Abstract
The phenotype of hematopoietic cells transformed by the BCR/ABL oncoprotein of the Philadelphia chromosome is characterized by growth factor-independent proliferation, reduced susceptibility to apoptosis, and altered adhesion and motility. The mechanisms underlying this phenotype are not fully understood, but there is evidence that some of the properties of BCR/ABL-expressing cells are dependent on the activation of downstream effector molecules such as RAS, PI-3k, and bcl-2. We show here that the small GTP-binding protein Rac is activated by BCR/ABL in a tyrosine kinase-dependent manner. Upon transfection with a vector carrying the dominant-negative N17Rac, BCR/ABL-expressing myeloid precursor 32Dcl3 cells retained the resistance to growth factor deprivation-induced apoptosis but showed a decrease in proliferative potential in the absence of interleukin-3 (IL-3) and markedly reduced invasive properties. Moreover, compared with BCR/ABL-expressing cells, fewer BCR/ABL plus N17Rac double transfectants were capable of homing to bone marrow and spleen. Consistent with these findings, survival of SCID mice injected with the BCR/ABL plus N17Rac double transfectants was markedly prolonged as compared with that of mice injected with BCR/ABL-expressing cells. Together, these data support the important role of a Rac-dependent pathway(s) controlling motility in BCR/ABL-mediated leukemogenesis.
Bcr/abl is a chimeric oncogene generated from a chromosomal translocation between chromosomes 9 and 22 (Philadelphia chromosome), which juxtaposes sequences from the bcr gene with sequences upstream of the second exon of c-abl. Bcr/abl genes encode two proteins: p185, which contains a smaller BCR portion (amino acids 1 to 426) and is associated with acute lymphocytic leukemia (1); and p210, which contains a larger BCR portion (amino acids 1–902 or 926) and is found in most cases of chronic myelogenous leukemia (2). Both BCR/ABL chimeric proteins are constitutively active tyrosine kinases that can transform fibroblasts and hematopoietic cells in culture (3–5) and induce leukemia in mice (6, 7). BCR/ABL-dependent transformation of hematopoietic cells is associated with the activation of several downstream effector molecules (8), some of which appear to be essential for leukemogenesis both in vitro and in vivo. For example, BCR/ABL-dependent leukemogenesis is suppressed when antisense strategies, expression of proteins with dominant-negative activity, or specific chemical inhibitors are used to inhibit the function of RAS, RAF, PI-3k, Akt, and bcl-2 (9–13). Suppression of the activity of these BCR/ABL-regulated molecules is associated with inhibition of proliferation and enhanced susceptibility to apoptosis. In addition to growth factor-independent proliferation and reduced susceptibility to apoptosis, the phenotype of BCR/ABL-expressing cells is characterized also by altered trafficking, which may reflect defects in cell–cell communication and cell–extracellular matrix interactions (14, 15). Specific downstream effectors might regulate the trafficking of BCR/ABL-expressing cells.
In this study, we show that the activity of Rac, a small G protein recently shown to function as a regulator of the integrin-dependent motility and invasiveness of epithelial tumor cells (16, 17), is enhanced in 32Dcl3 growth factor-dependent myeloid precursor cells ectopically expressing wild-type BCR/ABL. Upon transfection with a dominant-negative Rac, these cells exhibit a marked suppression of invasive properties, while proliferation was only marginally affected and there were no changes in apoptosis susceptibility. In vivo, the leukemogenic potential of 32Dcl3 cells coexpressing wild-type BCR/ABL and dominant-negative Rac was markedly impaired and correlated with a reduced ability of these cells to home to bone marrow and spleen of mice.
Together, these studies support the essential role of RAC as a regulator of BCR/ABL-dependent leukemogenesis by mechanisms that promote enhanced motility and invasiveness.
MATERIALS AND METHODS
Retroviral Constructs.
The p210 BCR/ABLbcr exon 3/abl exon 2 cDNA was cloned into the EcoRI–HindIII sites of the pSRaMSVtkneo retroviral vector (12). In this vector, the BCR/ABL cDNA is under the control of the long terminal repeat of the murine sarcoma virus (MSV), whereas the neomycin resistance gene is driven by the herpes simplex thymidine kinase (tk) promoter.
The N17Rac and V12Rac cDNAs were obtained from C. J. Der (University of North Carolina, Chapel Hill, NC) and L. Van Aelst (Cold Spring Harbor Laboratory), respectively. Both mutants were cloned into the BamHI site of the LXSP retroviral vector. In the LXSP vector, the Rac cDNA is under the control of the long terminal repeat of the Moloney murine leukemia virus, whereas the puromycin resistance gene is driven by the simian virus 40 (SV40) early promoter.
32Dcl3 Cell Transfection.
N17Rac was electroporated into p210BCR/ABL-32Dcl3 murine myeloid precursor cells (12) grown in Iscove’s modified Dulbecco medium (IMDM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin/streptomycin (100 μg/ml each), and 15% WEHI-conditioned medium (WEHI-CM) as a source of IL-3. N17Rac-expressing clones were selected in puromycin (2 μg/ml) and were maintained in IMDM-CM.
Northern Blot Analysis.
Total cellular RNA (10 μg/lane) was electrophoresed on agarose gels and blotted onto a nitrocellulose PROTRAN membrane (Schleicher & Schuell). Full-length N17Rac cDNA labeled with [α-32P]dCTP (NEN) was used as probe.
Infection of Bone Marrow Cells.
Helper-free retroviral stocks were prepared as described (18, 19). Bone marrow cells from C57BL/6TacfBR mice (The Jackson Laboratory) treated with 5-fluorouracil (150 mg/kg body weight) 6 days before cell harvest were infected with BCR/ABL and/or Rac retroviruses or the insert-less retrovirus as described (19). BCR/ABL and Rac transcripts were detected by reverse transcriptase-PCR by using total RNA isolated from 105-infected cells. The BCR/ABL transcript (332 bp) was amplified by using the 5′ (BCR) primer: CACAGCATTCCGCTGACCATCA and the 3′ (ABL) primer: GCTTCACACCATTCCCCATTGT, which span the BCR/ABL junction. The LXSP-Rac transcript (199 bp) was amplified with the 5′ (LXSP) primer: CGATCCTCCCTTTATCCAGCC and the 3′ (Rac) primer: TCTACCATAACATTGG-CAGA. The β-actin transcript (210 bp) also was detected in each sample as control for RNA loading and integrity (11).
Rac-1 GDP/GTP Exchange.
The coding region of the murine Rac-1 gene was amplified by reverse transcriptase-PCR from 32Dcl3 RNA and cloned in-frame at the EcoRI and XhoI sites of pGTX-4T-1 (Pharmacia). After transformation of Escherichia coli BL21 cells, purified GST-Rac-1 protein was bound to [3H]GDP (1.05 μCi) in 180 μl of binding buffer (20 m Tris, pH 7.5/1 mM Mg Cl2/1 mM DTT/100 mM NaCl,/40 μg/ml BSA) for 1 h at 32°C. Cell extracts of 5 × 107 parental or BCR/ABL-expressing 32Dcl3 cells starved for 5 h in IMDM supplemented with 0.1% BSA, and 2 mM l-glutamine was immunoprecipitated with antibody against Vav, a protooncogene shown to act as a guanine exchange factor for Rac (20), which is constitutively active in BCR/ABL-expressing cells (21). Anti-Vav immunoprecipitates were incubated with [3H]GDP-Rac-GST (≈0.25 μg in 180 μl) and cold GTP (250 μM) at 32°C for 40 min. Samples (30 μl) were collected every 10 min and diluted with 500 μl of ice-cold binding buffer. Bound [3H]GDP was measured by scintillation counting after filtering the samples through 0.4-μm nitrocellulose filters (Schleicher & Schuell).
Clonogenic Assay.
Bone marrow cells (105) were plated in MethoCult H4230 semisolid methyl-cellulose medium (Stem Cell Technologies, Vancouver, CA) in the presence or absence of threshold concentrations of recombinant murine IL-3 (rmuIL-3; Genetics Institute, Cambridge, MA) as described (9). Colonies were scored on day 12.
Proliferation Assay.
Cells (104/0.1 ml) were plated in a 96-well plate with or without WEHI-CM. The number of living cells was scored daily by Trypan blue exclusion test.
Apoptosis Assay.
Cells (2 × 105/ml) were incubated in IMDM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and penicillin/streptomycin (100 μg/ml each) for 24, 48, and 72 h. The percentage of apoptotic cells was determined by using the TACS1 Klenow in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) according to the manufacturer’s protocol.
Invasion Assay.
For the invasion assay, 32Dcl3 cells (106) expressing wild-type BCR/ABL or coexpressing BCR/ABL and N17Rac were suspended in 2 ml of IMDM and placed in the upper chamber of a Biocoat Matrigel invasion chamber (Becton Dickinson); the lower chamber was filled with 2 ml of IMDM-CM as chemoattractant. Cells migrating into the lower chamber were counted 24 h later. Cells remaining on the membrane were counted after washing and staining the membrane according to the manufacturer’s protocol.
Homing Assay.
Cells were labeled with [3H]uridine (NEN), (0.5 μCi/ml IMDM-CM medium) for 24 h at 37°C, washed, and injected i.v. into SCID mice (5 × 106 cells/mouse) (22). Bone marrow (from both femurs) and spleen cells were collected 24 h later and deposited onto glass microfiber filters (Whatman). Cell-associated radioactivity was measured in a β-scintillation counter.
Leukemogenesis in SCID Mice.
C.B-17/IcrTac-SCID male mice (Taconic Farms) were injected i.v. with 5 × 106 32Dcl3 cells expressing BCR/ABL or BCR/ABL plus N17Rac proteins. Mice were killed 3 weeks after cell injection, and leukemia development was monitored by histopathological analysis.
RESULTS
Activation of Rac in BCR/ABL-Expressing 32Dcl3 Myeloid Precursor Cells.
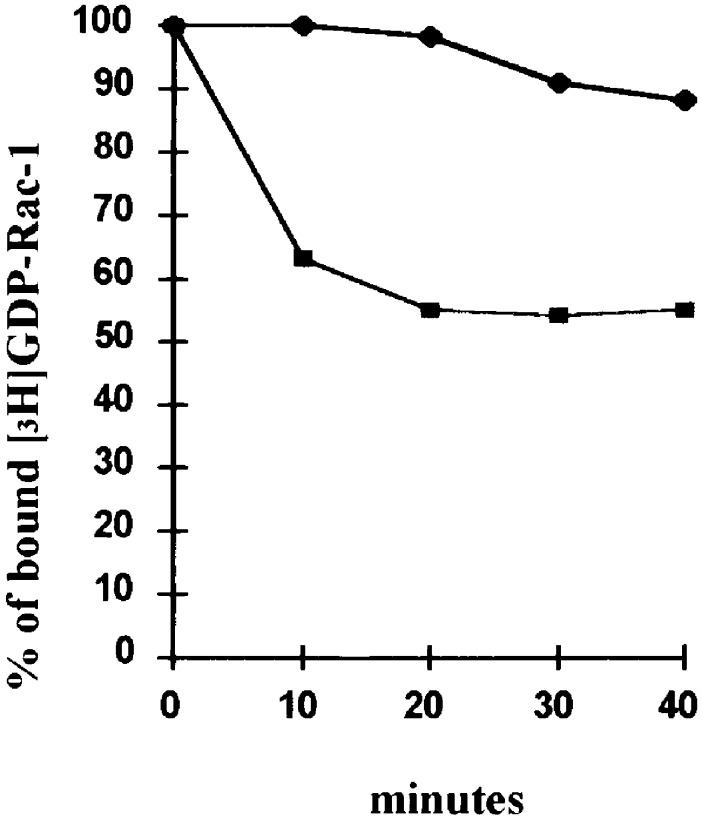
The Vav protooncogene has been recently shown to function as a guanine exchange factor for Rac (21). Moreover, Vav is activated by tyrosine phosphorylation in growth factor-stimulated hematopoietic cells and in cells expressing the BCR/ABL oncoprotein (22). Thus, we assessed Vav-dependent Rac activation in a guanine nucleotide release assay by using anti-Vav immunoprecipitates and [3H]GDP-Rac as substrate. The exchange of [3H]GDP for GTP was taken as a measure of Vav-dependent Rac activation. An early decline of bound [3H]GDP was detected in samples incubated with anti-Vav immunoprecipitates from IL-3-starved BCR/ABL-expressing cells, whereas the [3H]GDP release was considerably less and delayed in samples incubated with anti-Vav immunoprecipitates from IL-3-starved 32Dcl3 cells (Fig. 1). Results with parental 32Dcl3 cells were similar to those obtained using anti-Vav immunoprecipitates from 32Dcl3 cells expressing a kinase-deficient (K1172R) BCR/ABL mutant (not shown). Thus, Rac undergoes Vav-dependent activation in BCR/ABL-expressing 32Dcl3 cells. BCR/ABL-dependent Rac activation was accompanied by increased activity of the Rac-regulated c-Jun NH2-terminal kinase (JNK); such enhanced JNK activity was suppressed in cells coexpressing wild-type BCR/ABL and dominant negative N17Rac (not shown).
Figure 1.
Kinetics of Rac-1 [3H]GDP/GTP exchange in the presence of anti-Vav immunoprecipitates. Bound Rac-1 [3H]GDP was measured at 10-min intervals by a filter immobilization assay in which samples were filtered through nitrocellulose filters and filter-associated radioactivity was assessed by liquid scintillation counting. ♦, 32Dcl3 cells; ■, BCR/ABL-expressing 32Dcl3 cells. Results are representative of two experiments.
Requirement for Rac Activity in Proliferation but Not Survival of Growth Factor-Deprived BCR/ABL-Expressing Cells.
Two different clones of 32Dcl3 cells expressing wild-type BCR/ABL were electroporated with an insert-less vector or with a plasmid carrying a mutant cDNA encoding the N17Rac protein, which exhibits dominant-negative activity. After selection in puromycin (2 μg/ml), clones were screened for Rac expression, and three of them with similar levels of Rac transcripts (data not shown) were analyzed for growth factor-independent proliferation and apoptosis susceptibility.
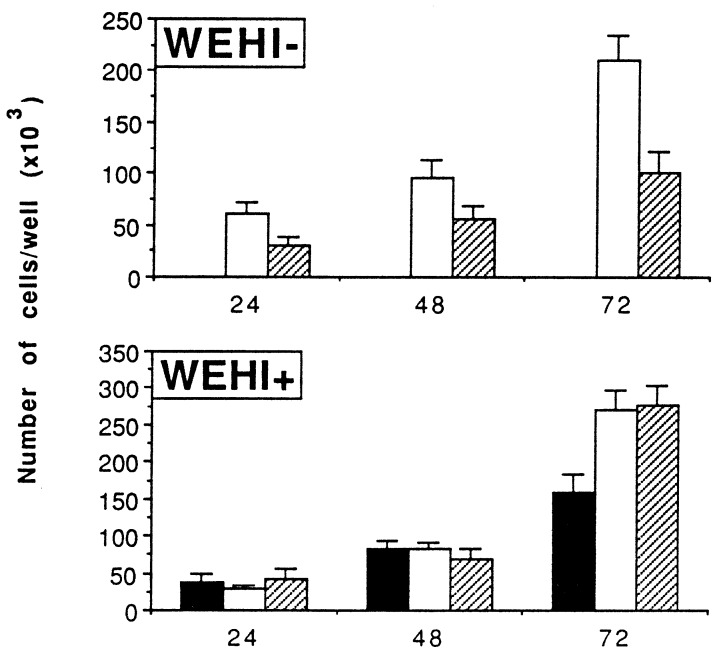
In the absence of IL-3-containing WEHI-CM, BCR/ABL-expressing 32Dcl3 cells proliferated faster than cells coexpressing wild-type BCR/ABL and N17Rac (Fig. 2 Upper), whereas no difference in proliferation rate was detected when cells were cultured in the presence of WEHI-CM (Fig. 2 Lower). As expected, 32Dcl3 cells transfected with the insert-less vector did not proliferate in the absence of IL-3-containing medium and grew more slowly than BCR/ABL-expressing cells in IL-3-containing medium. Analysis of 32Dcl3 transfectants for colony-forming ability in methylcellulose revealed slightly reduced numbers of colonies arising from cells coexpressing BCR/ABL and dominant-negative N17Rac in the absence of IL-3 and no effect on colony formation in plates supplemented with IL-3-containing medium (not shown). The size of the colonies arising from 32Dcl3 cells coexpressing wild-type BCR/ABL and N17Rac was smaller of that of 32Dcl3 cells expressing BCR/ABL only (not shown), consistent with the reduced proliferation of BCR/ABL and N17Rac-coexpressing cells in IL-3-deprived medium (Fig. 2 Upper). The 32Dcl3 cells expressing wild-type BCR/ABL only or coexpressing the dominant-negative RAC were completely resistant to apoptosis induced by growth factor deprivation (Fig. 3).
Figure 2.
Effect of N17Rac on proliferation of BCR/ABL-expressing 32Dcl3 cells. Cells were plated with (+) or without (−) WEHI-CM in 96-well plates. The number of cells in each well was scored after 24, 48, and 72 h. Results are representative of three independent experiments using two individual clones. ■, 32Dcl3 cells; □, BCR/ABL-expressing 32Dcl3 cells; and ▨, BCR/ABL- and N17Rac-positive 32Dcl3 cells.
Figure 3.
Effect of N17Rac on apoptosis of growth factor-deprived BCR/ABL-expressing 32Dcl3 cells. Cells were seeded without growth factors and the frequency of apoptotic cells was measured at 24, 48, and 72 h by using an in situ apoptosis detection kit. Results are representative of three independent experiments using two different clones; ■, 32Dcl3 cells; □, BCR/ABL-expressing 32Dcl3 cells; and ▨ BCR/ABL- and N17 Rac-expressing 32Dcl3 cells.
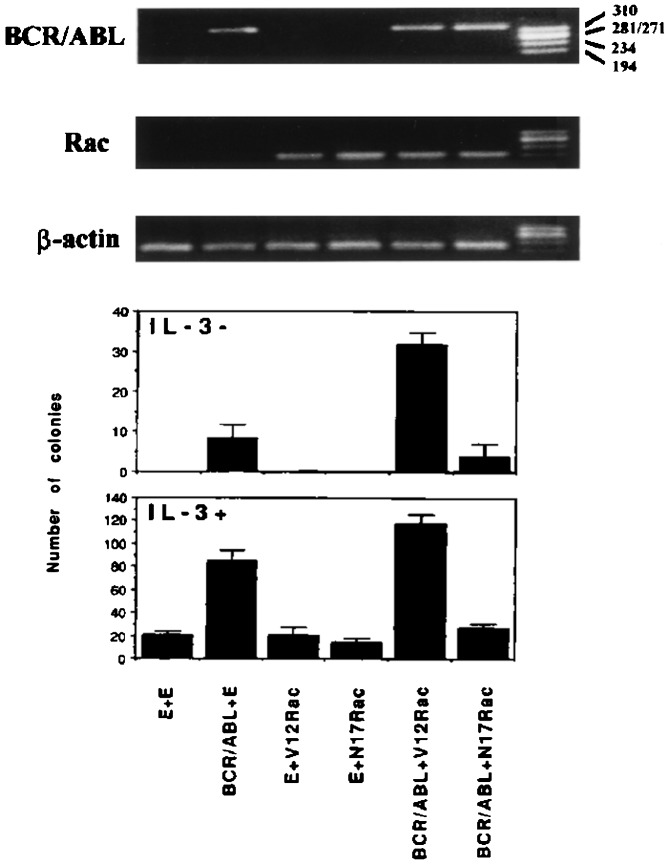
Further analysis of the requirement for Rac in proliferation of BCR/ABL-expressing cells by using primary mouse bone marrow cells infected with a retrovirus carrying the wild-type BCR/ABL or with both wild-type BCR/ABL and the dominant-negative N17Rac, showed that expression of the dominant-negative N17Rac reduced colony formation in both IL-3-deprived and IL-3-supplemented (0.1 unit/ml) cultures (Fig. 4). Colony formation was enhanced by coexpression of BCR/ABL and the constitutively active V12Rac, whereas expression of V12Rac alone had no effect (Fig. 4).
Figure 4.
Rac requirement for growth factor-independent colony formation of BCR/ABL-expressing mouse marrow cells. (Upper) Detection of BCR/ABL and mutant Rac mRNA transcripts in mouse bone marrow cells infected with BCR/ABL- and/or Rac-coding retroviruses. β-actin was detected as positive control for RNA loading and integrity. (Lower) Methylcellulose colony formation of mouse bone marrow cells infected with BCR/ABL- and/or Rac-coding retroviruses and cultured in the absence or in the presence of threshold concentrations (0.1 unit/ml) of recombinant murine IL-3. Results represent two independent experiments and are expressed as mean ± SD.
Effect of Dominant-Negative N17Rac Expression on Invasion and Homing of BCR/ABL-Expressing 32Dcl3 Cells.
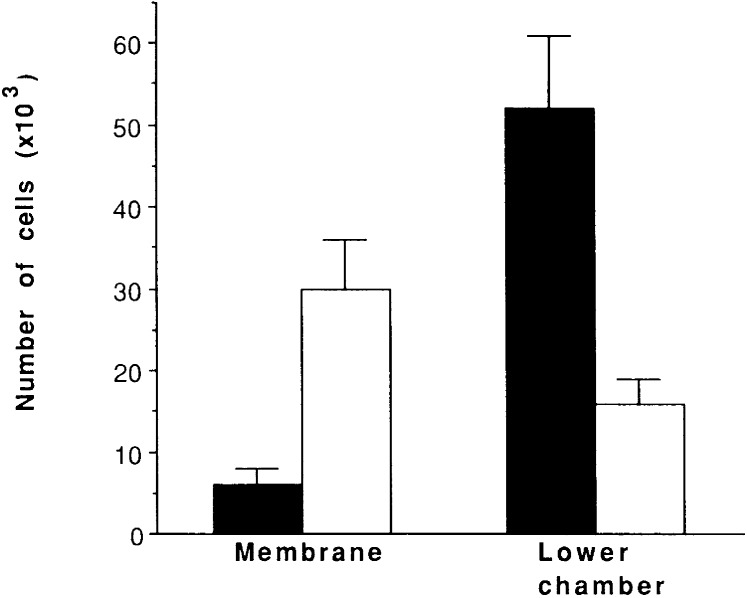
To assess the requirement for a functional Rac in the invasion of BCR/ABL-expressing cells, assays of invasion of artificial membranes were performed with 32Dcl3 cells expressing BCR/ABL only or coexpressing N17Rac. In the invasion assays, the number of BCR/ABL-expressing 32Dcl3 cells capable of passing through the artificial membrane was ≈3-fold less than that of 32Dcl3 cells coexpressing BCR/ABL and dominant-negative N17Rac (Fig. 5), whereas a high proportion of BCR/ABL and N17Rac expressing cells remained attached to the membrane (Fig. 5).
Figure 5.
Effect of N17Rac on invasive properties of BCR/ABL-expressing 32Dcl3 cells. Cells were placed in the upper chamber of a Biocoat Matrigel invasion chamber. Cells migrating into the lower chamber or remaining on the membrane were counted 24 h later. Results represent the mean ± SD of three experiments using two different clones. ■, BCR/ABL-expressing 32Dcl3 cells; and □, BCR/ABL -and N17Rac-expressing 32Dcl3 cells.
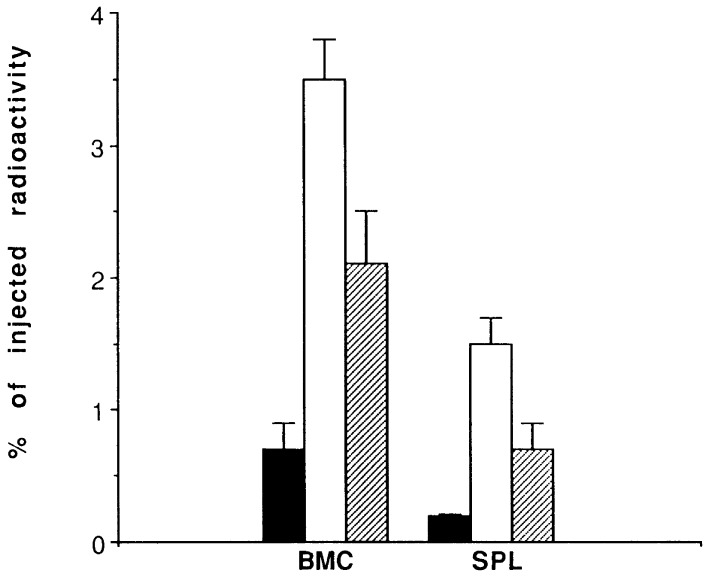
To determine whether the N17Rac-dependent effects on the invasion of BCR/ABL-expressing 32Dcl3 cells might be reflected in a reduced in vivo homing to hematopoietic tissues, 32Dcl3 transfectants were labeled with [3H]uridine, injected into SCID mice, and evaluated 24 h later for percent radioactivity in bone marrow and spleen. Expression of dominant-negative N17Rac reduced the homing ability of BCR/ABL-transfected 32Dcl3 cells, as indicated by a 50–75% decrease in bone marrow- and spleen-associated radioactivity (Fig. 6).
Figure 6.
In vivo homing of BCR/ABL- and BCR-ABL/N17 Rac-expressing cells. Single cell suspensions were prepared from bone marrow (BMC) and spleen (SPL) of mice 24 h after injection with [3H]uridine-labeled cells and deposited onto glass microfiber filters. Cell-associated radioactivity was determined as described in Materials and Methods. Results are expressed as the percentage of injected radioactivity (mean ± SD from three mice/group). ■, 32Dcl3 cells; □, BCR/ABL-positive 32Dcl3 cells (two clones); and ▨ BCR/ABL- and N17Rac-positive 32Dcl3 cells (two clones).
Requirement of Rac Activity for BCR/ABL-Mediated Leukemogenesis in Vivo.
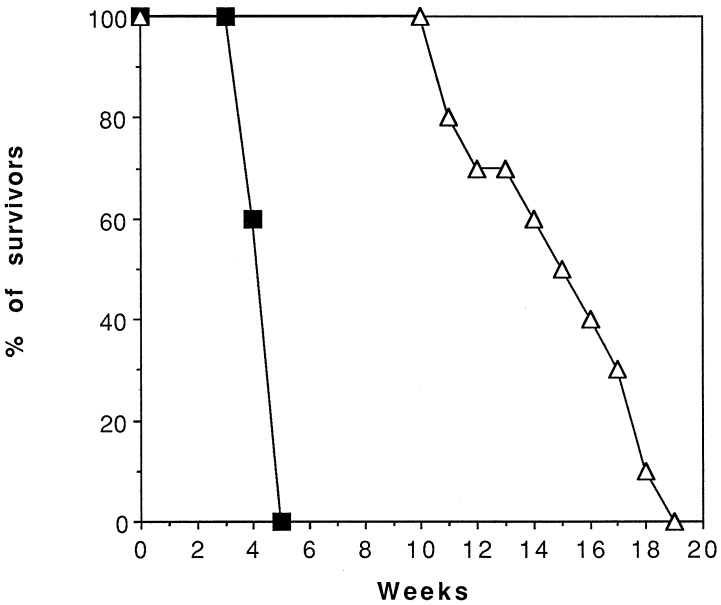
To determine whether Rac plays an essential role in BCR/ABL-mediated leukemogenesis in vivo, SCID mice were injected with 32Dcl3 cells expressing wild-type BCR/ABL only or coexpressing dominant-negative N17Rac, and 3 weeks later, various organs obtained from three mice of each injected group were evaluated by visual inspection and light microscopy. All three mice injected with wild-type BCR/ABL-expressing cells showed massive splenomegaly. Hematoxylin/eosin-stained sections demonstrated that the normal architecture of the spleen was extensively replaced by blasts with a low degree of myeloid maturation. Bone marrows were characterized by extensive chronic myelogenous leukemia-like myeloproliferation or overt acute myelogenous leukemia, although these cells usually demonstrated a higher degree of myeloid maturation than those in other organs. Acute myelogenous leukemia also involved livers in an intrasinusoidal infiltrate pattern, with occasional small parenchymal foci, and kidneys and lungs in two of three mice. By contrast, spleens of mice injected with 32Dcl3 cells coexpressing wild-type BCR/ABL and dominant-negative N17Rac were minimally enlarged; histopathologic analysis of various organs revealed normal architecture and no evidence of leukemic infiltrates in bone marrow, liver, kidneys, or lungs. Mild myelo-erythroid hyperplasia was detected in spleen (not shown). Consistent with these findings, the survival of mice injected with 32Dcl3 cells coexpressing wild-type BCR/ABL and dominant-negative N17Rac was ≈4-fold longer than that of mice injected with cells expressing wild-type BCR/ABL only (Fig. 7), although necropsy revealed splenomegaly and diffuse leukemia in various organs of mice injected with cells coexpressing wild-type BCR/ABL and dominant-negative N17Rac (not shown). Thus, Rac is required for the leukemogenic potential of wild-type BCR/ABL, but the disease process induced by wild-type BCR/ABL-expressing cells was not completely suppressed by inhibition of Rac activity.
Figure 7.
Survival of mice injected with BCR/ABL-expressing 32Dcl3 cells cotransfected with insert-less plasmid (■) or with plasmid carrying N17Rac cDNA (▵). SCID mice were injected i.v. with 5 × 106 cells from each of the two clones analyzed (5 mice/clone, 10 mice/group).
DISCUSSION
The phenotype of BCR/ABL-expressing cells is characterized by growth factor-independent proliferation (8, 23), reduced susceptibility to apoptosis induced by growth factor deprivation and by other stimuli (8, 24), and abnormal adhesion/invasion to artificial and physiological substrates that correlates with altered in vivo trafficking (14, 15, 22). The mechanisms whereby BCR/ABL induces this phenotype are only partially understood; moreover, it is unknown whether the various properties of BCR/ABL-expressing cells are under the control of distinct BCR/ABL-dependent pathways. For example, a functional RAS is required for both proliferation and survival of BCR/ABL-expressing cells (9, 25), and the BCR/ABL-dependent activation of the PI-3k/Akt pathway appears to transduce both survival and proliferative signals (12). In contrast, a BCR/ABL mutant lacking the SH3 domain renders transfected cells growth factor-independent and resistant to apoptosis but is defective in enhancing the invasive potential of transfected cells and in promoting their optimal homing to bone marrow and spleen. The aim of the present study was to identify a BCR/ABL downstream effector(s) involved in regulation of motility and to determine whether the regulation of this process was independent of the effects on proliferation and survival. An in vivo model of leukemia also was used to test whether interfering with a pathway regulating cell motility might adversely affect the leukemogenic potential of BCR/ABL.
The small GTP-binding protein Rac is critically important for the motility and the invasive phenotype of fibroblasts and epithelial cells (16, 17), raising the possibility that it also might be required for the trafficking of BCR/ABL-expressing cells. Indirect evidence for BCR/ABL-dependent Rac activation was obtained in experiments demonstrating that anti-Vav immunoprecipitates from BCR/ABL-expressing cells function as a guanine exchange factor for Rac (Fig. 1). As expected, Vav was tyrosine phosphorylated in BCR/ABL-expressing 32Dcl3 cells, but not in IL-3-starved parental cells or in cells transfected with a tyrosine kinase-deficient BCR/ABL mutant (not shown). The mechanism(s) of BCR/ABL-dependent Rac activation is unknown presently except for the requirement of a functional tyrosine kinase (not shown). In other models, Rac activation has been placed downstream of RAS, but it is not yet clear whether Rac is directly activated by RAS or requires PI-3k as a functional bridge between RAS and Rac (26, 27). The role of PI-3k in Rac activation also is unclear because it has been placed, functionally, both upstream and downstream of Rac (17, 18).
Because RAS and PI-3k are regulated independently by BCR/ABL and require for their activation the interaction of adapter molecules with more than one domain of the BCR/ABL oncoprotein (12, 28), it seems likely that the mechanisms of Rac activation are also complex. Assays of Rac activity in 32Dcl3 cells expressing BCR/ABL proteins defective in signaling via individual or multiple domains will help to dissect the regulatory pathways of Rac activation and to determine whether these pathways are interrelated with those that regulate Vav, PI-3k, and RAS activation.
In vitro, the functional consequences of inhibiting Rac activity in BCR/ABL-expressing cells were manifested in a reduced proliferative potential in growth factor-deprived cultures and in altered invasive properties. This phenotype was not surprising based on previous reports suggesting a role for Rac in cell cycle progression (29, 30) and in integrin-dependent cell adhesion and motility (16, 17). Interestingly, inhibition of Rac activity had no adverse effects on the survival of BCR/ABL-expressing cells, indicating that the regulation of cell growth by Rac is independent of effects on survival. This contrasts with the requirement for RAS in both proliferation and survival of BCR/ABL-expressing cells (9, 22). It seems unlikely that the altered invasive properties of 32Dcl3 cells expressing BCR/ABL and N17Rac are the consequence of the reduced proliferative potential of the doubly transfected cells; the reduced proliferation of these cells in growth factor-deprived cultures was modest, compared with the effects observed in the assays monitoring the invasive properties. The effects in invasion induced by dominant-negative Rac expression in BCR/ABL-expressing cells were not associated with changes in the levels of α1, α2, or β1 integrins (not shown), suggesting that Rac either functions as a mediator of integrin-generated signals (17) or induces changes in integrin’s affinity rather than in expression that might be responsible for the altered invasive properties of the double transfectants. Compared with wild-type BCR/ABL-expressing cells, BCR/ABL plus N17Rac transfectants showed markedly impaired leukemogenic potential. This might be the consequence, in part, of the reduced proliferation of the N17Rac-expressing cells; however, the defect in invasion that correlates with reduced homing to bone narrow and spleen is more likely to be the primary determinant of the lower leukemogenicity of the double-transfected cells. In SCID mice, levels of IL-3 and of other growth-promoting cytokines might be sufficient for optimal proliferation of BCR/ABL plus N17Rac-double-transfected cells, and yet the homing and the leukemogenic potential of these double transfectants were markedly suppressed. Thus, it is likely that separate Rac-dependent pathways exist for proliferation and motility. The lower leukemogenic potential of the double transfectants might depend not only on a reduced cell accumulation in hematopoietic and nonhematopoietic tissues as a consequence of altered adhesion and motility, but also on an impairment in the response to microenvironment-regulated signals that promote proliferation and survival (31).
In summary, we have identified Rac as a BCR/ABL downstream effector with an important role in leukemogenesis. The distinct involvement of Rac in pathways regulating cell trafficking as compared with cell survival points to the possibility of developing therapeutic strategies that combine compounds able to disrupt cell motility and promote apoptosis.
Acknowledgments
Supported in part by grants from National Institutes of Health and the American Cancer Society (B.C.), and by R29 CA70815 and Elsa U. Pardee (T.S.).
ABBREVIATIONS
- IMDM
Iscove’s modified Dulbecco medium
- IL-3
interleukin-3
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Clark S S, McLaughlin J, Timmonis M, Pendergast A M, Ben-Neriah Y, Dow L, Rovera G, Smith S D, Witte O N. Science. 1988;238:775–778. doi: 10.1126/science.3422516. [DOI] [PubMed] [Google Scholar]
- 2.Shtivelman E, Lifshitz B, Gale R P, Roe B A, Canaani E. Cell. 1986;47:277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- 3.Daley G Q, Baltimore D. Proc Natl Acad Sci USA. 1988;85:9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugo T G, Pendergast A M, Muller A J, Witte O N. Science. 1990;247:1079–1083. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 5.Gishizki M L, Witte O N. Science. 1992;256:836–839. doi: 10.1126/science.1375394. [DOI] [PubMed] [Google Scholar]
- 6.Daley G Q, Van Etten R, Baltimore D. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 7.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale P, Groffen J. Nature (London) 1990;344:251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 8.Cortez D, Kadlec L, Pendergast A M. Mol Cell Biol. 1995;15:5531–5541. doi: 10.1128/mcb.15.10.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skorski T, Kanakaraj P, Ku D H, Nieborowska-Skorska M, Canaani E, Zon G, Perussia B, Calabretta B. J Exp Med. 1994;179:1855–1865. doi: 10.1084/jem.179.6.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawyers C L, McLaughlin J, Witte O N. J Exp Med. 1995;181:307–315. doi: 10.1084/jem.181.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skorski T, Nieborowska-Skorska M, Szczylik C, Kanakaraj P, Perrotti D, Zon G, Gewirtz A, Perussia B, Calabretta B. Cancer Res. 1995;55:2275–2278. [PubMed] [Google Scholar]
- 12.Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi J K, Trotta R, Wlodarski P, Perrotti D, Chan T O, et al. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Garcia I, Grütz G. Proc Natl Acad Sci USA. 1995;92:5287–5291. doi: 10.1073/pnas.92.12.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon M Y, Dowding C R, Riley G P, Goldman J H, Greaves M T. Nature (London) 1987;328:342–345. doi: 10.1038/328342a0. [DOI] [PubMed] [Google Scholar]
- 15.Verfaillie C M, McCarthy J B, McGlave P B. J Clin Invest. 1992;90:1232–1238. doi: 10.1172/JCI115985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Nature (London) 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 17.Shaw L M, Rabionovitz I, Wang H H-F, Toker A, Mercurio A M. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 18.Muller A J, Young J C, Pendergast A M, Pondel M, Landau N R, Littman D R, Witte O N. Mol Cell Biol. 1991;11:1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skorski T, Nieborowska-Skorska M, Wlodarski P, Perrotti D, Martinez R, Wasik M A, Calabretta B. Proc Natl Acad Sci USA. 1996;93:13137–13142. doi: 10.1073/pnas.93.23.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crespo P, Schivebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Nature (London) 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 21.Matsuguchi T, Inhorn R C, Carlesso N, Xu G, Druker B, Griffin J D. EMBO J. 1995;14:257–265. doi: 10.1002/j.1460-2075.1995.tb06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 22.Skorski T, Nieborowska-Skorska M, Wlodarski P, Wasik M, Trotta R, Kanakaraj P, Salomoni P, Antonyak M, Martinez R, Majewski M, et al. Blood. 1998;91:406–418. [PubMed] [Google Scholar]
- 23.Cortez D, Reuther G, Pendergast A M. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- 24.Bedi A, Zehnbauer B A, Barber J P, Sharkis S J, Jones R J. Blood. 1994;83:2038–2044. [PubMed] [Google Scholar]
- 25.Cortez D, Stoica G, Pierce J H, Pendergast A M. Oncogene. 1996;13:2589–2594. [PubMed] [Google Scholar]
- 26.Qiu R G, Chen J, Korn D, McCormick F, Symons M. Nature (London) 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 27.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goga A, McLaughlin J, Afar D E H, Saffran D C, Witte O N. Cell. 1995;82:981–988. doi: 10.1016/0092-8674(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 29.Olson M F, Ashworth A, Hall A. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 30.Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 31.Ruoslahti E, Reed J C. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]