Abstract
The apposed membranes of myelinating Schwann cells are joined by several types of junctional specializations known as autotypic or reflexive junctions. These include tight, gap, and adherens junctions, all of which are found in regions of noncompact myelin: the paranodal loops, incisures of Schmidt-Lanterman, and mesaxons. The molecular components of autotypic tight junctions have not been established. Here we report that two homologues of Discs Lost–multi PDZ domain protein (MUPP)1, and Pals-associated tight junction protein (PATJ), are differentially localized in myelinating Schwann cells and associated with different claudins. PATJ is mainly found at the paranodal loops, where it colocalized with claudin-1. MUPP1 and claudin-5 colocalized in the incisures, and the COOH-terminal region of claudin-5 interacts with MUPP1 in a PSD-95/Disc Large/zona occludens (ZO)-1 (PDZ)-dependent manner. In developing nerves, claudin-5 and MUPP1 appear together in incisures during the first postnatal week, suggesting that they coassemble during myelination. Finally, we show that the incisures also contain four other PDZ proteins that are found in epithelial tight junctions, including three membrane-associated guanylate-kinase proteins (membrane-associated guanylate-kinase inverted-2, ZO-1, and ZO-2) and the adaptor protein Par-3. The presence of these different tight junction proteins in regions of noncompact myelin may be required to maintain the intricate cytoarchitecture of myelinating Schwann cells.
Keywords: tight junction; MUPP1; PATJ; Schmidt-Lanterman incisures; paranodal loops
Introduction
The myelin membrane can be divided into two structurally and biochemically distinct regions, compact and noncompact myelin. In compact myelin, the external leaflets of two adjacent plasma membranes are closely apposed (the interperiod line), and the cytoplasm amounts to a thin band (the major dense line). In noncompact myelin, found in the paranodal loops, Schmidt-Lanterman incisures, and the inner and outer mesaxons, the adjacent plasma membranes are also apposed, but the volume of cytoplasm is considerably increased. Areas of noncompact myelin contain junctional specializations found in epithelial cells, including tight, gap, and adherens junctions (Mugnaini and Schnapp, 1974; Fannon et al., 1995; Balice-Gordon et al., 1998; Spiegel and Peles, 2002). However, whereas in epithelia these junctions are formed between different cells, in myelinating glia they are found between membrane lamellae of the same cell, and are thus termed autotypic (Fannon et al., 1995) or reflexive (Balice-Gordon et al., 1998) junctions. Autotypic adherens junctions contain the calcium-dependent cell adhesion molecule E-cadherin, as well as the cytoplasmic protein β-catenin, which connects the former to the actin filaments (Trapp et al., 1989; Fannon et al., 1995; Gumbiner, 2000). Autotypic GAP junctions contain connexin (Cx)32 and Cx29 (Scherer et al., 1995; Altevogt et al., 2002). Tight junctions have been observed in the peripheral nervous system (PNS)* myelin sheath by transmission, and especially by freeze fracture electron microscopy (Mugnaini and Schnapp, 1974; Sandri et al., 1977; Tetzlaff, 1978, 1982). These tight junction strands are comprised of linear rows of intermembranous particles between adjacent cell membranes in the inner and outer mesaxon, paranodal loops, and the incisures. Autotypic tight junctions were proposed to function as a mechanical link and as a permeability barrier, separating the extracellular space outside the myelin sheath from the intramyelinic space between the lamellae (Revel and Hamilton, 1969; Hall and Williams, 1971; Mugnaini and Schnapp, 1974; Tabira et al., 1978; MacKenzie et al., 1984).
Studies in epithelial and endothelial cells revealed a complex molecular composition of tight junctions consisting of several adhesion molecules: occludin (Furuse et al., 1993), claudins (Furuse et al., 1998a; Morita et al., 1999a), and junctional adhesion molecules (JAMs) (Martin-Padura et al., 1998), as well as a growing list of peripheral membrane proteins (Gonzalez-Mariscal et al., 2000; Zahraoui et al., 2000; Tsukita et al., 2001). Claudins are integral membrane proteins with four transmembrane domains that form tight junction strands (Furuse et al., 1998b). They comprise of a large protein family, consisting of ∼20 different members that are expressed in different tissues (Tsukita et al., 2001). The COOH-terminal tails of claudins, occludin, and JAM, interact with several domain-containing proteins found at the cytoplasmic surface of tight junctions (Furuse et al., 1994; Fanning et al., 1998; Haskins et al., 1998; Itoh et al., 1999, 2001; Bazzoni et al., 2000; Hamazaki et al., 2002; Patrie et al., 2002; Roh et al., 2002a). The presence of PSD 95/Disc Large/zona occludens (ZO)1 (PDZ) proteins at tight junctions allows the formation of large macromolecular complexes that control the localization of various proteins at the cell membrane, and provides a link between tight junction fibrils and the actin cytoskeleton (Fanning et al., 1998; Cordenonsi et al., 1999; Itoh et al., 1999; Gonzalez-Mariscal et al., 2000; Patrie et al., 2002; Roh et al., 2002a). However, in contrast to the complex molecular composition of epithelial tight junctions, the constituents of autotypic tight junctions in myelinating Schwann cells are still unknown. In this study, we report the identification and subcellular distribution of new autotypic tight junction components in myelinating Schwann cells; these include: three members of the claudin family (claudin-1, claudin-2, and claudin-5); two multi-PDZ domain proteins (MUPPs) (MUPP1 and protein associated with Lin-7 [Pals]1-associated tight junction protein [PATJ]); three membrane-associated guanylate-kinase (MAGUK) proteins (MAGUK inverted [MAGI]-2, ZO-1, and ZO-2); and the adaptor protein Par-3. Furthermore, we demonstrate that autotypic tight junctions present in different aspects of noncompact myelin contain distinct junctional complexes. This unique arrangement may be required to maintain the intricate cytoarchitecture of myelinating Schwann cells and, in turn, normal nerve conduction.
Results
Discs Lost homologues, MUPP1, and Pals1-associated tight junction protein are found at tight junctions in epithelial cells
In the fly, Discs Lost (DLT) binds to neurexin-IV, a protein essential for the formation of septate junctions between ensheathing glial cells (Baumgartner et al., 1996; Bhat et al., 1999). The mammalian homologues of DLT include two proteins, MUPP1 (Ullmer et al., 1998) and PATJ (Roh et al., 2002b), also known as hInaDL (Philipp and Flockerzi, 1997). We originally isolated PATJ and MUPP1 cDNA in a screen for proteins that bind the cytoplasmic tail of Caspr2, a mammalian homologue of neurexin-IV (unpublished data). To determine whether the mammalian homologues of DLT are found in myelinated nerves, we generated polyclonal antibodies directed to the third PDZ domain (Ab M3 and Ab PJ3) or against a more unique region located between the fifth and sixth PDZ domains of each one of these proteins (Fig. 1 A). These antibodies precipitated a major band of 250- and 200-kD proteins from HEK-293 cells transfected with MUPP1 or PATJ, respectively (Fig. 1 B). Similar results were obtained using rat brain lysates (unpublished data). Immunoprecipitation experiments also showed that, despite the high sequence similarity between PATJ and MUPP1, only some minor crossreactivity was detected using the PJ3 and M3 antibodies (Fig. 1 B).
Figure 1.
Domain organization and subcellular localization of MUPP1 and PATJ in epithelial cells. (A) Schematic structure of Drosophila DLT and its mammalian homologues, MUPP1 and PATJ. All three proteins are composed of several PDZ domains (numbered squares), as well as a Lin7 binding domain (ellipses) in their NH2 terminus. The regions used to generate different antibodies against PATJ and MUPP1 are indicated, along with the name of each antibody. (B) Immunoprecipitation of MUPP1 and PATJ. HEK-293 cells transfected with MUPP1 (left) or PATJ (right) were incubated with preimmune serum (CS) or the indicated antibodies (IP Ab:). Precipitated proteins were immunoblotted using an antibody to MUPP1 or PATJ as indicated. The multiple bands detected in the left panel are degradation products of MUPP1. (C) PATJ and MUPP1 are localized at tight junctions in MDCK cells. Double immunofluorescence staining of MDCK cells using rabbit antisera (green) against PATJ or MUPP1 and monoclonal antibodies (red) against E-cadherin (Ecad), or using mouse antisera (green) against PATJ or MUPP1 and rabbit antisera (red) against ZO-1 or claudin-1 as indicated. The merged images are shown in the right panels (C, F, I, and L); insets show higher magnification of the small square labeled in each panel. Note that MUPP1 and PATJ colocalize with claudin-1 and ZO-1 but not E-cadherin. Bar, 10 μm.
We next determined the localization of endogenous MUPP1 and PATJ proteins in polarized MDCK cells by double labeling with antibodies to components of tight junctions (claudin-1 or ZO-1) or adherens junctions (E-cadherin). Both MUPP1 and PATJ were colocalized with ZO-1 and claudin-, but not with E-cadherin (Fig. 1 C). Further, confocal microscopy analysis showed that, in Z sections, MUPP1 and PATJ were found above E-cadherin (unpublished data). These results are in agreement with recent findings, published during the course of this study, identifying MUPP1 and PATJ as components of tight junctions (Hamazaki et al., 2002; Lemmers et al., 2002; Roh et al., 2002b).
Localization of MUPP1 to Schmidt-Lanterman incisures in myelinating Schwann cells
Antibodies to MUPP1 labeled the Schmidt-Lanterman incisures in rat sciatic nerve, as shown by colabeling for known incisures markers, such as myelin-associated glycoprotein (MAG), E-cadherin, and Cx32 (Fig. 2; unpublished data). Notably, although these marker proteins also labeled the paranodal loops (Fannon et al., 1995; Scherer et al., 1995), in the adult rat sciatic nerve, MUPP1 was barely detected at this site. MUPP1 antibodies also labeled isolated rings that were frequently located adjacent to the narrow base of the incisures (Fig. 2 F, inset). In addition, there was a thin line of MUPP1 staining along the outer aspect of the myelin sheath, linking adjacent incisures, which likely corresponds to the outer mesaxon (Fig. 2 I, inset). In contrast to the rat, paranodal staining for MUPP1 was conspicuous in the mouse, as revealed by double immunofluorescence labeling of mouse sciatic nerve for MUPP1 and Na+ channels (Fig. 2, J–L).
Figure 2.
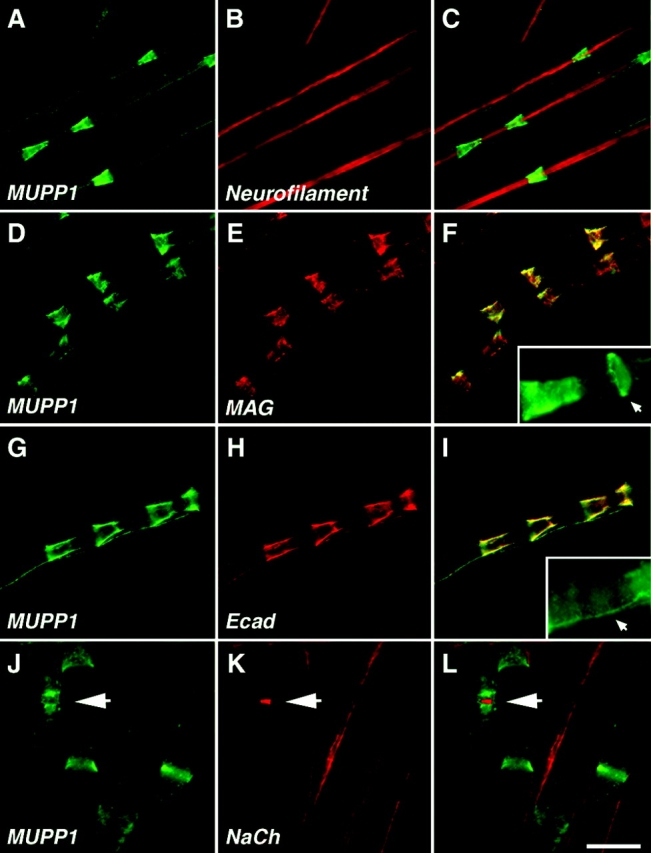
MUPP1 is located at Schmidt-Lanterman incisures. (A–I) Images of teased fibers from adult rat sciatic nerves, double labeled with an antiserum against MUPP1 (green) and a monoclonal antibody (red) against neurofilament, MAG, or E-cadherin (Ecad) as indicated. For each labeling, the right panel shows the merge image. The insets in F and I show the expression of MUPP1 in annular ribbons and the mesaxon, respectively. (J–L) Images of teased fibers from mouse sciatic nerve labeled with an antiserum against MUPP1 (green) and a monoclonal antibody (red) to voltage-gated Na+ channels (NaCh). MUPP1 was detected in incisures and paranodes; the nodal Na+ channels are indicated (arrow). The merged image is shown in L. Bars: A–I, 20 μm; J–L, 15 μm.
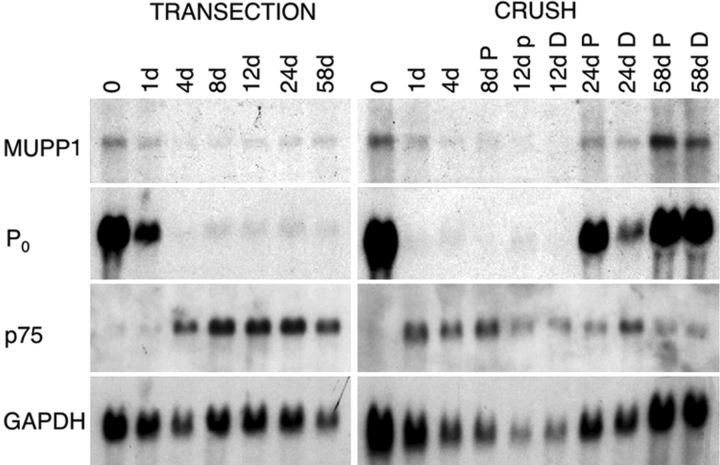
Regulated expression of MUPP1 mRNA in lesioned sciatic nerve
To determine whether axon–Schwann cell interactions regulate the expression of MUPP1 mRNA, we compared the effects of permanent transection with those of nerve-crush. The transections were designed to prevent axonal regeneration so that the effects of permanent axotomy could be examined in isolation. Nerve-crush also causes Wallerian degeneration, but allows axonal regeneration. In this way, the effects of axonal regeneration can be inferred by comparing the distal nerve-stumps of transected and crushed nerves at corresponding times. To facilitate this analysis, the distal nerve-stumps of crushed nerves were divided into two segments, a more proximal one (P) and a more distal one (D). Because axons regenerate in a proximal-to-distal manner, changes due to axonal regeneration should first be evident in the proximal segment, and subsequently in the distal segment. As shown in Fig. 3, the level of MUPP1 mRNA decreased by 4 d posttransection and postcrush, but increased at 24 and 58 d postcrush, but not posttransection. These changes mirror those of P0 mRNA, which encodes the major protein of compact myelin (Fig. 3). The expression of p75 (the low-affinity neurotrophin receptor) has a reciprocal pattern as expected from genes expressed by embryonic Schwann cells (Fig. 3). Thus, similar to other myelin-related genes, MUPP1 expression in Schwann cells is regulated by axonal contact (Scherer and Salzer, 2001).
Figure 3.
Northern blot analysis of MUPP1 expression after transection and crush injury. Each lane contains an equal amount (10 μg) of total RNA isolated from the distal stumps of sciatic nerves that had been transected or crushed. The number of days after each of these lesions are indicated; the 0 time point is from unlesioned adult nerves. In crushed nerves, the distal nerve-stumps were divided into proximal (P) and distal (D) segments of equal lengths. The blot was sequentially probed with radiolabeled cDNA for MUPP1, p75, GAPDH, and P0, and exposed to film for 14 d, 14 d, 4 d, and 4 h, respectively.
PATJ is found at the paranodal loops in rat sciatic nerve
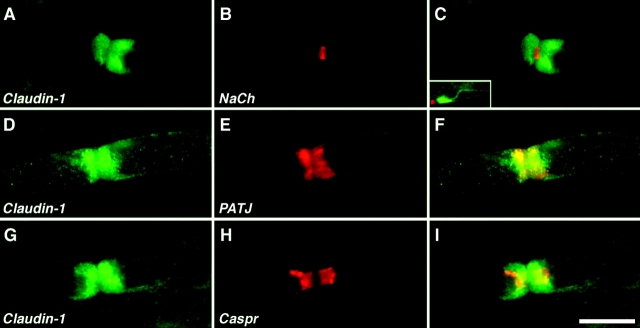
The reduced paranodal expression of MUPP1 in the rat suggested that another multi-PDZ domain protein could be located at this site. Thus, we examined the localization of the other DLT homologue we found, PATJ, in myelinated peripheral nerves. As depicted in Fig. 4, PATJ antibodies intensely labeled the paranodal region. PATJ was expressed as a thick ring surrounding Caspr, which is found in the axonal paranodal membrane (Einheber et al., 1997). Similar localization of PATJ was observed using mouse sciatic nerve (see Fig. 6). Strong PATJ staining was also frequently detected at the incisures closest to the paranodes (Fig. 4, G–I), and some weak staining was occasionally detected in parts of the more distant incisures (Fig. 4, J–L). These results showed that the expression pattern of PATJ was clearly distinct from that of MUPP1, demonstrating that these two related multi-PDZ domain proteins are differentially distributed in myelinating Schwann cells.
Figure 4.
PATJ is mainly found in the paranodal loops and only weakly in Schmidt-Lanterman incisures. Images of teased fibers from adult rat sciatic nerves, double labeled with an antiserum to PATJ (green) and a monoclonal antibody (red) to neurofilament, Caspr, or E-cadherin (Ecad). The merged images are shown on the right panel of each row. PATJ labeling surrounded that of Caspr, which marks the paranodal membrane of axons. PATJ antibody often labeled the incisures closest to the node (G and I, arrows), but was missing from all other E-cadherin–labeled incisures (I). Some weak staining of incisures was occasionally detected (J and L, arrows). Bar, 10 μm.
Figure 6.
Claudin-1 is located at the paranodal loops and along the mesaxon. Double immunofluorescence labeling of teased mouse sciatic nerve fibers using antibodies to claudin-1 (green) and Na+ channels (A–C, NaCh), PATJ (D–F), or Caspr (G–I), all in red. The right panel of each row shows the merged image. Claudin-1 was found in the paranodal region at both sides of the node (A–C), and colocalized with PATJ (D–F). Note that claudin-1 and Caspr are localized in distinct parts of the paranode (G–I). The inset in panel C shows internodal staining of claudin-1; this was more pronounced in smaller axons. Bar, 15 μm.
Developmental analysis of MUPP1 and PATJ
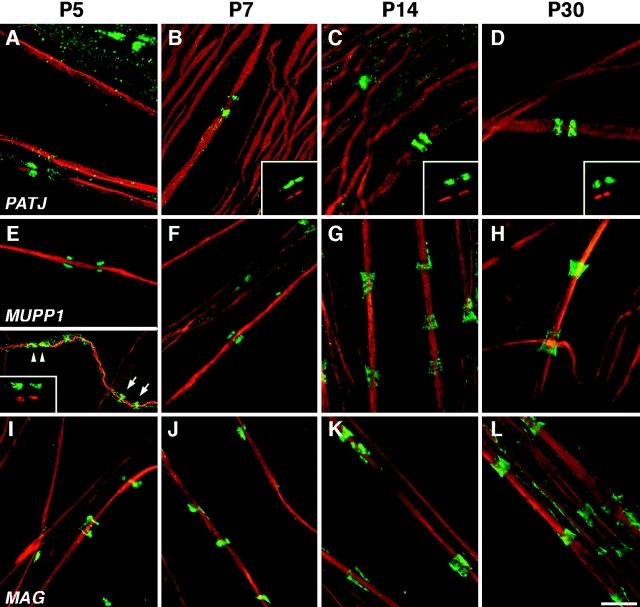
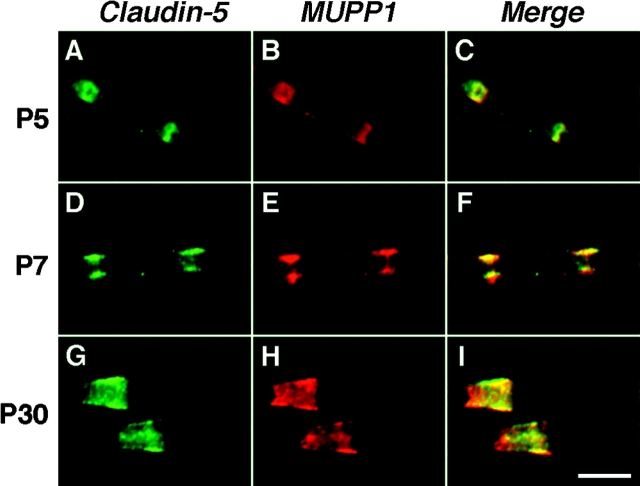
To determine how the differential localization of MUPP1 and PATJ evolved, we examined their distribution in developing rat sciatic nerve (Fig. 5). Starting from the fifth postnatal day (P5) onward, PATJ was localized in all paranodal loops, as detected by double labeling with an antibody to Caspr. At earlier days, we could detect only sporadic expression of PATJ in the paranodal region (unpublished data). At P5, MUPP1 (Fig. 5, E–H) and MAG (Fig. 5, I–L) were present within incisures, although the funnel shape of mature incisures was not evident until the second postnatal week. These results correlate well with older morphological data showing that incisures develop relatively late in PNS myelin sheaths (Small et al., 1987). At P5 and P7, MUPP1 was also detected in the paranodal region (inset in Fig. 5 E), but largely disappeared after the first week, indicating that its paranodal localization is developmentally regulated.
Figure 5.
Developmental analysis of MUPP1 and PATJ in myelinated peripheral nerve. Images of teased fibers from rat sciatic nerves at the indicated ages, double labeled with a monoclonal antibody to neurofilaments (red) and an antiserum to PATJ, MUPP1, or MAG (green). PATJ was present in the paranodal loops at all ages examined. At P5 and P7, strong MUPP1 staining (green) was detected in the paranodal region (arrowheads in lower half of E) and incisures (arrows). Note that during the first postnatal week, the incisures were not fully developed and appear as narrow rings. As development progressed, MUPP1 staining remains strong in incisures but not in paranodes. The insets in B-E show shifted images to illustrate that PATJ or MUPP1 staining (green) surrounds Caspr staining (red). Bar, 10 μm.
Differential distribution of claudins in autotypic tight junctions
The multidomain structure of MUPP1 and PATJ suggests that they may serve as a scaffold, organizing membrane complexes by binding to cell adhesion molecules. Tight junctions in epithelial cells contain several adhesion molecules, including occludin (Furuse et al., 1994), claudin (Furuse et al., 1998a; Morita et al., 1999a), and JAM (Martin-Padura et al., 1998). Occludin is expressed in sciatic nerve, but it is found in the perineurium and not in myelinating Schwann cells (Nagaoka et al., 1999). Similarly, we could not detect JAM in sciatic nerve using four different antibodies (Table I). The claudins are a large family of tight junction proteins that are differentially expressed in many tissues (Tsukita et al., 2001). In order to determine whether claudins are present in peripheral nerve, we performed reverse transcription–PCR on mouse sciatic nerve RNA, using primer pairs for claudin-1–16. The reaction products were cloned and sequenced, and claudin-1, -2, -5, -10, and -15 were identified.
Table I. Localization of tight junction proteins at Schmidt-Lanterman incisures and paranodal loops in myelinating Schwann cells.
| Proteins | Schmidt-Lanterman incisures | Paranodal loops | Antibody source/reference |
|---|---|---|---|
| Transmembrane | |||
| JAM | − | − | Bazzoni et al. (2000) |
| Occludin | − | − | Nagaoka et al. (1999) |
| Claudin-1 | − | +++ | Zymed |
| Claudin-2 | + | − | Zymed |
| Claudin-5 | +++ | − | Zymed and Morita et al. (1999c) |
| Claudin-11 | − | − | Morita et al. (1999b) |
| MUPP1 | +++ | − | Material and methods |
| Peripheral cytoplasmic | |||
| PATJ | + | +++ | Material and methods |
| ZO-1 | +++ | +++ | Zymed |
| ZO-2 | +++ | +++ | Bruce Stevenson |
| AF-6 | − | − | Transduction Laboratories |
| Par-3 | +++ | +++ | Lin et al. (2000) |
| Pals-1 | − | − | Kamberov et al. (2000) |
| MAGI-1 | − | − | Sigma-Aldrich |
| S-SCAM/MAGI-2 | +++ | − | Unpublished data; Sigma-Aldrich |
| Slipr/MAGI-3 | − | − | Unpublished data; Sigma-Aldrich |
| Cingulin | − | − | Citi et al. (1988) |
Except for cingulin, all peripheral proteins contain PDZ domains. Out of three transmembrane proteins of TJ that bind PDZ domains (JAM, occludin, and claudin), only members of the claudin family are found in myelinating Schwann cells.
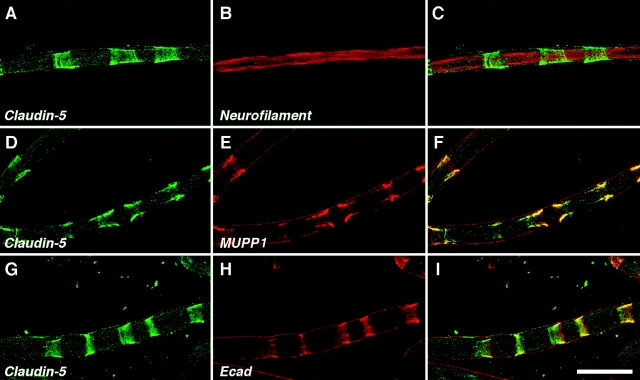
Because peripheral nerve contains several cell types, we used antibodies against claudin-1, -2, and -5 to determine whether they are expressed in myelinating Schwann cells. As depicted in Figs. 6–8, these claudins were localized at distinct sites. Claudin-1 was found at the paranodal region (Fig. 6, A–C), where it colocalized with PATJ at a ring that surrounded Caspr (Fig. 6, D–I). In addition, claudin-1 was found in the outer mesaxon along the internodes (Fig. 6 C, inset). Notably, this internodal staining of claudin-1 was more prominent in small than large diameter fibers (unpublished data). Claudin-2 was localized in a distinct ring surrounding the nodal voltage-gated Na+ channels (Fig. 7, A–C). Claudin-2 overlapped with ezrin and moesin (Fig. 7, D–I), two ERM proteins located in Schwann cell microvilli (Melendez-Vasquez et al., 2001; Scherer et al., 2001). In addition, claudin-2 was infrequently detected at the incisures (unpublished data). Claudin-5 was primarily detected at Schmidt-Lanterman incisures, as shown by double labeling with MUPP1 and E-cadherin (Fig. 8). Like MUPP1, claudin-5 was only rarely detected in the paranodal loops in adult rat sciatic nerve (unpublished data). During the development of peripheral nerve, claudin-5 colocalized with MUPP1, showing the same dynamic changes in expression at incisures (Fig. 9), as well as in the paranodes (unpublished data).
Figure 8.
Colocalization of claudin-5 and MUPP1 in incisures. Images of teased fibers from adult mouse sciatic nerves, double labeled for claudin-5 (green) and neurofilaments, MUPP1 or E-cadherin (Ecad), all in red. The right panel shows the merged images. Note that claudin-5 and MUPP1 are highly colocalized in incisures. The coating of MUPP1 and E-cadherin on the outside of the teased fibers is nonspecific, resulting from staining mouse teased fibers with a mouse monoclonal antibody. Bar, 25 μm.
Figure 7.
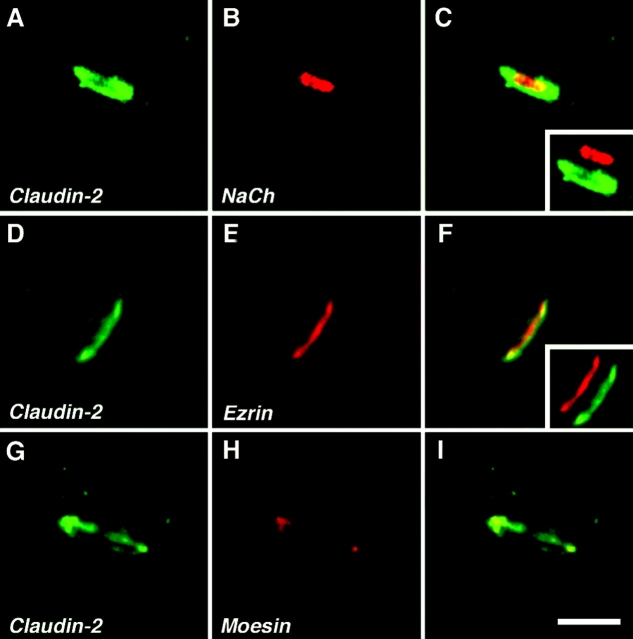
Localization of claudin-2 to the nodal region in peripheral nerves. Images of teased fibers from adult mouse sciatic nerve, double labeled for claudin-2 (green) and voltage-gated Na+ channels (A–C, NaCh), ezrin (D–F), or moesin (G–I), all in red. The left panel of each row shows the merged images. The insets in C and F show merged images in which the red and the green channels were shifted apart. Note that nodal membranes were labeled for NaCh, and were surrounded by a ring of claudin-2 staining. Claudin-2 colocalized with ezrin and moesin, which labeled Schwann cell microvilli. Bar, 5 μm.
Figure 9.
MUPP1 and claudin-5 appear together in developing myelinated nerves. These are images of teased fibers from rat sciatic nerves at the indicated ages, double labeled for MUPP1 (green) and claudin-5 (red). Note that claudin-5 and MUPP1 colocalized in incisures at all ages. Bar, 10 μm.
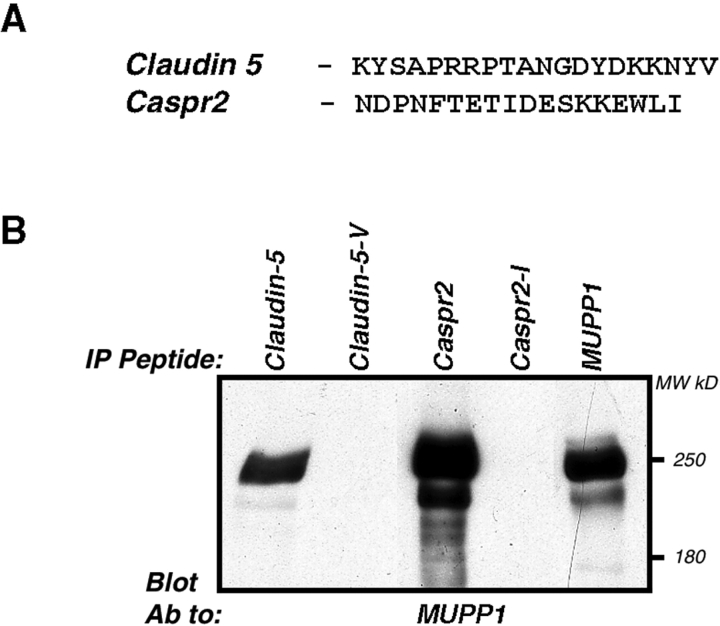
The colocalization of MUPP1 and claudin-5 suggested that they could associate. As shown in Fig. 10, peptides corresponding to the cytoplasmic tail of claudin-5 precipitated MUPP1 from HEK293 cells expressing this protein. As a positive control, we used the cytoplasmic domain of Caspr2, which interacts with various PDZ domain-containing proteins (Spiegel et al., 2002), including PATJ and MUPP1 (unpublished data). Removing the last amino acid from each of the peptides used completely abolished their binding, indicating that they interact with one or more PDZ domains present in MUPP1. Taken together, these data suggest that claudin-5 and MUPP1, as well as claudin-1 and PATJ may constitute tight junctional protein complexes that are coassembled during myelination in the PNS. This conclusion is further supported by recent studies, showing that the cytoplasmic tail of claudin-1 binds to PATJ (Roh et al., 2002a) and MUPP1 (Hamazaki et al., 2002).
Figure 10.
MUPP1 bind to the carboxyl terminus of claudin-5. (A) Amino acid sequences of the COOH-termini of claudin-5 and Caspr2 used in the binding experiments. Note that both proteins contain a sequence that may bind type-II PDZ domains. (B) The immobilized peptides shown in A, or the same peptides lacking the last amino acid, were mixed with lysates of HEK-293 cells expressing MUPP1. Purified complexes were separated on SDS-gel and immunoblotted using an antibody to MUPP1. Immunoprecipitation with MUPP1 antibody (MUPP1 IP) was used as a positive control. The location of molecular mass markers in kD is shown on the right.
Identification of additional tight junction components in myelinating Schwann cells
The above results prompted us to look for other tight junction components (Tsukita et al., 2001) by immunostaining. We found that myelinating Schwann cells also express other peripheral membrane proteins of tight junctions containing PDZ domains, such as the cell polarity protein Par3/ASIP (Joberty et al., 2000; Ebnet et al., 2001; Itoh et al., 2001), and the adaptor proteins MAGI-2/S-SCAM (Wu et al., 2000), and ZO-2 (Jesaitis and Goodenough, 1994; Itoh et al., 1999) in the different aspects of noncompact myelin (Table I). In addition, we found that ZO-1, which was previously reported to reside between the axon and the adaxonal Schwann cell membrane (Parmantier et al., 1999), was also located in the incisures and the paranodal loops (unpublished data). ZO-2 was similarly localized as ZO-1 (unpublished data). Other tight junction components, including cingulin, Pals1, MAGI-1, MAGI-3, and AF-6 were not detected, suggesting that tight junction complexes in myelinating Schwann cells may contain additional proteins yet to be identified.
Discussion
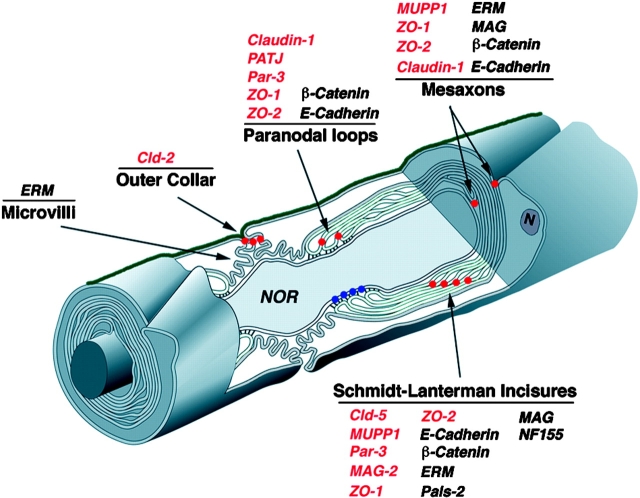
In the present study we have identified new components of autotypic tight junctions in myelinating Schwann cells (Table I; Fig. 11). Nine of these proteins, three claudins (claudin-1, -2, and -5), two multi-PDZ domain proteins (MUPP1 and PATJ), three MAGUK proteins (ZO-1, ZO-2, and MAGI-2), and the adaptor protein Par-3 were localized at tight junctions in epithelial cells (Furuse et al., 1998a; Izumi et al., 1998; Morita et al., 1999c; Hamazaki et al., 2002; Roh et al., 2002b). These proteins were found in different aspects of noncompact myelin, suggesting that myelinating Schwann cells have distinct autotypic tight junctions, each composed of a unique set of proteins. For example, in the adult rat sciatic nerve, MUPP1 and claudin-5 colocalized in the Schmidt-Lanterman incisures, whereas PATJ and claudin-1 were found at the paranodes. This complementary pattern of expression is particularly interesting, as PATJ and MUPP1 share similar domain organization and both interact with claudin-1 (Hamazaki et al., 2002; Lemmers et al., 2002; Roh et al., 2002a). In addition, the COOH-terminal tail of claudin-5 interacts with MUPP1 in a PDZ-dependent manner, suggesting that claudin-1/PATJ and claudin-5/MUPP1 are found in a complex at the paranodal loops and the incisures, respectively. Furthermore, the ability of distinct PDZ domains present in MUPP1 and PATJ to interact with different proteins, (Barritt et al., 2000; Becamel et al., 2001; Roh et al., 2002b) suggests that they serve as scaffolding proteins that organize specific membrane complexes at different autotypic junctions. Interestingly, DLT, the Drosophila homologue of MUPP1 and PATJ, binds to neurexin-IV, a protein that is essential for the formation of septate junctions between ensheathing glial cells in the fly (Baumgartner et al., 1996; Bhat et al., 1999). Thus, the presence of MUPP1 and PATJ in myelinating Schwann cells illustrates the way molecules that are involved in glial ensheathment of axons in invertebrates have evolved to generate the complex architecture of myelinated fibers in vertebrates.
Figure 11.
Location of autotypic junction proteins in myelinating Schwann cells. The organization of peripheral myelinated nerve is shown schematically. The location of autotypic junctions in the Schmidt Lanterman incisures, paranodal loops, mesaxons and the outer aspect of the nodal gap are marked with red dots. The heterotypic septate-like junction formed between the axon and the paranodal loops of the myelinating Schwann cell is labeled with blue dots. The basal lamina covering the Schwann cell-axon unit is shown in green. The localization of different proteins discussed in this study is listed. Tight junction proteins are labeled in red. Note that autotypic tight junctions present in different aspects of noncompact myelin contain distinct junctional complexes (modified from Spiegel and Peles [2002] and used with permission of Taylor and Francis, www.tandf.co.uk).
Claudins are integral membrane proteins with four transmembrane domains that are exclusively localized at tight junction strands (Tsukita and Furuse, 2000). Furthermore, the expression of claudins appears to be necessary and sufficient for the formation of tight junctions (Furuse et al., 1998b). Although some cells, including oligodendrocytes (Morita et al., 1999b), contain only one claudin, most cell types express more than two claudins (Tsukita et al., 2001). Here we showed that myelinating Schwann cells express at least three different claudins. However, whereas in simple epithelia multiple claudins are found at the same tight junction strand, in myelinating Schwann cells they are found in different locations. This is reminiscent of the differential distribution of the GAP junction proteins Cx32 and Cx29 in different aspects of non-compact myelin (Altevogt et al., 2002). Claudin-1 was found in the paranodal loops and the mesaxon, whereas claudin-5 was located at the incisures of Schmidt-Lanterman. The localization of these claudins agrees well with freeze-fracture EM data demonstrating tight junctional strands in the paranodal loops, Schmidt-Lanterman incisures and the mesaxon (Mugnaini and Schnapp, 1974; Sandri et al., 1977; Tetzlaff, 1978). Furthermore, the stronger mesaxonal and paranodal expression of claudin-1 in smaller than in larger fibers is consistent with morphological data, reporting more tight junctions in small nerve fibers in the PNS (Shinowara et al., 1980; Tetzlaff, 1982).
Claudin-2 appeared as a ring that surrounded Na+ channels at the nodes of Ranvier and colocalized with ERM proteins, which are present in the microvilli (Melendez-Vasquez et al., 2001; Scherer et al., 2001). The microvilli, which encapsulate the nodes, are emanating from the outer aspect of the Schwann cell membrane. At this region, the outer collars of two meeting Schwann cells are joined by tight junctions (Berthold and Rydmark, 1983). The presumption that claudin-2 forms these tight junctions needs to be confirmed by immuno-EM. This location is analogous to the site of tight junctions in epithelial cells, which are found just below the microvilli that are emanating from the apical membrane. The function of claudin-2 at this site is not clear; especially as it was shown that the nodal gap is permeable to horseradish peroxidase applied outside the nerve fibers, indicating that it is not sealed off by tight junctions (Hall and Williams, 1971). Further studies using Schwann cell–specific claudin-2 knockout mice will be required to resolve its function in peripheral nerve.
Tight junctions are generated through multiple interactions between cell adhesion molecules and several adapter proteins, which in turn recruit additional peripheral membrane proteins to the tight junction plaques (Tsukita et al., 2001). MUPP1 and PATJ appeared together with claudins during development of myelinating Schwann cells, making it difficult to conclude whether claudins recruit MUPP1 and PATJ to autotypic junctions. In addition, we cannot rule out the existence of other membrane proteins, which nucleate a complex that recruits PATJ, MUPP1, and the claudins. Although PATJ interacts with claudin-1 through its eighth PDZ domain, it is recruited to tight junctions by binding to ZO-3 (Roh et al., 2002a). In mammalian epithelial junctions, PATJ is also associated with the adapter protein Pals-1 (Roh et al., 2002a). Although Pals-1 was not detected in myelinating Schwann cells, we did observe the presence of its related protein Pals-2 in Schmidt-Lanterman incisures (unpublished data), suggesting that similar protein complexes containing claudins, PATJ or MUPP1, Pals-2, and possibly ZO-3 are involved in the formation of tight junction complexes in the paranodal loops and the incisures. The function of the different PDZ adaptor proteins described here may extend beyond tight junctions, as some of them (i.e., members of the ZO and MAGI families) were shown to be present at both tight and adherens junctions (Ide et al., 1999; Nishimura et al., 2000).
In epithelia, tight junctions are specialized cell–cell contact sites that act as a selective permeability barrier (gate function) and as an intramembranous fence that restricts the movement of lipids and proteins from specific membrane domains (Mitic and Anderson, 1998). In agreement with these roles, tight junctions between the paranodal terminal loops appear to prevent large molecules from entering the potential space between the layers of the myelin sheath (Revel and Hamilton, 1969; Hall and Williams, 1971; Tabira et al., 1978; MacKenzie et al., 1984). Tight junctions may also serve to keep the membrane components of incisures and paranodes distinct from compact myelin. In central nervous system (CNS) myelin sheaths, tight junctions are predominantly found in a series of radially arranged intralamellar strands in compact myelin (the so-called radial component), and have been proposed to increase its mechanical strength (Tabira et al., 1978; Dermietzel et al., 1980; Gow et al., 1999; Morita et al., 1999b). The importance of this radial component was demonstrated using claudin-11–null mice, which lack tight junction strands in CNS myelin and exhibit slower conduction velocity (Gow et al., 1999). Freeze-fracture EM suggests that gap junctions are enclosed between tight junction strands in incisures and paranodes (Sandri et al., 1977). These gap junctions provide a relatively short pathway through the myelin sheath that links the membrane closer to the axon (adaxonal) and the perinuclear region of the Schwann cell (Balice-Gordon et al., 1998). Disruption of this radial pathway may lead to development of demyelination in X-linked form of Charcot-Marie-Tooth disease (Bergoffen et al., 1993). The presence of tight junction components in the Schmidt-Lanterman incisures may contribute to maintaining the radial pathway by keeping the myelin membranes attached at the point where the compact myelin opens up to enclose the cytoplasm, as well as by providing a barrier that restricts the movement of compact myelin proteins. Similarly to the proposed function of adherens junctions (Fannon et al., 1995), tight junctions present in the paranodal loops, outer mesaxon and the Schmidt-Lanterman incisures may stabilize newly formed wraps of myelin during development of the nerve. Finally, as tight junctions are involved in polarized vesicle transport (Zahraoui et al., 2000), autotypic tight junctions at the Schmidt-Lanterman incisures may also function as a sorting center for protein trafficking. Such a mechanism would insure fast and efficient replacement of proteins and lipids from the myelin sheath. The identification of autotypic junction components in myelinating Schwann cells described here provides a further step towards the understanding of the way the complex structure of these cells is formed and maintained.
Materials and methods
Northern blot and PCR analysis
Adult rat sciatic nerve lesions, RNA extraction, and Northern blots were performed as described previously (Scherer et al., 1995). Full-length rat MUPP1 cDNA (Ullmer et al., 1998), was used as a probe. Identification of claudins expressed in sciatic nerve was done by RT-PCR reaction using total RNA isolated from mouse sciatic nerve. The following primer sets made according to the sequence of mouse claudin 1-16 were used: 5′cld1-acgagggactgtggatgtc, 3′cld1-tcaaggggtcatagaattcttg; 5′cld2-tgcgacacacagcacaggc, 3′cld2-ccagcggcgagtagaagtc; 5′cld3-gtggatgaactgcgtggtgc, 3′cld3-ctgggcctcgggcaccaac; 5′cld4-gagggcctctggatgaactg, 3′cld4-cacatagggttgtagaagtc; 5′cld5-gaaggggctgtggatgtcg, 3′cld5-accgtcggatcatagaactcg; 5′cld6-gggaggggctgtggtgtc, 3′cld6-ccaccaaggggttgtagaagtc; 5′cld7-gtacaaggggctctggatgg, 3′cld7-gtctgtgacaatctgatgacc; 5′cld8-gggaaggcttgtggatgaattg, 3′cld8- caccagtgggttgtagaagtc; 5′cld9-gggaggggctgtggtgtc, 3′cld9-ggcttccgcaaccagtggg; 5′cld10-gccaacctgtggaagatctg, 3′cld10-gtttgcatacagggaacagcc; 5′cld11- ggcctgtgggctgactgcg, 3′cld11-ccagatggtggcgacaatgg; 5′cld12-gagatgtccacgcagccac, 3′cld12-tcataccgggcacacttcac; 5′cld13-catttgggagggtctgtggc, 3′cld13-caagcaatgggttaaagaagcc; 5′cld14-cctgaagggactgtggatgg, 3′cld14-atgccactgggcagcagcg; 5′cld15-ggcttcttcatgtcagccctg, 3′cld15-ctgcaatggccagcagcttg; and 5′cld16-gagaggaactacccttatgcc, 3′cld16-attgagtaattaaacgggacagg. The reaction products were isolated from an agarose gel, cloned in pCR2 TA cloning vector (Invitrogen) and sequenced.
Antibodies
Antibodies to PATJ and MUPP1 were generated by immunizing rabbits with GST fusion proteins (Amersham Biosciences) containing the third PDZ domain of PATJ (Ab PJ3; aa 320–450), MUPP1 (Ab M3; aa 363–466), the region between the fifth and sixth PDZ domains of MUPP1 (Ab M56; aa 766–999), or the same region of PATJ (Ab PJ56; aa 780–1062). Mouse polyclonal antibodies were also generated using the same GST fusion proteins as antigens. A polyclonal antibody that reacted with both MAGI2 and MAGI3 was generated by immunizing rabbits with a GST fusion protein containing the third and fourth PDZ domains of rat Slipr (Ab M23; aa 424–607). Affinity purification was performed by first removing the antibodies against GST by passing the anti-serum through a column of Sepharose-GST (Pierce Chemical Co.) and then on a column of the different GST fusion proteins used for immunization. Antibodies against Caspr (Poliak et al., 2001), Na+ channels (Rasband et al., 1999), Cx32 (Goodenough et al., 1988), Pals1 and Pals2 (Kamberov et al., 2000; Roh et al., 2002b), and cingulin (Citi et al., 1988) have been described previously. Antibodies to neurofilament (a mixture of anti-NF 68, 160, and 200), ezrin and MAGI3/Slipr were obtained from Sigma-Aldrich, monoclonal antibodies to E-cadherin and moesin from Transduction Laboratories, anti-MAG from Roche, and anti-claudin 5 and claudin-1 and -2 antibodies from Zymed Laboratories.
Immunoprecipitation, peptide pulldown, and immunoblot analysis
Immunoprecipitation and immunoblotting analyses of transfected HEK-293 cells were done essentially as previously described (Poliak et al., 1999). Pulldown experiments were done essentially as described (Spiegel et al., 2002), using HEK-293 cells transfected with the full-length rat MUPP1 (Ullmer et al., 1998). Cells were solubilized in TNTG (20 mM Tris 7.5, 0.3% Triton X-100, 150 mM NaCl, 10% glycerol and protease inhibitors), and the lysates were incubated with 40 μg of biotinylated peptides coupled to Neutravidin beads (Pierce Chemical Co.). Bound proteins were washed twice with HNTG (20 mM Hepes 7.0, 0.1% Triton X-100, 150 mM NaCl, 10% glycerol), once in PBS, and then separated on SDS gels and immunoblotted with anti-MUPP1 antibody. The following peptides were used: claudin 5-NGDYDKKNYV-COOH, Caspr2CT-NFTETIDESKKEWLI, or the corresponding peptides lacking the last amino acid.
Immunofluorescence
Sciatic nerves were dissected and immersed in 4% paraformaldehyde or Zamboni's fixative for 10 min. After desheathing, nerves were teased on gelatin-coated slides, dried for 2 h and frozen at –20°C. The preparations were postfixed/permeabilized in methanol or acetone –20°C for 20 min or permeabilized in PBS/0.5% Triton X-100 for 10 min. Slides were washed and blocked for 30 min with PBS containing 10% normal goat serum, 0.1% Triton X-100 and 1% glycine. The samples were incubated overnight at 4°C with the different primary antibodies described above, diluted in blocking solution. Antibodies M3 and PJ3 were used to detect MUPP1 and PATJ, respectively. Slides were washed three times for 5 min in PBS and incubated for 1 h in secondary antibodies: anti–mouse-Cy3 or biotinylated-anti-rabbit (Jackson ImmunoResearch Laboratories). The latter was washed in PBS and reacted with Streptavidin-Alexa-488 (Molecular Probes) for 45 min, and was further processed as described previously (Poliak et al., 2001). Immunofluorescence was viewed and analyzed using a BioRad confocal microscope, or a Zeiss Axioplan microscope equipped with SPOT-II (Diagnostic Instruments) cooled CCD camera.
Acknowledgments
We thank Shoichiro Tsukita (Kyoto University, Japan) Roberto Bruzzone (Pasteur Institute, Paris), Ben Margolis (University of Michigan Medical School, Ann Arbor, MI), Gianfranco Bazzoni and Elisabetta Dejana (Instituto di Recerche Farmacologiche Mario Negri, Milano, Italy), Tony Pawson (Mount Sinai Hospital, Toronto, Ontario, Canada), and Bruce Stevenson (University of Alberta, Edmonton, Canada) for different reagents. We are especially grateful to Sergey Zozulya for his help with the T7 library screening, and Susan Shumas for technical assistance.
This research was supported by The Israel Science Foundation, the United States-Israel Science Foundation, Dr. Pearl H. Levine Foundation for Research in the Neurosciences, and the Pasteur-Weizmann Joint Research Program. E. Peles is an Incumbent of the Madeleine Haas Russell Career Development Chair.
Footnotes
Abbreviations used in this paper: CNS, central nervous system; Cx, Connexin; DLT, Discs Lost; ERM, Ezrin–Radixin–Moesin; JAM, junctional adhesion molecule; MAGI, membrane-associated guanylate-kinase inverted; MAGUK, membrane-associated guanylate-kinase; MUPP, multi-PDZ protein; P, postnatal day; Pals, protein associated with Lin-7; PATJ, Pals1-associated tight junction protein; PDZ, PSD-95/Disc Large/ZO-1; PNS, peripheral nervous system; ZO, zona occludens.
References
- Altevogt, B.M., K.A. Kleopa, F.R. Postma, S.S. Scherer, and D.L. Paul. 2002. Cx29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J. Neurosci. 15:6458–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon, R.J., L.J. Bone, and S.S. Scherer. 1998. Functional gap junctions in the Schwann cell myelin sheath. J. Cell Biol. 142:1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt, D.S., M.T. Pearn, A.H. Zisch, S.S. Lee, R.T. Javier, E.B. Pasquale, and W.B. Stallcup. 2000. The multi-PDZ domain protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J. Cell. Biochem. 79:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner, S., J.T. Littleton, K. Broadie, M.A. Bhat, R. Harbecke, J.A. Lengyel, R. Chiquet-Ehrismann, A. Prokop, and H.J. Bellen. 1996. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 87:1059–1068. [DOI] [PubMed] [Google Scholar]
- Bazzoni, G., O.M. Martinez-Estrada, F. Orsenigo, M. Cordenonsi, S. Citi, and E. Dejana. 2000. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275:20520–20526. [DOI] [PubMed] [Google Scholar]
- Becamel, C., A. Figge, S. Poliak, A. Dumuis, E. Peles, J. Bockaert, H. Lubbert, and C. Ullmer. 2001. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J. Biol. Chem. 276:12974–12982. [DOI] [PubMed] [Google Scholar]
- Bergoffen, J., S.S. Scherer, S. Wang, M.O. Scott, L.J. Bone, D.L. Paul, K. Chen, M.W. Lensch, P.F. Chance, and K.H. Fischbeck. 1993. Connexin mutations in X-linked Charcot-Marie tooth disease. Science. 262:2039–2042 [DOI] [PubMed] [Google Scholar]
- Berthold, C.H., and M. Rydmark. 1983. Electron microscopic serial section analysis of nodes of Ranvier in lumbosacral spinal roots of the cat: ultrastructural organization of nodal compartments in fibres of different sizes. J. Neurocytol. 12:475–505. [DOI] [PubMed] [Google Scholar]
- Bhat, M.A., S. Izaddoost, Y. Lu, K.O. Cho, K.W. Choi, and H.J. Bellen. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845. [DOI] [PubMed] [Google Scholar]
- Citi, S., H. Sabanay, R. Jakes, B. Geiger, and J. Kendrick-Jones. 1988. Cingulin, a new peripheral component of tight junctions. Nature. 333:272–276. [DOI] [PubMed] [Google Scholar]
- Cordenonsi, M., F. D'Atri, E. Hammar, D.A. Parry, J. Kendrick-Jones, D. Shore, and S. Citi. 1999. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J. Cell Biol. 147:1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel, R., A.G. Leibstein, and D. Schunke. 1980. Interlamellar tight junctions of central myelin: A freeze-fracture and cytochemical study on their component of the tight junction. J. Cell Sci. 109:2287–2298. [DOI] [PubMed] [Google Scholar]
- Ebnet, K., A. Suzuki, Y. Horikoshi, T. Hirose, M.K. Meyer Zu Brickwedde, S. Ohno, and D. Vestweber. 2001. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J. 20:3738–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber, S., G. Zanazzi, W. Ching, S. Scherer, T.A. Milner, E. Peles, and J.L. Salzer. 1997. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 139:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning, A.S., B.J. Jameson, L.A. Jesaitis, and J.M. Anderson. 1998. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273:29745–29753. [DOI] [PubMed] [Google Scholar]
- Fannon, A.M., D.L. Sherman, G. Ilyina-Gragerova, P.J. Brophy, V.L. Friedrich, Jr., and D.R. Colman. 1995. Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J. Cell Biol. 129:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, M., T. Hirase, M. Itoh, A. Nagafuchi, S. Yonemura, and S. Tsukita. 1993. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123:1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, M., M. Itoh, T. Hirase, A. Nagafuchi, S. Yonemura, and S. Tsukita. 1994. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 127:1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, M., K. Fujita, T. Hiiragi, K. Fujimoto, and S. Tsukita. 1998. a. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141:1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, M., H. Sasaki, K. Fujimoto, and S. Tsukita. 1998. b. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal, L., A. Betanzos, and A. Avila-Flores. 2000. MAGUK proteins: structure and role in the tight junction. Semin. Cell Dev. Biol. 11:315–324. [DOI] [PubMed] [Google Scholar]
- Goodenough, D.A., D.L. Paul, and L. Jesaitis. 1988. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J. Cell Biol. 107:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow, A., C.M. Southwood, J.S. Li, M. Pariali, G.P. Riordan, S.E. Brodie, J. Danias, J.M. Bronstein, B. Kachar, and R.A. Lazzarini. 1999. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 99:649–659. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2000. Regulation of cadherin adhesive activity. J. Cell Biol. 148:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, S.M., and P.L. Williams. 1971. The distribution of electron-dense tracers in peripheral nerve fibres. J. Cell Sci. 8:541–555. [DOI] [PubMed] [Google Scholar]
- Hamazaki, Y., M. Itoh, H. Sasaki, M. Furuse, and S. Tsukita. 2002. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J. Biol. Chem. 277:455–461. [DOI] [PubMed] [Google Scholar]
- Haskins, J., L. Gu, E.S. Wittchen, J. Hibbard, and B.R. Stevenson. 1998. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 141:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, N., Y. Hata, H. Nishioka, K. Hirao, I. Yao, M. Deguchi, A. Mizoguchi, H. Nishimori, T. Tokino, Y. Nakamura, and Y. Takai. 1999. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 18:7810–7815. [DOI] [PubMed] [Google Scholar]
- Itoh, M., K. Morita, and S. Tsukita. 1999. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J. Biol. Chem. 274:5981–5986. [DOI] [PubMed] [Google Scholar]
- Itoh, M., H. Sasaki, M. Furuse, H. Ozaki, T. Kita, and S. Tsukita. 2001. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 154:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi, Y., T. Hirose, Y. Tamai, S. Hirai, Y. Nagashima, T. Fujimoto, Y. Tabuse, K.J. Kemphues, and S. Ohno. 1998. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 143:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis, L.A., and D.A. Goodenough. 1994. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila Discs Large tumor suppressor protein. J. Cell Biol. 124:949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty, G., C. Petersen, L. Gao, and I.G. Macara. 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2:531–539. [DOI] [PubMed] [Google Scholar]
- Kamberov, E., O. Makarova, M. Roh, A. Liu, D. Karnak, S. Straight, and B. Margolis. 2000. Molecular cloning and characterization of Pals, proteins associated with mLin-7. J. Biol. Chem. 275:11425–11431. [DOI] [PubMed] [Google Scholar]
- Lemmers, C., E. Medina, M.H. Delgrossi, D. Michel, J.P. Arsanto, and A. Le Bivic. 2002. hINADl/PATJ, a homolog of Discs Lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J. Biol. Chem. 277:25408–25415. [DOI] [PubMed] [Google Scholar]
- Lin, D., A.S. Edwards, J.P. Fawcett, G. Mbamalu, J.D. Scott, and T. Pawson. 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2:540–547. [DOI] [PubMed] [Google Scholar]
- MacKenzie, M.L., Z. Shorer, M.N. Ghabriel, and G. Allt. 1984. Myelinated nerve fibres and the fate of lanthanum tracer: an in vivo study. J. Anat. 138:1–14. [PMC free article] [PubMed] [Google Scholar]
- Martin-Padura, I., S. Lostaglio, M. Schneemann, L. Williams, M. Romano, P. Fruscella, C. Panzeri, A. Stoppacciaro, L. Ruco, A. Villa, et al. 1998. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez, C.V., J.C. Rios, G. Zanazzi, S. Lambert, A. Bretscher, and J.L. Salzer. 2001. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proc. Natl. Acad. Sci. USA. 98:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic, L.L., and J.M. Anderson. 1998. Molecular architecture of tight junctions. Annu. Rev. Physiol. 60:121–142. [DOI] [PubMed] [Google Scholar]
- Morita, K., M. Furuse, K. Fujimoto, and S. Tsukita. 1999. a. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA. 96:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, K., H. Sasaki, K. Fujimoto, M. Furuse, and S. Tsukita. 1999. b. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J. Cell Biol. 145:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, K., H. Sasaki, M. Furuse, and S. Tsukita. 1999. c. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 147:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini, E., and B. Schnapp. 1974. Possible role of zonula occludens of the myelin sheath in demyelinating conditions. Nature. 251:725–727. [DOI] [PubMed] [Google Scholar]
- Nagaoka, T., M. Oyamada, S. Okajima, and T. Takamatsu. 1999. Differential expression of gap junction proteins connexin26, 32, and 43 in normal and crush-injured rat sciatic nerves. Close relationship between connexin43 and occludin in the perineurium. J. Histochem. Cytochem. 47:937–948. [DOI] [PubMed] [Google Scholar]
- Nishimura, W., T. Iizuka, S. Hirabayashi, N. Tanaka, and Y. Hata. 2000. Localization of BAI-associated protein1/membrane-associated guanylate kinase-1 at adherens junctions in normal rat kidney cells: polarized targeting mediated by the carboxyl-terminal PDZ domains. J. Cell. Physiol. 185:358–365. [DOI] [PubMed] [Google Scholar]
- Parmantier, E., B. Lynn, D. Lawson, M. Turmaine, S.S. Namini, L. Chakrabarti, A.P. McMahon, K.R. Jessen, and R. Mirsky. 1999. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron. 23:713–724. [DOI] [PubMed] [Google Scholar]
- Patrie, K.M., A.J. Drescher, A. Welihinda, P. Mundel, and B. Margolis. 2002. Interaction of two actin-binding proteins, synaptopodin and a-actinin-4, with the tight junction protein MAGI-1. J. Biol. Chem. 277:30183–30190 [DOI] [PubMed] [Google Scholar]
- Philipp, S., and V. Flockerzi. 1997. Molecular characterization of a novel human PDZ domain protein with homology to INAD from Drosophila melanogaster. FEBS Lett. 413:243–248. [DOI] [PubMed] [Google Scholar]
- Poliak, S., L. Gollan, R. Martinez, A. Custer, S. Einheber, J.L. Salzer, J.S. Trimmer, P. Shrager, and E. Peles. 1999. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 24:1037–1047. [DOI] [PubMed] [Google Scholar]
- Poliak, S., L. Gollan, D. Salomon, E.O. Berglund, R. Ohara, B. Ranscht, and E. Peles. 2001. Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J. Neurosci. 21:7568–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband, M.N., J.S. Trimmer, E. Peles, S.R. Levinson, and P. Shrager. 1999. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J. Neurocytol. 28:319–331. [DOI] [PubMed] [Google Scholar]
- Revel, J.-P., and D.W. Hamilton. 1969. The double nature of the intermediate dense line in peripheral nerve myelin. Anat. Rec. 163:7–16. [DOI] [PubMed] [Google Scholar]
- Roh, M.H., C.J. Liu, S. Laurinec, and B. Margolis. 2002. a. The carboxy-terminus of zona Occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J. Biol. Chem. 277:27501–27509 [DOI] [PubMed] [Google Scholar]
- Roh, M.H., O. Makarova, C.J. Liu, K. Shin, S. Lee, S. Laurinec, M. Goyal, R. Wiggins, and B. Margolis. 2002. b. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 157:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri, C., J.M. Van Buren, and K. Akert. 1977. Membrane morphology of the vertebrate nervous system. A study with freeze-etch technique. Prog. Brain Res. 46:1–384. [PubMed] [Google Scholar]
- Scherer, S.S., and J. Salzer. 2001. Axon-Schwann cell interactions in peripheral nerve degeneration and regeneration. In Glial Cell Development. K.R. Jessen and W.D. Richardson, editors. Oxford University Press, Oxford. 299–330.
- Scherer, S.S., S.M. Deschenes, Y.T. Xu, J.B. Grinspan, K.H. Fischbeck, and D.L. Paul. 1995. Connexin32 is a myelin-related protein in the PNS and CNS. J. Neurosci. 15:8281–8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, S.S., T. Xu, P. Crino, E.J. Arroyo, and D.H. Gutmann. 2001. Ezrin, radixin, and moesin are components of Schwann cell microvilli. J. Neurosci. Res. 65:150–164. [DOI] [PubMed] [Google Scholar]
- Shinowara, N.L., W.B. Beutel, and J.P. Revel. 1980. Comparative analysis of junctions in the myelin sheath of central and peripheral axons of fish, amphibians and mammals: a freeze-fracture study using complementary replicas. J. Neurocytol. 9:15–38. [DOI] [PubMed] [Google Scholar]
- Small, J.R., M.N. Ghabriel, and G. Allt. 1987. The development of Schmidt-Lanterman incisures: an electron microscope study. J. Anat. 150:277–286. [PMC free article] [PubMed] [Google Scholar]
- Spiegel, I., and E. Peles. 2002. Cellular junctions of myelinated nerves. Mol. Membr. Biol. 19:95–101. [DOI] [PubMed] [Google Scholar]
- Spiegel, I., D. Salomon, B. Erne, N. Schaeren-Wiemers, and E. Peles. 2002. Caspr3 and Caspr4, two novel members of the Caspr family are expressed in the nervous system and interact with PDZ domains. Mol. Cell. Neurosci. 20:283–297. [DOI] [PubMed] [Google Scholar]
- Tabira, T.M.J.C., P.J. Reier, and H.D. Webster. 1978. An experimental analysis of interlamellar tight junctions in amphibian and mammalian C.N.S. myelin. J. Neurocytol. 7:489–503. [DOI] [PubMed] [Google Scholar]
- Tetzlaff, W. 1978. The development of a zonula occludens in peripheral myelin of the chick embryo. A freeze-fracture study. Cell Tissue Res. 189:187–201. [DOI] [PubMed] [Google Scholar]
- Tetzlaff, W. 1982. Tight junction contact events and temporary gap junctions in the sciatic nerve fibres of the chicken during Wallerian degeneration and subsequent regeneration. J. Neurocytol. 11:839–858. [DOI] [PubMed] [Google Scholar]
- Trapp, B.D., S.B. Andrews, A. Wong, M. O'Connell, and J.W. Griffin. 1989. Co-localization of the myelin-associated glycoprotein and the microfilament components, F-actin and spectrin, in Schwann cells of myelinated nerve fibres. J. Neurocytol. 18:47–60. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., and M. Furuse. 2000. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann. NY Acad. Sci. 915:129–135. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., M. Furuse, and M. Itoh. 2001. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2:285–293. [DOI] [PubMed] [Google Scholar]
- Ullmer, C., K. Schmuck, A. Figge, and H. Lubbert. 1998. Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett. 424:63–68. [DOI] [PubMed] [Google Scholar]
- Wu, X., K. Hepner, S. Castelino-Prabhu, D. Do, M.B. Kaye, X.J. Yuan, J. Wood, C. Ross, C.L. Sawyers, and Y.E. Whang. 2000. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl. Acad. Sci. USA. 97:4233–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui, A., D. Louvard, and T. Galli. 2000. Tight junction, a platform for trafficking and signaling protein complexes. J. Cell Biol. 151:F31–F36. [DOI] [PMC free article] [PubMed] [Google Scholar]