Abstract
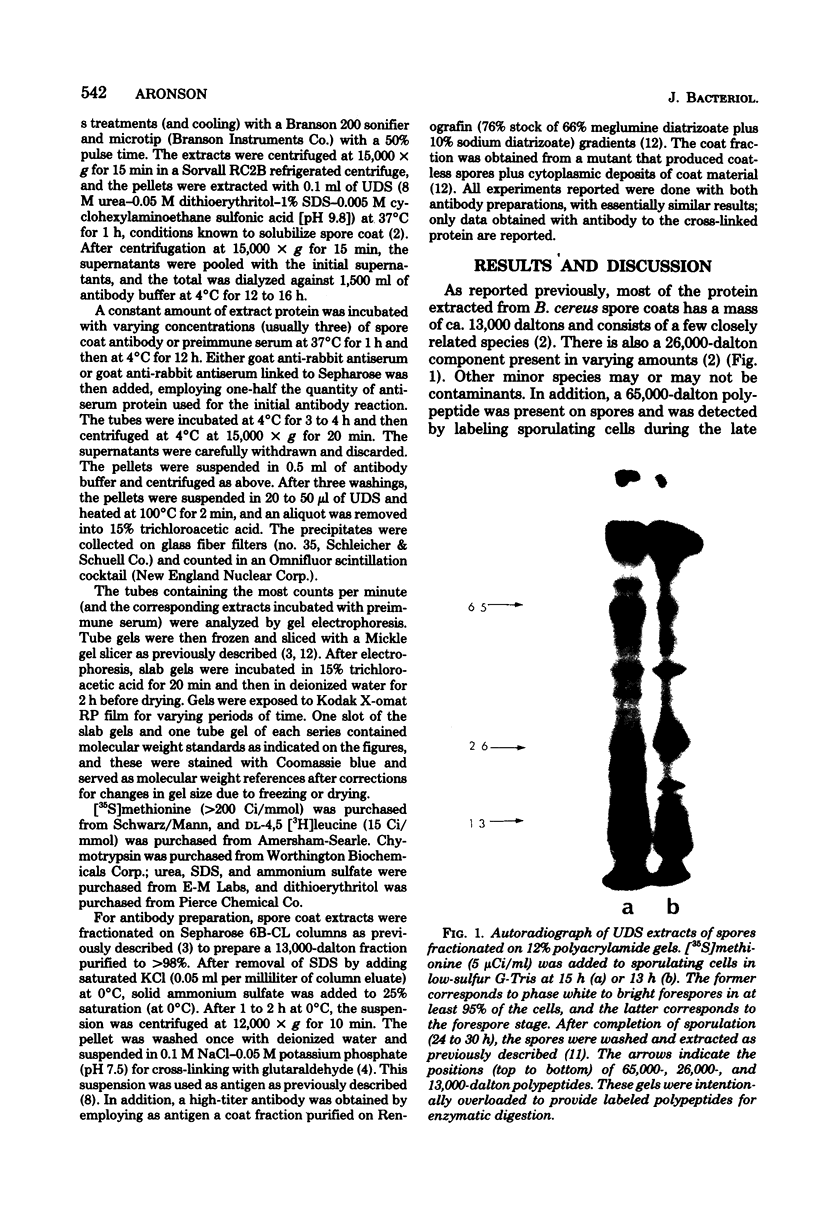
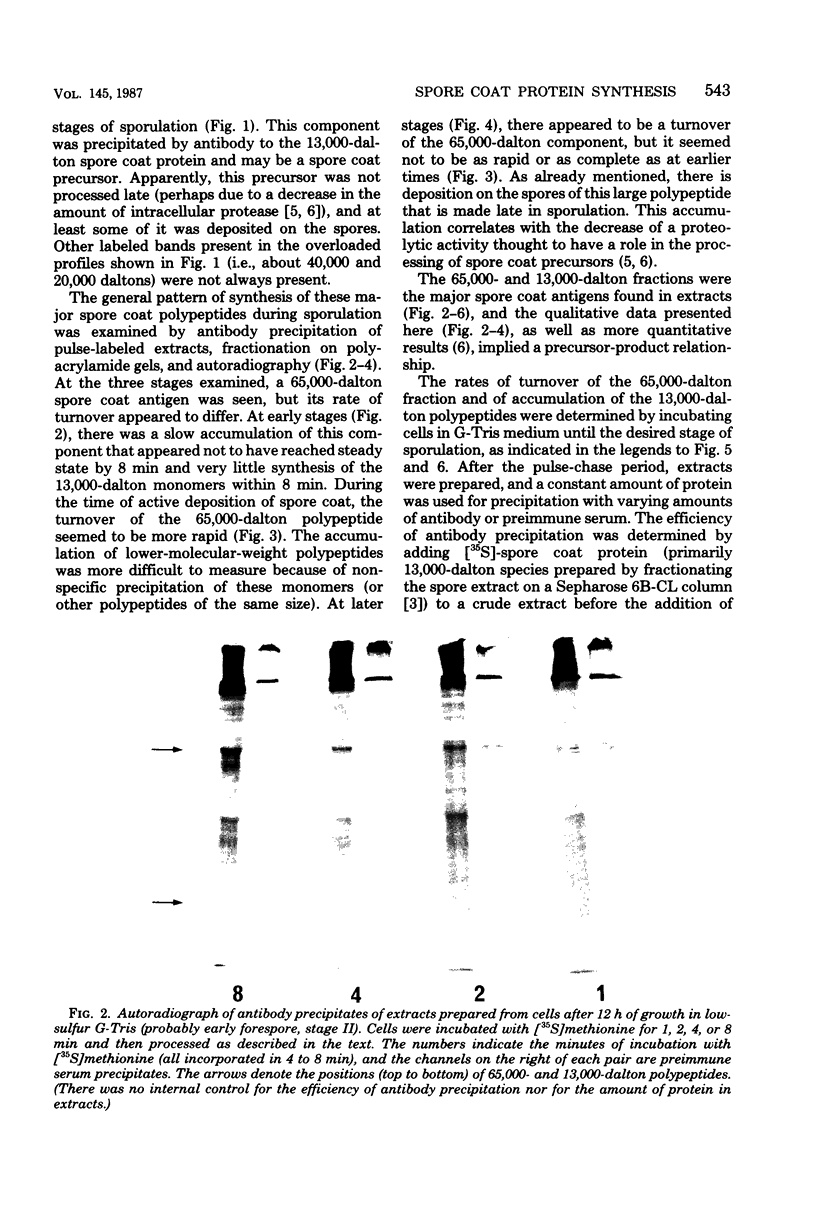
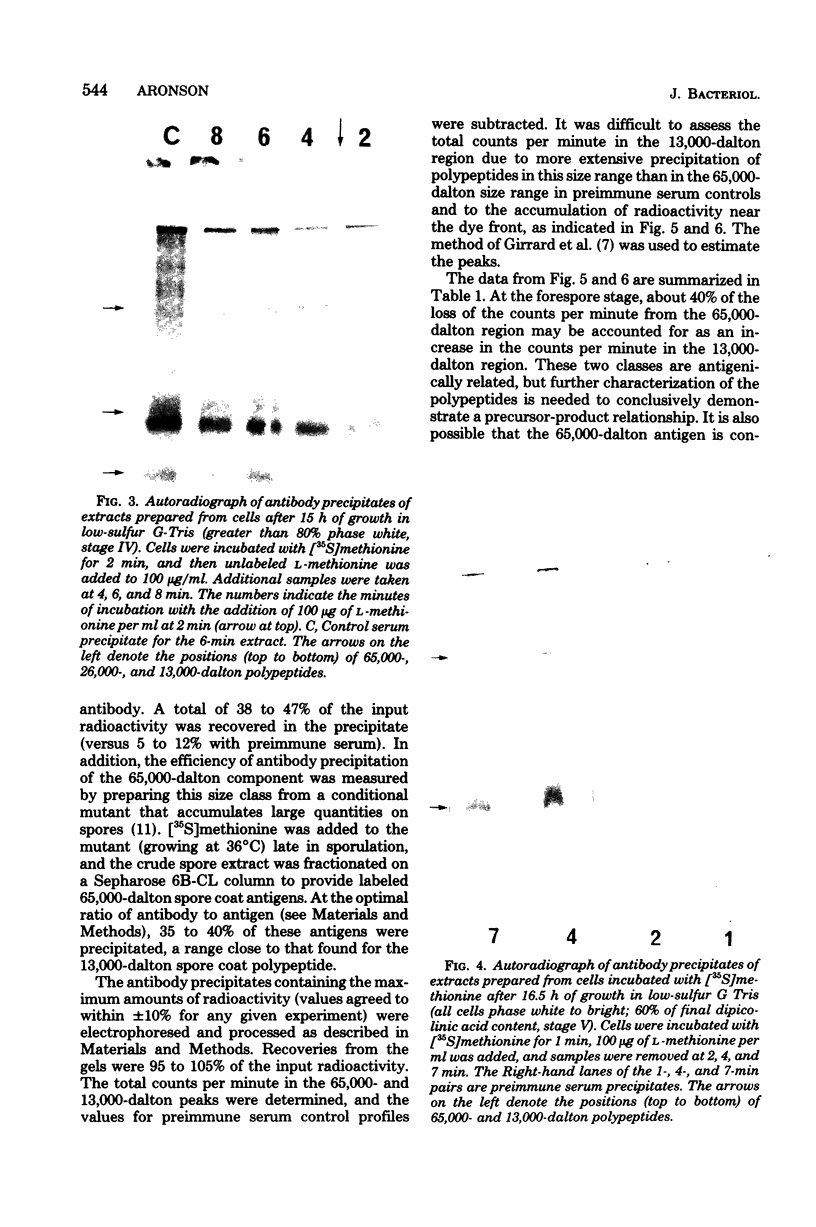
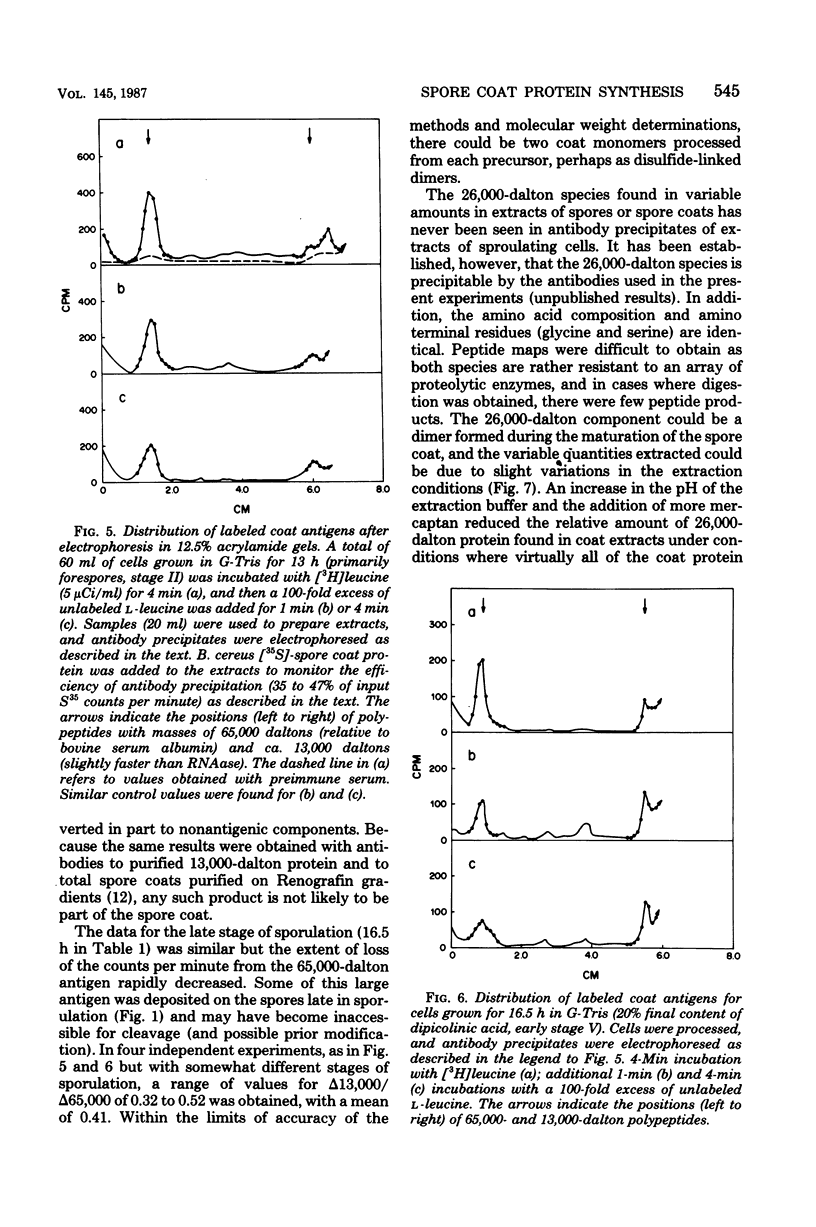
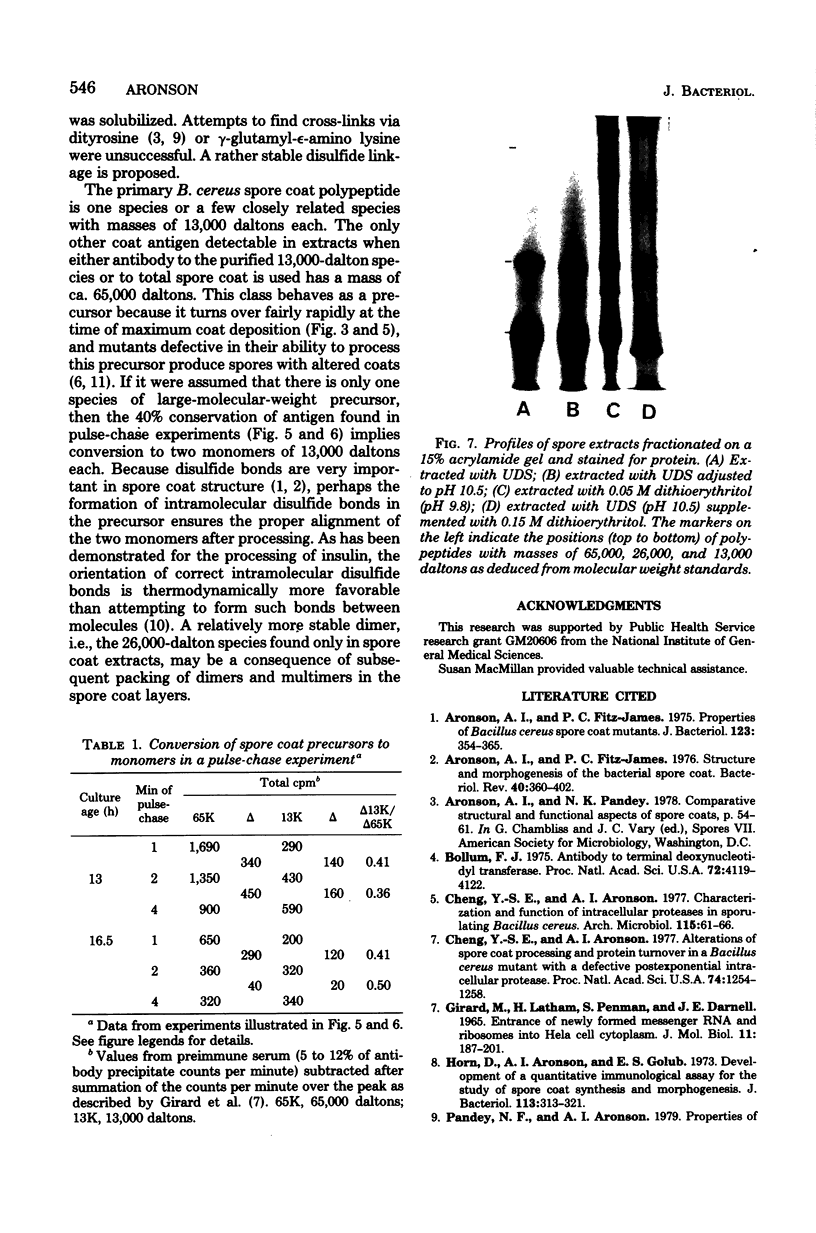
The major structural protein of Bacillus cereus spore coats was synthesized, commencing 1 to 2 h after the end of exponential growth, as a precursor with a mass of ca. 65,000 daltons. About 40% of this precursor, i.e. 26,000 daltons, was converted to spore coat monomers of 13,000 daltons each, perhaps as disulfide-linked dimers. The rate of conversion varied, being initially slow, most rapid at the time of morphogenesis of the coat layers, and then slow again late in sporulation, coincident with a decrease in intracellular protease activity. There was a second major spore coat polypeptide of about 26,000 daltons that was extractable from mature spores in variable amounts. This protein had a peptide profile and a reactivity with spore coat protein antibody that were very similar to those of the 13,000-dalton monomers. It is probably a disulfide-linked dimer that is not readily dissociated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. C. Properties of Bacillus cereus spore coat mutants. J Bacteriol. 1975 Jul;123(1):354–365. doi: 10.1128/jb.123.1.354-365.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4119–4122. doi: 10.1073/pnas.72.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Characterization and function of intracellular proteases in sporulating Bacillus cereus. Arch Microbiol. 1977 Oct 24;115(1):61–66. doi: 10.1007/BF00427846. [DOI] [PubMed] [Google Scholar]
- GIRARD M., LATHAM H., PENMAN S., DARNELL J. E. ENTRANCE OF NEWLY FORMED MESSENGER RNA AND RIBOSOMES INTO HELA CELL CYTOPLASM. J Mol Biol. 1965 Feb;11:187–201. doi: 10.1016/s0022-2836(65)80050-x. [DOI] [PubMed] [Google Scholar]
- Horn D., Aronson A. I., Golub E. S. Development of a quantitative immunological assay for the study of spore coat synthesis and morphogenesis. J Bacteriol. 1973 Jan;113(1):313–321. doi: 10.1128/jb.113.1.313-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey N. K., Aronson A. I. Properties of the Bacillus subtilis spore coat. J Bacteriol. 1979 Mar;137(3):1208–1218. doi: 10.1128/jb.137.3.1208-1218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. C. A Bacillus cereus mutant defective in spore coat deposition. J Gen Microbiol. 1980 Jan;116(1):173–185. doi: 10.1099/00221287-116-1-173. [DOI] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. Properties of Bacillus cereus temperature-sensitive mutants altered in spore coat formation. J Bacteriol. 1978 Jun;134(3):1157–1170. doi: 10.1128/jb.134.3.1157-1170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]