation was omitted. Detailed specifications for different experiments are given in the online supplemental material (available at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1). Images were captured using MetaMorph software (Universal Imaging Corp.).
Deconvolution microscopy was performed using a widefield optical sectioning microscope (Deltavision; Applied Precision), or using Huygens Essential software (Scientific Volume Imaging) after acquisition on a Carl Zeiss MicroImaging, Inc. Axioplan 2 using MetaMorph software.
The method for measuring kinetochore fluorescence (Fig. 9) with the use of MetaMorph imaging software and primary 12-bit images was described in detail by Hoffman et al. (2001). Results were obtained from two independent experiments (Fig. 9 B, 1 and 2). In each experiment, in-focus images of 10 cells were taken for each data set. Computer-generated 10 × 10 (0.65 μm × 0.65 μm) and 14 × 14 (0.91 μm × 0.91 μm) pixel regions were centered over kinetochores to be analyzed. For each cell, the six most strongly labeled kinetochores not belonging to the same pair of sister chromatids were measured, and the average integrated intensity for the checkpoint protein was set in relation to the average integrated intensity of the CREST signal. Two-tailed t tests were performed for each data set.
Chromosome spreads
Chromosome spreading was performed as previously described (Gimenez-Abian et al., 1995), with minor modifications. In brief, cells were harvested by mitotic shake off and hypotonically swollen in 40% medium/60% Vienna tap water for 5 min at room temperature. Cells were fixed with freshly made Carnoy's solution (75% methanol, 25% acetic acid), and the fixative was changed several times. For spreading, cells in Carnoy's solution were dropped onto glass slides and dried at 37°C. Slides were stained with 5% Giemsa (Merck) at pH 6.8 for 7 min, washed briefly in tap water, air dried, and mounted with Entellan (Merck).
Live cell imaging
The video light microscopy conditions used in this study have been previously detailed (Rieder and Cole, 1998). In brief, Rose chamber cultures of actively growing PtK1 cells were treated with 500 nM Hesperadin for up to several hours before filming. The chambers were maintained at 37°C during imaging by either placing them into a heating block that was mounted on the stage of the microscope or on the stage of a microscope contained in a warm room. A prophase cell was located within the culture, and its progress was followed by either differential interference contrast or phase contrast light microscopy. The video parameters used included shuttered 546-nm light from a 100-W tungsten source, a framing rate of 4/min, and an exposure of 1 s/frame. All images were processed by Image Pro or Image 1 (Universal Imaging Corp.) and stored on the hard drive.
Online supplemental material
Supplemental figures (Figs. S1–S4) and movies (Videos 1 and 2) are available online at http://www.jcb.org/cgi/content/full/jcb.200208092/DC1. The supplemental information includes data on the inhibition of different kinases by Hesperadin (Fig. S1), kinetic information for the experiment in Fig. 5 (Fig. S2), raw data used for the quantification in Fig. 7 C (Fig. S3), and data on the localization of checkpoint proteins in logarithmically growing HeLa cells treated with Hesperadin (Fig. S4). Furthermore, movies of HeLa (Video 1) or PtK1 cells (Video 2; stills in Fig. 6) treated with Hesperadin are shown. The online supplemental material also contains detailed methods for the experiments in Figs. 2 D, 5, 7, 8, A–D, and 9.
Supplemental Material
Acknowledgments
We thank Martin Steegmaier for communicating unpublished results, Wolfgang Rettig, Peter Swetly, and Bernd Wetzel for generous support, and Juan F. Giménez-Abián, Alexey Khodjakov, Kim Nasmyth, and Michael Glotzer for stimulating discussions and comments. We also thank Hartmut Vodermaier for conversations motivating the naming of Hesperadin, Alexei Mikhailov, Tilman Voss, Horst Ahorn, and Martin Lenter for their help, Kelly Hust, Karin Paiha, and Beate Peters for excellent technical support, Tim Yen and Masanori Mishima for antibodies, and Manuel Mendoza for advice on kinase assays.
Research in the laboratory of J.-M. Peters is supported by Boehringer Ingelheim and by grants from the European Molecular Biology Organization and the Austrian Science Fund.
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: APC, anaphase-promoting complex; NEB, nuclear envelope breakdown; RNAi, RNA interference; siRNA, small inhibitory RNA.
References
- Abruzzi, K.C., M. Magendantz, and F. Solomon. 2002. An α-tubulin mutant demonstrates distinguishable functions among the spindle assembly checkpoint genes in Saccharomyces cerevisiae. Genetics. 161:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, R.R., M. Carmena, and W.C. Earnshaw. 2001. a. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11:49–54. [DOI] [PubMed] [Google Scholar]
- Adams, R.R., H. Maiato, W.C. Earnshaw, and M. Carmena. 2001. b. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153:865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault, J.G., and C.L. Rieder. 1992. Chromosome mal-orientation and reorientation during mitosis. Cell Motil. Cytoskeleton. 22:155–159. [DOI] [PubMed] [Google Scholar]
- Biggins, S., and A.W. Murray. 2001. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15:3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins, S., F.F. Severin, N. Bhalla, I. Sassoon, A.A. Hyman, and A.W. Murray. 1999. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 13:532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.H. 2002. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, S.P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield, C., V.L. Johnson, A. Tighe, R. Ellston, C. Haworth, T. Johnson, A. Mortlock, N. Keen, and S.S. Taylor. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. [DOI] [PubMed] [Google Scholar]
- Giet, R., and D.M. Glover. 2001. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Abian, J.F., D.J. Clarke, A.M. Mullinger, C.S. Downes, and R.T. Johnson. 1995. A postprophase topoisomerase II–dependent chromatid core separation step in the formation of metaphase chromosomes. J. Cell Biol. 131:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayne, C., G. Tzivion, and Z. Luo. 2000. Raf-1/MEK/MAPK pathway is necessary for the G2/M transition induced by nocodazole. J. Biol. Chem. 275:31876–31882. [DOI] [PubMed] [Google Scholar]
- Hoffman, D.B., C.G. Pearson, T.J. Yen, B.J. Howell, and E.D. Salmon. 2001. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell. 12:1995–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna, S., M. Mendoza, V. Jantsch-Plunger, and M. Glotzer. 2000. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10:1172–1181. [DOI] [PubMed] [Google Scholar]
- Kaitna, S., P. Pasierbek, M. Jantsch, J. Loidl, and M. Glotzer. 2002. The Aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 12:798–812. [DOI] [PubMed] [Google Scholar]
- Kallio, M.J., M.L. McCleland, P.T. Stukenberg, and G.J. Gorbsky. 2002. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 12:900–905. [DOI] [PubMed] [Google Scholar]
- Kanda, T., K.F. Sullivan, and G.M. Wahl. 1998. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8:377–385. [DOI] [PubMed] [Google Scholar]
- Kapoor, T.M., T.U. Mayer, M.L. Coughlin, and T.J. Mitchison. 2000. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150:975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, J.M., and R.B. Nicklas. 2000. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J. Cell Sci. 113(Pt 21):3815–3823. [DOI] [PubMed] [Google Scholar]
- Li, X., and R.B. Nicklas. 1995. Mitotic forces control a cell-cycle checkpoint. Nature. 373:630–632. [DOI] [PubMed] [Google Scholar]
- Mayer, T.U., T.M. Kapoor, S.J. Haggarty, R.W. King, S.L. Schreiber, and T.J. Mitchison. 1999. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 286:971–974. [DOI] [PubMed] [Google Scholar]
- Mishima, M., S. Kaitna, and M. Glotzer. 2002. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell. 2:41–54. [DOI] [PubMed] [Google Scholar]
- Mitchison, T., and M. Kirschner. 1984. Dynamic instability of microtubule growth. Nature. 312:237–242. [DOI] [PubMed] [Google Scholar]
- Murata-Hori, M., and Y. Wang. 2002. The kinase activity of Aurora B is required for kinetochore-microtubule interactions during mitosis. Curr. Biol. 12:894–899. [DOI] [PubMed] [Google Scholar]
- Murata-Hori, M., M. Tatsuka, and Y.L. Wang. 2002. Probing the dynamics and functions of Aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell. 13:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K. 2001. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35:673–745. [DOI] [PubMed] [Google Scholar]
- Nicklas, R.B. 1997. How cells get the right chromosomes. Science. 275:632–637. [DOI] [PubMed] [Google Scholar]
- Nicklas, R.B., J.C. Waters, E.D. Salmon, and S.C. Ward. 2001. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J. Cell Sci. 114:4173–4183. [DOI] [PubMed] [Google Scholar]
- Nigg, E.A. 2001. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2:21–32. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., and R.W. Cole. 1998. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J. Cell Biol. 142:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., and E.D. Salmon. 1998. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 8:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., E.A. Davison, L.C. Jensen, L. Cassimeris, and E.D. Salmon. 1986. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 103:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., A. Schultz, R. Cole, and G. Sluder. 1994. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127:1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., R.W. Cole, A. Khodjakov, and G. Sluder. 1995. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, U.P. 1976. Light and electron microscopy of rat kangaroo cells in mitosis. III. Patterns of chromosome behavior during prometaphase. Chromosoma. 54:363–385. [DOI] [PubMed] [Google Scholar]
- Shah, J.V., and D.W. Cleveland. 2000. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell. 103:997–1000. [DOI] [PubMed] [Google Scholar]
- Shannon, K.B., J.C. Canman, and E.D. Salmon. 2002. Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell. 13:3706–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp-Baker, H., and R.H. Chen. 2001. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 153:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias, D.A., P.R. Andreassen, F.B. Lacroix, L. Wilson, and R.L. Margolis. 2001. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA. 98:4492–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, B.M., and A.W. Murray. 2001. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 11:1462–1467. [DOI] [PubMed] [Google Scholar]
- Tanaka, T.U., N. Rachidi, C. Janke, G. Pereira, M. Galova, E. Schiebel, M.J. Stark, and K. Nasmyth. 2002. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 108:317–329. [DOI] [PubMed] [Google Scholar]
- Taylor, S.S., D. Hussein, Y. Wang, S. Elderkin, and C.J. Morrow. 2001. Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J. Cell Sci. 114:4385–4395. [DOI] [PubMed] [Google Scholar]
- Waizenegger, I.C., S. Hauf, A. Meinke, and J.M. Peters. 2000. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 103:399–410. [DOI] [PubMed] [Google Scholar]
- Walter, R., A. Heckel, G.J. Roth, J. Kley, G. Schnapp, M. Lenter, J.C.A. van Meel, W. Spevak, and U. Weyer-Czernilofsky, inventors and assignees. 2002 May 10. Sulfonylamino substituted 3-(aminomethylide)-2-indolinones as cell proliferation inhibitors. Patent Cooperation Treaty, International Publication Number WO 02/36564.
- Warren, C.D., D.M. Brady, R.C. Johnston, J.S. Hanna, K.G. Hardwick, and F.A. Spencer. 2002. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell. 13:3029–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, J.C., R.H. Chen, A.W. Murray, and E.D. Salmon. 1998. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, J.H., E. Munar, D.R. Jameson, P.R. Andreassen, R.L. Margolis, R. Seger, and E.G. Krebs. 1999. Mitogen-activated protein kinase kinase activity is required for the G(2)/M transition of the cell cycle in mammalian fibroblasts. Proc. Natl. Acad. Sci. USA. 96:11335–11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.