Abstract
Akey step in lipolytic activation of adipocytes is the translocation of hormone-sensitive lipase (HSL) from the cytosol to the surface of the lipid storage droplet. Adipocytes from perilipin-null animals have an elevated basal rate of lipolysis compared with adipocytes from wild-type mice, but fail to respond maximally to lipolytic stimuli. This defect is downstream of the β-adrenergic receptor–adenylyl cyclase complex. Now, we show that HSL is basally associated with lipid droplet surfaces at a low level in perilipin nulls, but that stimulated translocation from the cytosol to lipid droplets is absent in adipocytes derived from embryonic fibroblasts of perilipin-null mice. We have also reconstructed the HSL translocation reaction in the nonadipocyte Chinese hamster ovary cell line by introduction of GFP-tagged HSL with and without perilipin A. On activation of protein kinase A, HSL-GFP translocates to lipid droplets only in cells that express fully phosphorylatable perilipin A, confirming that perilipin is required to elicit the HSL translocation reaction. Moreover, in Chinese hamster ovary cells that express both HSL and perilipin A, these two proteins cooperate to produce a more rapidly accelerated lipolysis than do cells that express either of these proteins alone, indicating that lipolysis is a concerted reaction mediated by both protein kinase A–phosphorylated HSL and perilipin A.
Keywords: lipolysis; adipocytes; ADRP/adipophilin; HSL; lipid storage droplets
Introduction
Fatty acids stored as triacylglycerols (TAG)* in adipose cells constitute the primary energy reserves in animals. The lipolytic reaction in adipose cells governs the breakdown of TAG and the release of fatty acids that are transported in the plasma to supply energy needs to various tissues. Dysregulation of the lipolytic pathway has been one of the major hypotheses linking insulin resistance to hyperlipidemia in metabolic disease, obesity, and diabetes mellitus (Bergman and Mittelman, 1998; Reaven et al., 1988). Lipolysis is controlled primarily by cAMP-dependent protein kinase/protein kinase A (PKA). Lipolysis in primary adipose cells correlates closely with steady-state levels of PKA activation as measured in cells treated with a variety of ligands for both stimulatory and inhibitory receptors linked to adenylyl cyclase (Honnor et al., 1985).
Hormone-sensitive lipase (HSL) is an important enzyme in this PKA-activated process (Londos et al., 1999b; Holm et al., 2000), but the meager activation of HSL by PKA in vitro, usually less than twofold, cannot account for the large increases (30–100-fold) in lipolysis observed on elevation of PKA activity in mammalian adipocytes. Rather, we have proposed that PKA-mediated translocation of HSL from the cytosol to the surface of the lipid droplet after lipolytic stimulation drives lipolytic activation (Egan et al., 1992; Brasaemle et al., 1999; Londos et al., 1999a). Thus, substrate access, rather than an increase in specific activity of HSL, is likely to be the basis for the large PKA-stimulated lipolysis in cells.
An important clue to the HSL translocation process came from an analysis of the lipolytic reaction in the perilipin-null mouse (Tansey et al., 2001). Adipocytes from these animals exhibited elevated basal lipolysis, which reflects the loss of the well-established protective effect of perilipin against TAG breakdown under conditions of quiescent PKA (Souza et al., 1998; Brasaemle et al., 2000; Tansey et al., 2003). But, adipocytes lacking perilipin also exhibited a near total loss of β-adrenergic receptor–stimulated lipolysis, despite the presence of normal levels of HSL protein per cell (Tansey et al., 2001). Wild-type (wt) animals respond with the expected robust reduction in respiratory exchange ratio after injection of a β-3 adrenergic agonist (Tansey et al., 2001), whereas perilipin-null animals fail to respond to the β-3 adrenergic agonist with a lowering of their respiratory exchange ratio. Another notable feature of the perilipin-null mouse is that the lipid droplets in its adipocytes are coated with adipose differentiation–related protein (ADRP), a perilipin-related protein (Tansey et al., 2001). These data suggest that perilipin is not only required to maintain adipose cells in the quiescent state, but that perilipin is also required to elicit a functional lipolytic activation in adipose cells. To test the latter hypothesis mechanistically, we examined HSL translocation in adipocytes derived from embryonic fibroblasts of wt and perilipin-null mice, and show that HSL fails to translocate to lipid droplets in cells lacking perilipin. Further, we have introduced a normal and an unstimulatable mutant variant of perilipin A into CHO fibroblasts and examined the ability of GFP-tagged HSL to translocate after PKA activation. We show that only cells expressing native perilipin are permissive for the PKA-regulated translocation of HSL. Furthermore, CHO cells expressing both perilipin and HSL are able to mount a greater and more rapid response to a lipolytic stimulus than when either of these proteins is expressed separately.
Results
Adipocytes from perilipin-null mice exhibit a defect in lipolysis at a point downstream of cAMP generation
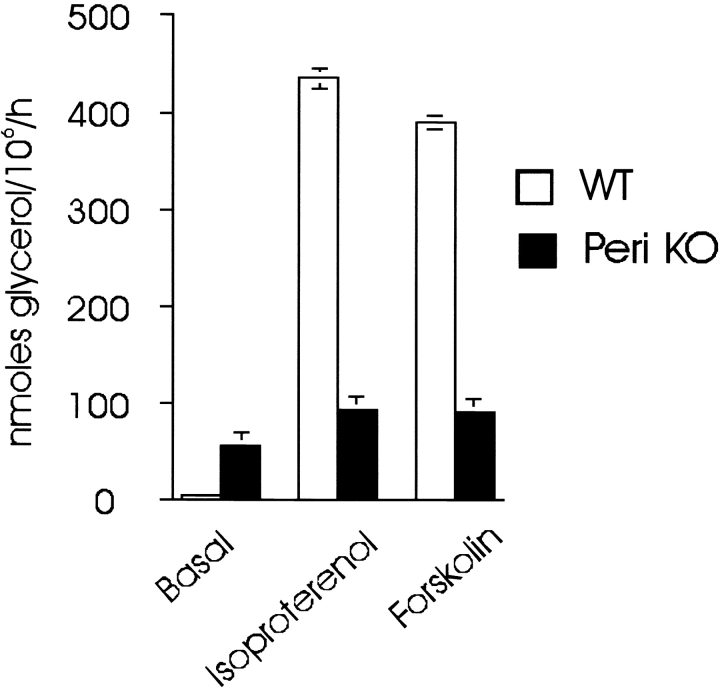
Previously, we observed two aberrations in lipolysis of adipocyte from perilipin-null mice: (1) increased basal activity; and (2) a near total loss of stimulated activity (Tansey et al., 2001). Those earlier works were performed in adipocytes from 17-wk-old mice in a mixed (50/50) 129Sv/EvTac and C57BL/6J background. To eliminate the potential for both genetic background and cell size effects, we have now studied 6-wk-old mice in a pure 129Sv/EvTac background. The results agree with those of the earlier work in that basal activity in cells from the perilipin-null mice was 10-fold greater than the wt cells, whereas isoproterenol-stimulated lipolysis in the perilipin-null cells was decreased by ∼75% (Fig. 1). Cell sizes for wt and perilipin-null adipocytes were nearly identical in these works, which eliminates the possibility that size differences contribute to their different lipolytic activities (see legend to Fig. 1).
Figure 1.
Isolated adipocytes from perilipin -null mice respond poorly to lipolytic stimuli. Incubations to measure lipolytic activity contained 1 U/ml adenosine deaminase and 100 nM PIA for the basal condition, or for stimulated conditions, 10 μM isoproterenol or 10 μM forskolin. Values represent the means ± SEM of triplicate determinations of nmol glycerol release per 106 cells in 60 min. Values are from two separate experiments in which incubations were performed in triplicate (n = 6). For all differences in basal and stimulated glycerol release between cells from control and perilipin-null mice, P < 0.001. Adipocytes from control and perilipin-null mice used in these experiments did not differ significantly in size, i.e., control cells, 61.7 ± 9.3 μm; null cells, 57.9 ± 2.2 μm (n = 100) in diameter.
To test if this reduced ability to stimulate TAG hydrolysis in adipocytes from perilipin-null mice cells represents a defect at the receptor level or downstream from the β-adrenergic receptor, we also compared the actions of the β-adrenergic agonist, isoproterenol, with those of forskolin, an activator of the adenylyl cyclase catalytic component (Tesmer et al., 1999). The results reveal that when cells are stimulated by forskolin, perilipin-null cells are also weakly responsive (Fig. 1). Thus, the impairment in maximally stimulated lipolysis is due to a defect downstream from the β-adrenergic receptor–G-protein–adenylyl cyclase complex at the plasma membrane. We have compared the lipolytic activity in homogenates of fat pads from wt and perilipin-null animals, and find no differences in their activities when normalized for the amount of HSL as determined by immunoblotting (Table I). Thus, the HSL of the null animal is of no greater specific activity than the HSL in the wt animals, and as we demonstrated previously, both wt and perilipin-null animals have equivalent amounts of HSL on a per-cell basis (Tansey et al., 2001). These results reinforce our earlier conclusion that elevated basal activity in perilipin-null cells represents the loss of protection of cellular TAG by perilipin. Accordingly, we focused on the relationship between HSL and perilipin.
Table I.
Adipose tissue of perilipin -null animal contains HSL of normal specific activity
| Animal | Wild type | perilipin-null |
|---|---|---|
| Densitometry (arbitrary units) |
16,900 | 13,000 |
| Activity (nmol/h × mg protein−1) |
686 | 518 |
| Specific activity | 25% | 25% |
Aliquots of infranatants from adipose tissue of wt and perilipin-null mice were tested for lipolytic activity as described under Materials and methods. Equivalent cellular aliquots were electrophoresed under SDS-PAGE, im-munoblotted with affinity-purified anti-HSL, and scanned with a den-sitometer. The values for densitometric scans were divided by the activity values to give specific activities, revealing that the samples from both types of animals had identical specific activities.
HSL translocation is compromised in adipocytes derived from perilipin-null embryonic fibroblasts
A key step in lipolytic activation is the translocation of HSL from the cytosol to the surface of the lipid storage droplet, a process not easily observed visually in the primary adipocytes where the cytosolic space is occupied by a large unilocular lipid droplet. As an alternative, we examined primary embryonic fibroblasts from perilipin-null and wt mice that were differentiated into adipocytes in culture; such adipocytes have a multilocular lipid droplet, and are thus more amenable to microscopic examination of HSL subcellular location. Previously, we found that the loss of perilipin in the perilipin-null mouse is accompanied by an increase in the accumulation ADRP protein in adipose cells (Tansey et al., 2001). ADRP is a ubiquitous protein found at the surface of lipid droplets in most cultured cells (Brasaemle et al., 1997) and is also present in early stages of differentiating 3T3-L1, but is excluded from mature 3T3-L1 adipocytes, where perilipin alone coats the lipid droplet (Brasaemle et al., 1997).
Adipocytes derived from embryonic fibroblasts of wt and perilipin-null mice were costained for both perilipin and ADRP (Fig. 2). In cells from wt animals, lipid droplets are uniformly coated with perilipin; a few undifferentiated cells present some small lipid droplets coated with ADRP, and very few cells were positive for both perilipin and ADRP. Perilipins are expressed most strongly in adipocytes, to a much lesser extent in steroidogenic cells, and only minimally elsewhere (Londos et al., 1999b). The expression of perilipin by the majority of wt cells indicates that these are adipocytes, and the Nomarski images show the prominent adipocyte lipid droplets. In the supplemental material, we show that these cells also express HSL and GLUT4, further confirming their adipocyte character. By contrast, all cells from perilipin-null mice do not express perilipin, but showed ADRP staining. Thus, as with the mature, primary adipose cells of the perilipin-null mice (Tansey et al., 2001), cultured cells differentiated from embryonic fibroblasts of these animals express ADRP on their lipid droplets.
Figure 2.
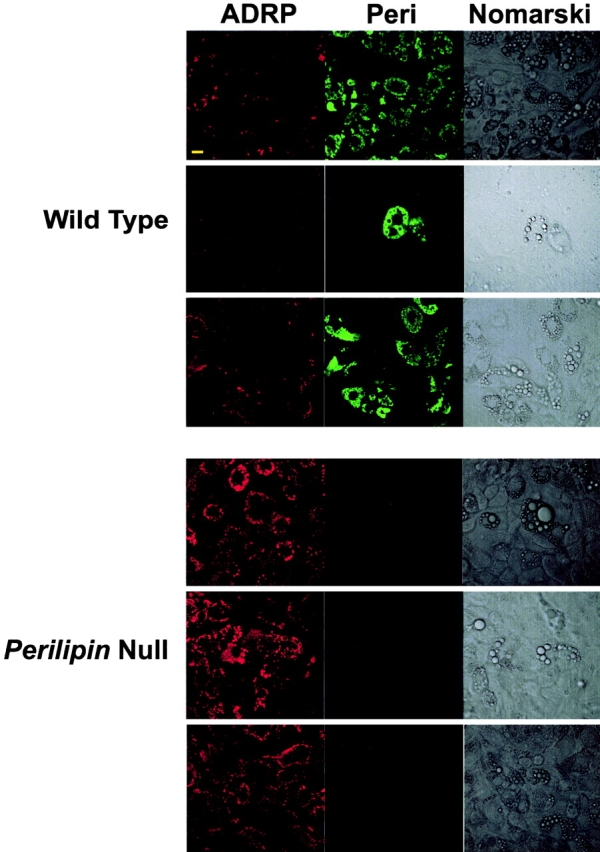
ADRP replaces perilipin on lipid droplets in embryonic fibroblasts derived from perilipin -null mice. Embryonic fibroblasts from wt and perilipin-null mice were differentiated into adipocytes and loaded with oleic acid as described under Materials and methods. The cells were fixed and coimmunostained with goat anti-perilipin and rabbit anti-ADRP antibodies (for perilipin, FITC-conjugated donkey anti–goat antibodies; and for ADRP, Cy5-conjugated donkey anti–rabbit antibodies). Top, middle, and bottom rows show representative cells from three different embryos of each phenotype. Bar, 10 μm.
To determine if HSL translocation was compromised in adipocytes of perilipin-null animals, we examined the subcellular location of HSL in the cultured adipocytes derived from the embryonic fibroblasts. As is clear from Fig. 3, the majority of both wt and perilipin-null cells expressed HSL, confirming their differentiated state. In the unstimulated state, HSL in wt cells was dispersed throughout the cytoplasm. On stimulation with isoproterenol, HSL translocation to perilipin-coated lipid droplets was readily evident by the bright, uniform rings of HSL staining around lipid droplets, and this pattern was observed in the majority of cells examined from the wt animals.
Figure 3.
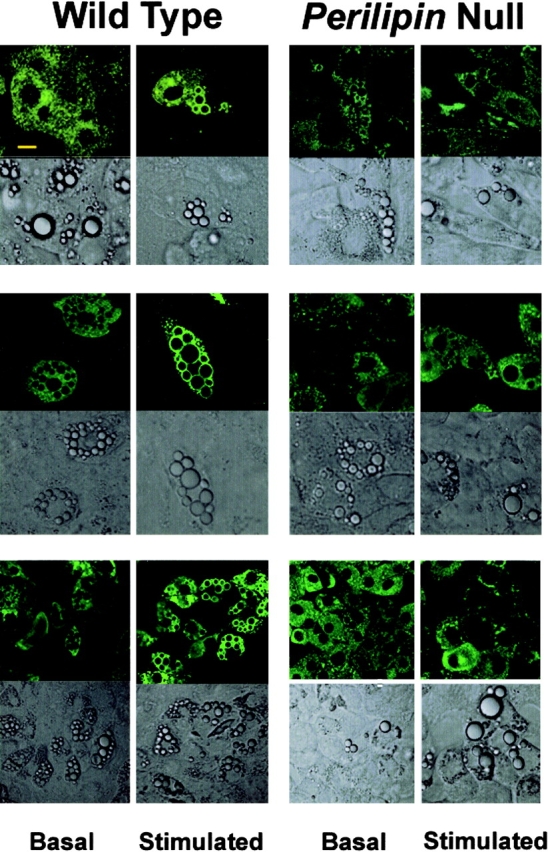
HSL fails to translocate in adipocytes differentiated from perilipin -null embryonic fibroblasts on stimulation. Embryonic fibroblasts from wt and perilipin-null mice were differentiated into adipocytes as described under Materials and methods. Basal cells were incubated with 100 nM PIA and stimulated cells for 10 min with 10 μM isoproterenol. After 10 min of stimulation, cells were fixed and immunostained with affinity-purified rabbit anti-HSL antibodies and FITC-conjugated goat anti–rabbit antibodies. Bar, 10 μm. Top, middle, and bottom rows show representative cells from three of four rounds of differentiation performed using fibroblasts derived from four different embryos of each phenotype.
HSL translocation indicated by uniform rings of staining at lipid droplet surfaces
In unstimulated perilipin-null cells, some HSL staining is associated with lipid droplets (Fig. 3). This may account for the elevated basal lipolysis seen in the absence of stimulation. By contrast, no increase in HSL association with lipid droplets was observed in any cells derived from the perilipin-null mice after stimulation with isoproterenol, indicating that perilipin is required to elicit the PKA-dependent HSL translocation reaction in adipocytes. For the data shown in Fig. 3, we performed four differentiation experiments using fibroblasts from each of 12 wt and 12 perilipin-null embryos. The fields depicted are representative of three cohorts of cells from three different embryos of each phenotype and, as evidenced by expression of HSL in most cells derived from either wt or perilipin-null embryos, the cells had differentiated into adipocytes. Unlike the cells from the wt embryos, whose adipocyte nature is strongly confirmed by their perilipin expression, the cells from the perilipin-null embryos express ADRP on their lipid droplets, similar to the finding of ADRP on lipid droplets of primary mature adipose cells from perilipin-null mice. To further confirm the adipocyte nature of the differentiated embryonic perilipin-null cells, we costained for ADRP and GLUT4, an adipocyte marker, and show that the lipid-laden, cultured adipocytes from the null cells also express the glucose transporter GLUT4 (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200210169/DC1; MacDougald and Lane, 1995). Further, we show that both differentiated wt and perilipin-null cells exhibit insulin-stimulated translocation of GLUT4 from an intracellular compartment to the plasma membrane (Fig. S2; see Online supplemental material) Thus, the increased association of HSL in wt cells with lipid storage droplets on stimulation parallels the activated lipolytic reaction (Fig. 1). Similarly, the elevated basal but attenuated stimulation of perilipin-null cells also follows the relative HSL association with lipid storage droplets in the stimulated state.
HSL-GFP translocation occurs only in CHO cells that express fully phosphorylatable perilipin A
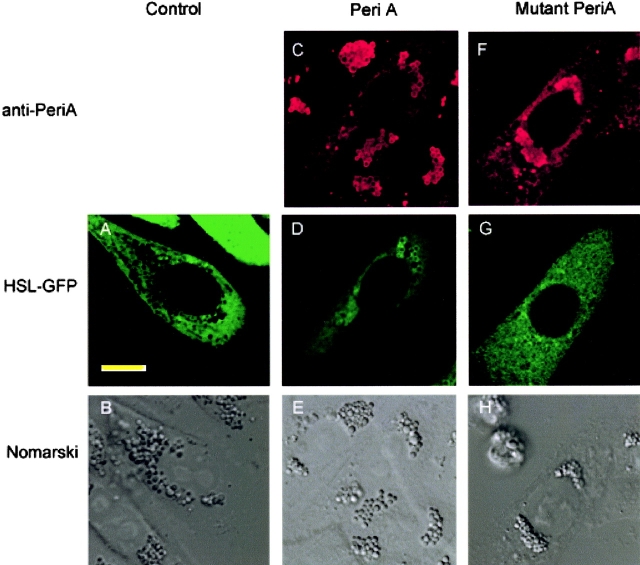
To further test the relationship between perilipin and HSL, we examined HSL translocation in nonadipogenic CHO cells. These included control CHO cells and CHO cells that were stably transfected to express either wt perilipin A or a mutated perilipin A in which the serine residues within the three most NH2-terminal PKA sites had been mutated to alanines. The metabolic characteristics of these cell lines are described in detail by Tansey et al. (2003). Control CHO cells contain lipid droplets that are coated with ADRP (Tansey et al., 2003), just as with the droplets in the adipocytes derived from embryonic fibroblasts of the perilipin-null animals. The control CHO cells and those expressing the various perilipins were then transiently transfected with a construct to express HSL-GFP. We first tested if HSL-GFP in CHO behaved similar to native HSL in adipocytes (i.e., would this species translocate to perilipin-coated lipid droplets on activation of PKA?). Indeed, on stimulation of PKA activity in these cells, HSL-GFP translocated to lipid droplets, identified by their coating of perilipin (Fig. 4, C–E). By contrast, HSL-GFP did not translocate to lipid droplets on stimulation of control cells with ADRP-coated droplets (Fig. 4, A and B), nor in CHO cells that expressed the mutated perilipin A on their droplets (Fig. 4, F–H).
Figure 4.
HSL-GFP localizes to perilipin-coated lipid droplets in stimulated CHO cells. HSL-GFP was introduced transiently into CHO cells that were stably transfected to express perilipin A. Cells were stimulated for 10 min with 10 μM forskolin plus IBMX. The cells were fixed with 3% PFA and immunostained for perilipin, shown in red. HSL is shown in green. (A and B) Control cells without perilipin but with ADRP-coated droplet. (C–E) CHO cells stably transfected with native perilipin A. (F–H) CHO cells stably transfected to express perilipin A mutated in its three most NH2-terminal PKA sites. Red staining is Cy5 staining for perilipin; green is HSL-GFP. It is evident that HSL localized to the perilipin-coated droplets. Bar, 10 μm.
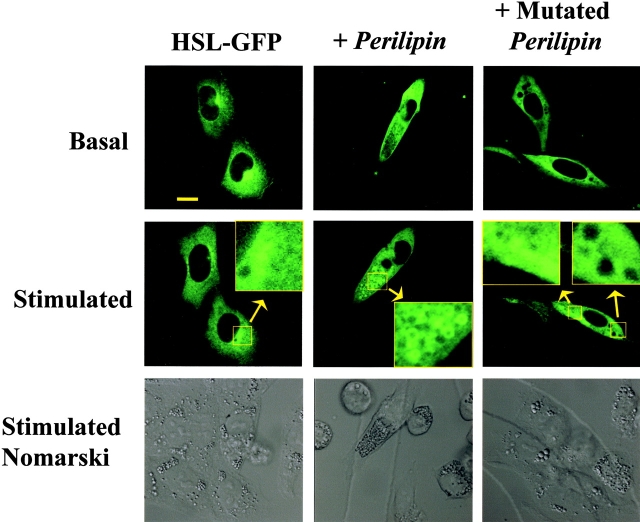
To further assess this translocation reaction in a more quantitative manner, cells were also plated on cell locator grids that permitted identification and imaging of a given cell both before and after stimulation with reagents to activate protein kinase A activity. No translocation of HSL-GFP was observed on activation of the control CHO cells that express ADRP on their lipid droplets (Fig. 5); only one of 27 cells examined showed HSL translocation on stimulation. By contrast, the majority of cells expressing native perilipin on their droplets exhibited HSL translocation on stimulation, with 24 of 36 cells showing translocation. No HSL translocation was observed in cells expressing perilipin A mutated at the three NH2-terminal PKA sites; none of the 20 cells examined exhibited translocation. The data in Fig. 4 and Fig. 5 establish that perilipin is required to elicit HSL-GFP translocation, and further, that PKA phosphorylation at selected PKA sites is necessary to support this reaction. Unlike the large lipid droplets in the adipocytes, the CHO cells contain much smaller droplets, despite having been loaded with oleic acid to enhance lipid deposition. Consequently, the images of translocated HSL in these fibroblastic cells do not show clear and distinct rings of staining as seen in the adipocytes. Additional images of translocated HSL-GFP in CHO cells are shown in Fig. S3.
Figure 5.
HSL-GFP translocates only to lipid droplet in CHO cells that are coated with fully phosphorylatable perilipin A constructs. HSL-GFP was introduced transiently into CHO cells that were either unmodified, in which case they express ADRP on their lipid droplets, or stably transfected to express perilipin A or perilipin A in which the three most NH2-terminal PKA sites were mutated on their lipid droplets. Cells expressing the perilipins have no ADRP on their lipid droplets. These cell lines are described in detail in Tansey et al. (2003). Cells were grown on a locator grid, identified, and photographed before stimulation. After 10 min of stimulation with 10 μM forskolin plus IBMX, the same cells were relocated and rephotographed. Inset for +Perilipin is enlarged 4×. Inset for +Mutated Perilipin is enlarged 6×. The cells shown in the figure were those stably transfected to express the perilipin A constructs described in Tansey et al. (2003). Additional images of HSL translocation are shown in Fig. S3.
HSL and perilipin act cooperatively to increase lipolysis in CHO cells
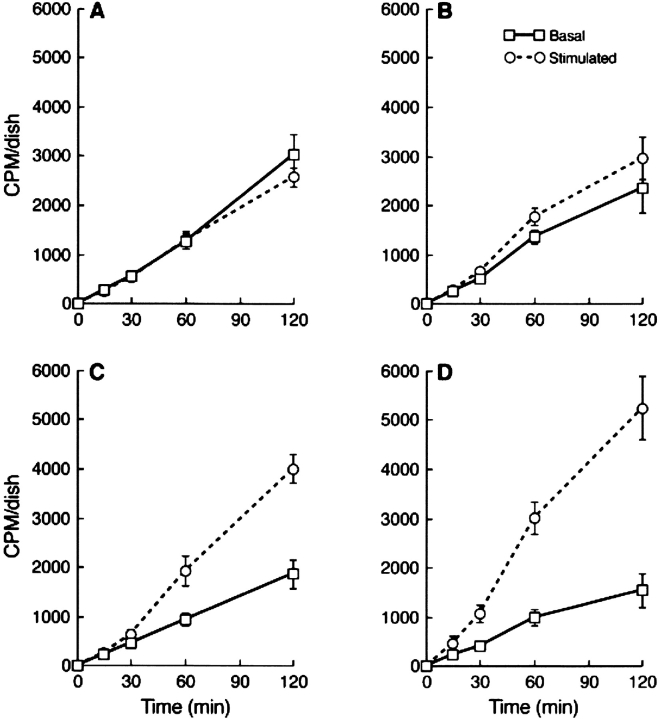
We also studied lipolysis in CHO cells that were stably transfected to express HSL-GFP at relatively high levels; total neutral lipase activities in homogenates was increased sixfold in the stably transfected cells relative to controls (176 ± 5.7 vs. 28.4 ± 1.6, n = 6) nmol fatty acid released per hour × mg protein−1. The expression of HSL-GFP and perilipin in such cells by immunoblotting is revealed in Fig. 6, which shows abundant HSL-GFP in transfected CHO cells and the absence of HSL in control CHO cells. For the metabolic works, we expressed perilipin A by adenovirus infection, in which case perilipin A expression was modestly lower than with the stably transfected cells used in the translocation works shown in Fig. 5. As we demonstrate in a separate paper, lipolysis in CHO cells can be readily assessed by loading cells with a mixture of unlabeled and radiolabeled oleic acid, and subsequently tracking the efflux of radiolabeled oleic acids to the medium (Tansey et al., 2003). Triacsin C was included to prevent re-esterification of fatty acids, and 1% fatty acid–free BSA was used to trap effluxed fatty acids. Under the conditions used herein, the released oleic acid derives solely from the pool of TGAs housed in lipid storage droplets. Lipolysis was measured both in the absence of PKA activation (basal) or in the presence of isobutylmethylxanthine (IBMX) and forskolin to elevate cAMP and activate PKA activity. Fig. 7 presents kinetics of efflux of free fatty acid in four different cell types: (1) regular CHO cells infected with Lac Z adenovirus; (2) CHO cells expressing HSL-GFP; (3) CHO cells expressing perilipin A; and finally, (4) CHO cells expressing HSL-GFP plus perilipin A. Expression of HSL-GFP alone had little effect on lipolysis, except for a modest stimulation after a 60-min incubation (Fig. 7 B). On the other hand, expression of perilipin A alone suppressed lipolysis by ∼30% in the basal state, but on stimulation, lead to increased lipolysis, which was preceded by a lag of 30 min (Fig. 7 C). However, in the presence of both perilipin A and HSL-GFP, there was greater lipolysis on stimulation than with either protein alone. This cooperativity between HSL and perilipin A largely reflects the rapid onset of stimulation when HSL is combined with perilipin A, in which case the 30-min lag was virtually eliminated (Fig. 7 D).
Figure 6.
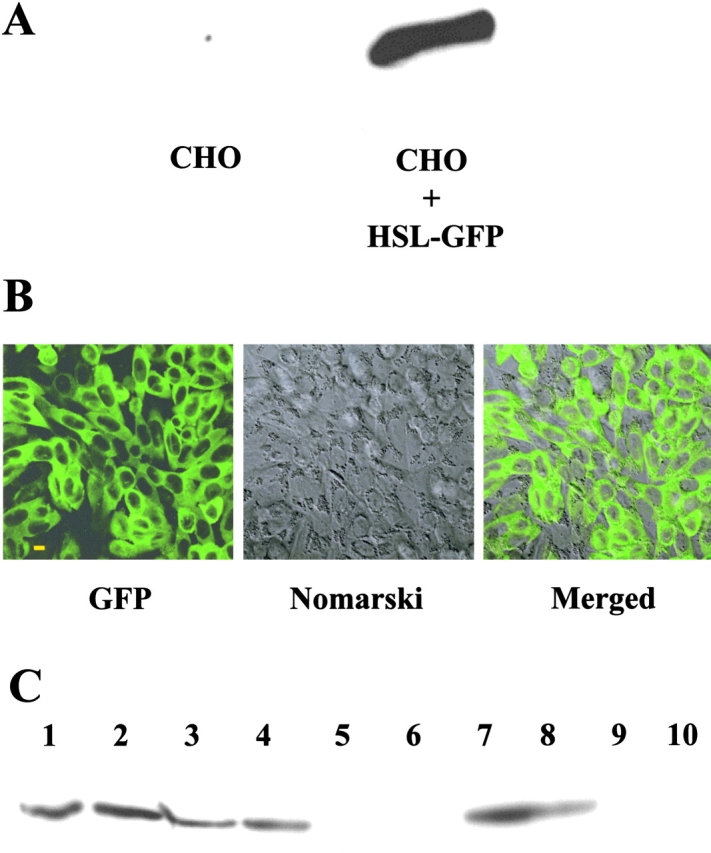
Expression of perilipin A by adenovirus infection of CHO cells also stably transfected to express HSL-GFP phenotype. (A) Immunoblotting for HSL in control CHO cells (left) and in CHO cells stably transfected to express HSL-GFP (right). (B) Immunofluorescence for HSL-GFP. CHO cells showing GFP staining, Nomarski staining, and the merged images. Bar, 10 μm. (C) Cell extracts were prepared and immunoblotted for perilipin A. Lanes 1 and 2 are cells stably transfected to express perilipin A. The GFP CHO HSL-GFP cells were further modified by infection with perilipin A adenovirus (lanes 3 and 4) or with Lac Z adenovirus (lanes 5 and 6); control CHO cells were infected with adenovirus perilipin A (lanes 7 and 8) or Lac Z adenovirus (lanes 9 and 10). Bar, 10 μm.
Figure 7.
HSL accelerates PKA-stimulated, perilipin-mediated lipolysis in CHO cells. Basal activities are shown with solid lines, and activities stimulated with IBMX and forskolin are shown in dashed lines. Note that stimulated lipolysis in CHO cells expressing perilipin A alone (B) occurs only after a lag of 30 min, during which time forskolin stimulation has no effect on fatty acid efflux from the cells. Cells not infected with adenovirus perilipin were infected with Lac Z adenovirus. (A) Control cells expressing neither perilipin nor HSL; (B) CHO cells expressing HSL-GFP; (C) CHO cells expressing perilipin A; (D) CHO cells expressing HSL-GFP plus perilipin A.
Discussion
The present paper underscores the essential relationship between perilipin and HSL during PKA-activated lipolysis. A major consequence of ablation of the perilipin gene is a loss of the ability to simulate lipolysis in isolated adipocytes (Tansey et al., 2001). This loss was also evident in the intact animal, as the perilipin-null mice failed to respond normally to an injection of a β-3 adrenergic receptor agonist (see Introduction). The present data demonstrate clearly that these defects are downstream of the receptor–G-protein–adenylyl cyclase complex at the adipocyte plasma membrane, and suggest a malfunction of the interaction of HSL with its substrate, the endogenous lipid storage droplet. Such data indicate that perilipin is required to elicit the response by HSL. The overall low adipose phenotype of the perilipin-null mouse likely results from chronically elevated basal lipolysis in adipose cells. Thus, the perilipin-null mouse serves to highlight two major actions of perilipin: (1) to protect cellular TAGs against hydrolysis in the unstimulated, basal state and thus permit TAG storage; and (2) to facilitate HSL translocation to lipid droplets and hydrolysis of TAGs in the stimulated state.
This latter role of perilipin is firmly established by data showing that HSL fails to translocate to lipid droplets in adipocytes differentiated from cultured embryonic fibroblasts of perilipin-null mice. Moreover, this translocation process may not require factors unique to adipocytes because it can be replicated by expressing both perilipin A and HSL in CHO fibroblasts. Also, phosphorylation of perilipin A at its three most NH2-terminal PKA sites is required to induce the HSL to translocate to lipid droplets. Previously, we had also shown that perilipin expression alone in CHO cells renders lipolysis in these cells strongly susceptible to activation by PKA, a phenomenon also eliminated by mutation of the same three NH2-terminal PKA sites (Tansey et al., 2003).
The reconstituted CHO cell system also made it possible to examine the functional consequences of simultaneously expressing both perilipin A and HSL, in which case HSL alone had little effect on lipolysis, whereas perilipin A alone mediated a much stronger effect on stimulated lipolysis. Addition of HSL to the perilipin A cell leads to cooperative response, largely the result of a temporally accelerated lipolytic response. Lipolysis in CHO cells without ectopic HSL must result from the action of some unknown neutral lipid lipase, which is apparently not HSL because we could find no PKA-stimulated lipase in homogenates of these cells, nor could we detect HSL by immunoblotting. Moreover, there is also a very strong suppression by perilipin A of lipolysis mediated by the endogenous neutral lipase of CHO cells (Tansey et al., 2003), and phosphorylation of the three NH2-terminal PKA sites on perilipin permits interaction of this lipase with the lipid within the core of the droplets. Thus, perilipin regulates the interactions of not only HSL with lipid droplets, but with other lipases as well (Souza et al., 2002). Clearly, the protein coating on lipid droplets is important for lipolysis and, indeed, the lipid droplet–associated proteins reside at the limiting surface of endogenous lipid droplets, the necessary site action of neutral lipid lipases. Droplets coated with ADRP neither facilitate HSL binding to the lipid droplet surface nor respond to elevated PKA activity, whereas droplets coated with perilipin A support both reactions. The accelerated lipolytic activity evident with perilipin A–coated droplets may result from increased accessibility of the lipase to TAG within droplets when perilipin is phosphorylated at its three most NH2-terminal PKA sites.
Recent works reveal that HSL contains three sites for PKA phosphorylation (ser563, 659, and 660) (Anthonsen et al., 1998), and mutational analysis has shown that ser659 and ser660 are responsible for the modest PKA-mediated activation of HSL. In a separate work, we show that HSL translocation proceeds normally when either of these two sites is mutated singly, but is abolished on simultaneous mutation of both sites (unpublished data). We consider it likely that the PKA-mediated translocation of HSL from the cytosol to the surface of lipid droplets is the basis for the large cellular response to lipolytic stimuli and to elevated cAMP (Egan et al., 1992; Brasaemle et al., 1999; Londos et al., 1999a). The demonstration that phosphorylation of HSL at selected PKA sites is required to effect translocation of the enzyme highlights the fact that HSL translocation is not merely secondary to phosphorylation of perilipin (unpublished data).
Initially, we proposed the HSL translocation hypothesis to explain the large discrepancy between the consequences of in vitro and in vivo phosphorylation of HSL on lipolysis, the latter being far greater than the former (Egan et al., 1992). The large stimulated cellular response is selectively lost in adipocytes of the perilipin-null mouse, and the data in the present paper demonstrate that perilipin is required to obtain PKA-mediated translocation of HSL to lipid droplets. The visualization of translocated HSL is a reflection of an increased concentration of the enzyme at the lipid droplet surface, which is clearly facilitated by perilipin A. Moreover, we demonstrate that perilipin must be fully phosphorylatable to carry out this function. Thus, perilipin is key to the PKA-dependent response in adipocytes primarily through its ability to induce phosphorylated HSL to translocate from the cytosol to the lipid droplet surface, and the data largely validate our translocation hypothesis. Importantly, the binding of HSL to the lipid droplet reflects the interaction of the enzyme with its TAG substrate because mutation of the catalytic site serine also eliminates the translocation reaction (unpublished data).
Two hypotheses are suggested by the current data on the adipocyte lipolytic reaction. Phosphorylated perilipin might directly recruit HSL to the lipid droplet surface by interacting with the enzyme. Alternatively, phosphorylated perilipin may modify the lipid droplet surface to indirectly facilitate interaction of HSL with the core TAG within the droplet. HSL translocation to the lipid droplet occurs rapidly, within 5 min (Brasaemle et al., 1999), and the initiation of HSL-stimulated lipolysis also occurs within this rapid time frame, as noted in Fig. 7 (Brasaemle et al., 1999). Initially, HSL and perilipin are colocalized on lipid droplets, but the perilipin slowly departs from large lipid droplets and is found on dispersed, much smaller droplets, as we noted previously (Londos et al., 1999b). This latter event occurs over a much slower time-course >30 min (unpublished data). Yet, even after the departure of perilipin, HSL remains bound to larger lipid droplets, now devoid of perilipin, where it continues to catalyze the hydrolysis of TAG (unpublished data). We cannot preclude the possibility of a rapid HSL–perilipin interaction in the initial docking of HSL on the lipid droplet, but maintenance of the lipolytic response occurs when the HSL and perilipin are found in separate subcellular compartments. Again, it should be emphasized that phosphorylation of perilipin facilitates the interaction of lipases other than HSL with TAG because it clearly fosters hydrolysis mediated by the endogenous neutral lipid lipase of CHO cells, which is neither HSL nor a substrate for PKA. Such data favor the “indirect” hypothesis posed in the online supplemental material. However, the two hypotheses are not mutually exclusive because both may apply to the lipolytic reaction. The works presented herein represent the initial demonstration of the basis for the interaction of HSL with its endogenous substrate, the intracellular lipid storage droplet, and provide a basis for further defining the lipolytic reaction in adipocytes, and structural information on the perilipin at the lipid droplet is required for a more definitive description of the role of this protein.
It is clear that HSL and perilipin constitute the core of the lipolytic reaction. Nonetheless, other factors in addition to HSL and perilipin may contribute to the lipolytic response. Adipocyte FABP is a potential candidate because the introduction of this fatty acid binding protein together with HSL enhances lipolysis, presumably by capturing and immobilizing the fatty acid products that may inhibit the lipase (Shen et al., 2001).
Materials and methods
Animals
The generation of mice with a targeted disruption of the perilipin gene has been previously reported (Tansey et al., 2001) in mice with a mixed 129Sv/EvTac/C57/B6 background. For the present works, we used perilipin-null and wt mice that had been generated a pure 129Sv/EvTac background. Mice were genotyped by PCR analysis of tail DNA as described previously (Tansey et al., 2001).
Antibodies
Antibodies used include the following: anti-PAT, which was raised in rabbits against the NH2-terminal ∼100 aa of murine perilipin A; anti-COT, which was raised in goats against the COOH-terminal ∼100 aa of murine perilipin A; and anti-ADRP, which was raised in rabbits against a peptide composed of the NH2-terminal 26 aa of murine ADRP.
Adipocyte isolation and lipolysis
Adipocytes were isolated from epididymal fat pads by collagenase digestion according to the Rodbell method as modified by Honnor et al. (1985) in solutions containing 500 nM adenosine. All incubations to measure lipolytic activity in primary adipocytes contained 1 U/ml adenosine deaminase plus 100 nM N6-phenylisopropyladenosine (PIA) for basal activity, or plus 10 μM isoproterenol or 1 μM forskolin for stimulated activity. Incubations were performed at 37°C for 60 min; glycerol released to the medium was determined by radiometric assay in microtiter plates, as described by Brasaemle et al. (1999). Data are normalized to cell number; cells were counted according to Fine and Di Giralamo (1997). Cell sizes were calculated using diameter measurements obtained with a confocal microscope (model LSM5; Carl Zeiss MicroImaging, Inc.). Total neutral lipids were measured gravimetrically, and cell numbers were calculated according to Fine and Di Giralamo (1997).
Cell culture
Cultured adipocytes from embryonic fibroblasts from wt and perilipin-null animals were developed according to Todaro and Green (1963) and Sethi et al. (2000). 16-d-old embryos were removed from pregnant mice of perilipin-null and wt animals (in a 129Sv/EvTac background), and after removal of heads and all internal organs, the embryos were digested with trypsin overnight, and cells were plated in T-175 flasks and incubated overnight. The cells were trypsinized and replated at high density (0.5 × 106 cells) on Petri dishes containing glass bottoms (MatTek Corporation). Cells were grown in the presence of DME (GIBCO BRL) supplemented with 10% FCS (HyClone), 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM glutamine (Biofluids, Inc.), and 1 mM pyruvic acid (Sigma-Aldrich). When cells reached confluence, differentiation was induced as described by Sethi et al. (2000) with 0.5 mM IBMX, 5 μg/ml insulin, and 1 μM ciglitizone (Biomol) for 4 d, after which cells were cultured for an additional 2 d with insulin and ciglitizone. Cells were then maintained at 500 ng/ml insulin plus 400 μM oleic acid complexed to 2% fatty acid–free BSA. Cultures were terminated at 10 d and used for immunocytochemistry. To examine HSL translocation, cells were incubated in Krebs Ringer Hepes, pH 7.4, 2 mM glucose, and 2% fatty acid–free BSA, and were treated for 1 h with either 200 nM PIA (basal) or 1 μM isoproterenol (stimulated).
CHO cell culture
CHO-K1 cells were cultured in Ham's F-12 medium supplemented with 10% FCS (HyClone), 100 U/ml penicillin, 100 μg/ml steptomycin, and 2 mM glutamine (Biofluids, Inc.). Clones of CHO-K1 cells overexpressing HSL-GFP were selected in the presence of 600 μg/ml G418. CHO cells overexpressing perilipins were cultured as described previously (Tansey et al., 2003). For immunofluorescence experiments, cells were plated on Petri dishes containing glass bottoms (MatTek Corporation). For cellular lipolysis studies, cells were plated in 24-multi-well dishes. All cells were cultured in a 5% CO2 atmosphere at 37°C.
Development, purification, and infection of recombinant adenovirus
The recombinant adenovirus-expressing murine perilipin A was constructed by using the method of homologous recombination in Escherichia coli described by He et al. (1998). In brief, the 1.9-kb KpnI-XbaI fragment of full-length mouse perilipin A cDNA in the pBlueScript® vector was subcloned into a viral shuttle vector pAdTrack-CMV (Stratagene), and, as control, a Lac Z fragment from plasmid pSVβgal (Promega) was used. The resulting plasmids were linearized by the PmeI restriction enzyme and cotransformed into E. coli BJ5183 competent cells, together with an adenoviral backbone plasmid pAdEasy™-1 (Stratagene). The replication-deficient recombinant adenoviral genome was created in E. coli. BJ5183 by in vivo homologous recombinant machinery. Positive clones containing the viral genome were identified, and the recombinant adenoviral genomic DNA was extracted and linearized with PacI restriction enzyme to remove unnecessary vector DNA. The linear adenovirus genomic DNA was transfected into 293 cells (American Type Culture Collection) grown in T-75 cm2 flasks to allow for packaging and amplification of the recombinant adenovirus. After transfection, the cells were incubated for ∼1 wk until cytopathic effect appeared and adenovirus was released by three or four cycles of freezing and thawing of the 293 cells, which were pelleted and resuspended in 2 ml of culture medium or PBS buffer. The cell lysate was clarified by centrifugation for 10 min at 5,000 g to remove cell debris, and the crude viron-containing supernatant was used to amplify the adenovirus by reinfecting fresh 293 cells at 90% confluence for 3 h with the crude viral mixture, after which fresh medium was added to the cells. This amplifying procedure was repeated for 3–5 rounds until the titer of the virus reached relative high levels in the crude cell lysate.
For the large-scale preparation of adenovirus, viruses were infected and amplified in 293 cells in 20–30 T-150 cm2 flasks for 3–5 d until >50% cells rounded up or floated. Then, the cells were harvested and lysed in 10 ml of 15% glycerol-PBS (pH 7.4) buffer by four cycles of freezing and thawing, and centrifuged at 5,000 g for 20 min to remove cells debris. 5.5 g CsCl were dissolved in 10 ml of the viral lysate to produce ∼11.5 ml of a CsCl solution at a density of 1.35 g/ml. This solution was centrifuged at 32,000 rpm for 20 h at 10°C in a rotor (SW41Ti; Beckman Coulter), and the white viral bands were collected by syringe in 0.5–1 ml volume by puncturing the side of the tube with a 16-G needle. The harvested virus was dialyzed against a large volume of 15% glycerol-PBS (pH 7.4) buffer at 4°C for 20 h with four changes of the fresh dialysis buffer. The titer of the adenovirus was determined by a limiting dilution plaque assay using 293 cells in 24-well plates, and a viral titer of ∼1011–1012 plaque-forming units/ml was obtained for use as a stock solution and frozen in aliquots at −80°C. When adenovirus was used to infect the cells in the experiments, the viral infection mixture was prepared in a small volume by directly adding adenovirus stock in the culture medium at a dose of multiplicity of infection of 50–100 plaque-forming units per cell. The cells were incubated with the viral mixture for 3 h, the mixture was removed, and fresh culture medium was added.
Transfection and infections
For transfection experiments, cells were transfected in the presence of LipofectAMINE™ Plus (GIBCO BRL), using amounts of DNA and LipofectAMINE™ according to the manufacturer's recommendations. For transient transfections of CHO cells with HSL, EGFP (from pEGFP) was fused in-frame to the carboxyl terminus of HSL by PCR. The HSL–EGFP fusion was subcloned, and DNA sequencing confirmed the precision of the in-frame fusion and the amino acid sequences of both HSL and GFP. For HSL translocation experiments, CHO cell recipients of HSL-GFP included control cells that contain ADRP on their lipid droplets or retrovirally modified, G418-resistant cells that expressed either native perilipin A or perilipin A in which the serine residues in the three most NH2-terminal protein kinase A sites had been mutated to alanine residues. These mutations have been shown to render perilipin A nonfunctional with respect to its ability to mediate lipolysis (Tansey et al., 2003). To examine the effects of exogenous HSL on lipolysis in CHO cells, clonal selection of HSL-EGFP cells was performed in the presence of G418 to obtain cells that expressed high levels of HSL; these cells were subsequently infected with adenovirus to introduce perilipin A.
Immunocytochemistry and Western blotting
For perilipin, ADRP, and HSL staining, cells were fixed in 3% PFA for 15 min at RT. After a brief rinse in PBS (pH 7.4), cells were blocked before staining with donkey chromopure IgG or goat chromopure IgG in presence of 0.1% of saponin for 2 h at RT. Double-labeled immunostainings were performed overnight at 4°C with appropriate antibodies. Primary antibodies were used in different combinations and dilutions as follows: 1:500 for rabbit anti-murine ADRP, 1:500 goat anti-murine perilipin (COT), 1:5 affinity-purified rabbit anti–rat HSL antibody. After four washes with PBS with 0.1% saponin, cells were incubated for 1 h with appropriate secondary antibodies; FITC-conjugated donkey anti–rabbit (1:200), cy5-conjugated donkey anti–goat (1:500). Cells were viewed with a confocal laser microscope (LSM510; Carl Zeiss MicroImaging, Inc.) using a 63× oil objective lens.
Translocation of HSL in CHO cells was assessed by plating cells on glass bottom dishes equipped with a cell locator grid (MatTek Corporation), and HSL-GFP movement was observed by confocal laser microscopy using a 63× water objective lens. Cells transiently expressing HSL-GFP were identified and photographed in the basal, unstimulated condition, were relocated, and were photographed 10 min after stimulation with IBMX and forskolin. Cellular extracts for immunoblotting were obtained by scraping cells in Laemmli sample buffer containing 5% SDS and 1 mM DTT. Each lane of SDS-PAGE gels was loaded with protein derived from a single well of a 24-multi-well dish.
In vitro HSL activity and cellular lipolysis
In vitro lipase activity was measured in cell homogenates by testing the hydrolysis of a phospholipid-stabilized emulsion of [3H]triolein (Amersham Biosciences) according to Holm et al. (1997). All analyses were performed in triplicate. Total protein in the homogenates was measured by the Pierce protein assay (Pierce Chemical Co.).
Cellular lipolysis in CHO cells
Lipolysis in CHO cells was performed according to Tansey et al. (2003) with minor modifications. Cells were incubated with 1 ml Ham's F12 medium in 24-multi-well plates, loaded overnight with 400 μM oleic acid plus 0.4 μCi/well [3H]oleic acid (NEN Life Science Products), and were maintained in a 37°C incubator with a 5% CO2 atmosphere. After loading, cells were washed three times with sterile PBS (pH 7.4) with medium supplemented with 4% de-fatted BSA to remove excess oleic acid, and the egress of [3H]oleic acid from the cells was followed by taking 50-μl aliquots of the medium over a 2-h period in medium further supplemented with 2.5 μM triacsin C to prevent re-esterification of released fatty acid. For stimulation of PKA activity, the medium was supplemented with 10 μM forskolin and 1 mM IBMX (Tansey et al., 2003). Triplicate wells were tested for each condition.
HSL-specific enzyme activity
Adipocytes were isolated from fat pads from wt and perilipin-null animals and homogenized according to Holm et al. (1997). The infranatant was removed from beneath the floating fat and aliquots from each preparation were assayed for enzymatic activity according to Holm et al. (1997) and immunoblotted with rabbit anti-HSL antibodies.
Online supplemental material
Fig. S1 shows that the adipocytes differentiated from both wt and perilipin-null fibroblasts, and are adipocytes by the criterion of GLUT4 expression (GLUT4 antibodies were a gift from Dr. Samuel W. Cushman, National Institutes of Health, Bethesda, MD). The adipocytes from the perilipin-null animals have ADRP on their lipid droplets, in contrast to those from the wt mice, which have perilipin on their droplets. Fig. S2 shows insulin-stimulated translocation of GLUT4 in adipocytes derived from wt and perilipin-null fibroblasts. The adipocytes from both wt and perilipin-null mice respond to insulin by translocating GLUT4 from an intracellular compartment to the plasma membrane. Fig. S3 shows the translocation of HSL-GFP to perilipin-coated lipid droplets in CHO cells expressing perilipin A on their lipid droplets after stimulation with IBMX and forskolin. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb. 200210169/DC1.
Supplemental Material
Acknowledgments
We thank Ms. Jai-Wei Gan, Ms. Nicole Richman, and Drs. Asmah Amleh and Joseph Brzostowski for valuable technical advice, and Dr. Samuel W. Cushman for careful review of the manuscript.
This work was supported, in part, by a grant to J.A. Contreras from the Swedish Research Council project 13010.
C. Sztalryd and G. Xu contributed equally to this paper.
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: ADRP, adipose differentiation–related protein; HSL, hormone-sensitive lipase; IBMX, isobutylmethylxanthine; PIA, N6-phenylisopropyladenosine; PKA, cAMP-dependent protein kinase; TAG, triacylglycerols; wt, wild type.
References
- Anthonsen, M.W., L. Ronnstrand, C. Wernstedt, E. Degerman, and C. Holm. 1998. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 273:215–221. [DOI] [PubMed] [Google Scholar]
- Bergman, R.N., and S.D. Mittelman. 1998. Central role of the adipocyte in insulin resistance. J. Basic Clin. Physiol. Pharmacol. 9:205–221. [DOI] [PubMed] [Google Scholar]
- Brasaemle, D.L., T. Barber, N.E. Wolins, G. Serrero, E.J. Blanchette-Mackie, and C. Londos. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38:2249–2263. [PubMed] [Google Scholar]
- Brasaemle, D.L., D.M. Levin, D.C. Adler-Wailes, and C. Londos. 1999. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of cytosolic hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim. Biophys. Acta. 1483:251–262. [DOI] [PubMed] [Google Scholar]
- Brasaemle, D.L., B. Rubin, I.A. Harten, J. Gruia-Gray, A.R. Kimmel, and C. Londos. 2000. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 275:38486–38493. [DOI] [PubMed] [Google Scholar]
- Egan, J.J., A.S. Greenberg, M.K. Chang, S.A. Wek, M.C. Moos, Jr., and C. Londos. 1992. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. USA. 89:8537–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine, J.B., and M. Di Giralamo. 1997. A simple method to predict cellular density in adipocyte metabolic incubations. Int. J. Obes. Relat. Metab. Disord. 21:764–768. [DOI] [PubMed] [Google Scholar]
- He, T.C., S. Zhou, L.T. da Costa, J. Yu, K.W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, C., J.A. Contreras, R. Verger, and M.C. Schotz. 1997. Large-scale purification and kinetic properties of recombinant hormone-sensitive lipase from baculovirus-insect cell systems. Methods Enzymol. 284:272–284. [DOI] [PubMed] [Google Scholar]
- Holm, C., T. Osterlund, H. Laurell, and J.A. Contreras. 2000. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu. Rev. Nutr. 20:365–393. [DOI] [PubMed] [Google Scholar]
- Honnor, R.C., G.S. Dhillon, and C. Londos. 1985. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J. Biol. Chem. 260:15130–15138. [PubMed] [Google Scholar]
- Londos, C., D.L. Brasaemle, C.J. Schultz, D.C. Adler-Wailes, D.M. Levin, A.R. Kimmel, and C.M. Rondinone. 1999. a. On the control of lipolysis in adipocytes. Ann. NY Acad. Sci. 892:155–168 [DOI] [PubMed] [Google Scholar]
- Londos, C., D.L. Brasaemle, C.J. Schultz, J.P. Segrest, and A.R. Kimmel. 1999. b. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 10:51–58. [DOI] [PubMed] [Google Scholar]
- MacDougald, O.A., and M.D. Lane. 1995. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 64:345–373. [DOI] [PubMed] [Google Scholar]
- Reaven, G.M., H. Chang, H. Ho, C.Y. Jeng, and B.B. Hoffman. 1988. Lowering of plasma glucose in diabetic rats by antilipolytic agents. Am. J. Physiol. 254:E23–E30. [DOI] [PubMed] [Google Scholar]
- Sethi, J.K., H. Xu, K.T. Uysal, S.M. Wiesbrock, L. Scheja, and G.S. Hotamisligil. 2000. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 469:77–82. [DOI] [PubMed] [Google Scholar]
- Shen, W.J., Y. Liang, R. Hong, S. Patel, V. Natu, K. Sridhar, A. Jenkins, D.A. Bernlohr, and F.B. Kraemer. 2001. Characterization of the functional interaction of adipocyte lipid-binding protein with hormone-sensitive lipase. J. Biol. Chem. 276:49443–49448. [DOI] [PubMed] [Google Scholar]
- Souza, S.C., L.M. de Vargas, M.T. Yamamoto, P. Lien, M.D. Franciosa, L.G. Moss, and A.S. Greenberg. 1998. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 273:24665–24669. [DOI] [PubMed] [Google Scholar]
- Souza, S.C., K.V. Muliro, L. Liscum, P. Lien, M.T. Yamamoto, J.E. Schaffer, G.E. Dallal, X. Wang, F.B. Kraemer, M. Obin, and A.S. Greenberg. 2002. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J. Biol. Chem. 277:8267–8272. [DOI] [PubMed] [Google Scholar]
- Tansey, J.T., C. Sztalryd, J. Gruia-Gray, D.L. Roush, J.V. Zee, O. Gavrilova, M.L. Reitman, C.X. Deng, C. Li, A.R. Kimmel, and C. Londos. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA. 98:6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey, J.T., A.M. Huml, R. Vogt, K.E. Davis, J.M. Jones, K.A. Fraser, D.L. Brasaemle, A.R. Kimmel, and C. Londos. 2003. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 278:8401–8406. [DOI] [PubMed] [Google Scholar]
- Tesmer, J.J., R.K. Sunahara, R.A. Johnson, G. Gosselin, A.G. Gilman, and S.R. Sprang. 1999. Two-metal-Ion catalysis in adenylyl cyclase. Science 285:756-760. [DOI] [PubMed] [Google Scholar]
- Todaro, G.J., and H. Green. 1963. Quantitative studies on the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.