Abstract
Regulatory proteins have been identified in embryonic development of the endocrine pancreas. It is unknown whether these factors can also play a role in the formation of pancreatic endocrine cells from postnatal nonendocrine cells. The present study demonstrates that adult human pancreatic duct cells can be converted into insulin-expressing cells after ectopic, adenovirus-mediated expression of the class B basic helix-loop-helix factor neurogenin 3 (ngn3), which is a critical factor in embryogenesis of the mouse endocrine pancreas. Infection with adenovirus ngn3 (Adngn3) induced gene and/or protein expression of NeuroD/β2, Pax4, Nkx2.2, Pax6, and Nkx6.1, all known to be essential for β-cell differentiation in mouse embryos. Expression of ngn3 in adult human duct cells induced Notch ligands Dll1 and Dll4 and neuroendocrine- and β-cell–specific markers: it increased the percentage of synaptophysin- and insulin-positive cells 15-fold in ngn3-infected versus control cells. Infection with NeuroD/β2 (a downstream target of ngn3) induced similar effects. These data indicate that the Delta-Notch pathway, which controls embryonic development of the mouse endocrine pancreas, can also operate in adult human duct cells driving them to a neuroendocrine phenotype with the formation of insulin-expressing cells.
Keywords: neurogenin 3; islets of langerhans; transdifferentiation; insulin; diabetes mellitus
Introduction
Several studies have suggested that adult β-cells might originate from duct or duct-associated cells (Slack, 1995; Bouwens and Pipeleers, 1998; Edlund, 1999; Bonner-Weir et al., 2000). Evidence for this concept is largely indirect, and the underlying mechanisms are unknown. It is also conceivable that acinar cells can become a source of new β-cells in view of their plasticity, allowing them to transdifferentiate into hepatocytes (Shen et al., 2000) and into duct cells (Rooman et al., 2000). If postnatal acinar and/or duct cells could form new β-cells, they would become a particularly useful target for therapies that aim β-cell replacement in diabetic patients (Keymeulen et al., 1998; Shapiro et al., 2000), since both cell types are abundantly available in the pancreas of these patients and in donor organs. To assess such potential, we examined whether expression of key embryonic transcription factors in adult human duct cells could induce their differentiation into insulin-expressing cells.
Experiments with transgenic mice have indicated key factors in the embryonic development of their endocrine pancreas. Analysis of null mutants for Pdx1/Ipf1, ngn3, NeuroD/β2, Pax4, Nkx2.2, Nkx6.1, or Pax6 has identified a hierarchy of transcription factors that control embryonic formation of pancreatic islets (for review see Sander and German, 1997; Jensen et al., 2000a; Edlund, 2001). It is unknown whether these factors play a role in the postnatal growth of the pancreatic β-cell mass and whether they can be used to induce formation of human β-cells from postnatal nonendocrine cells. In this perspective, we examined the endocrinogenic potential of the class B basic helix-loop-helix (bHLH)* transcription factor neurogenin 3 (ngn3), which seems to function as a major and timely switch in the rodent embryonic pancreas (Gradwohl et al., 2000). When expression of ngn3 is directed ectopically into the embryonic epithelium, pancreas precursor cells develop prematurely and exclusively into glucagon-producing cells (Apelqvist et al., 1999; Schwitzgebel et al., 2000). Similarly, ngn3 induced premature differentiation into glucagon- and somatostatin-producing cells when introduced into early chicken endoderm (Grapin-Botton et al., 2001). The failure to induce insulin-producing cells might indicate that these immature cells lack the competence to drive β-cell differentiation. The differentiating activity of ngn3 is under control of Notch signaling. Indeed, null mutant mice for the Notch ligand Dll1, for an intracellular mediator of Notch signaling RBP-Jκ (Apelqvist et al., 1999), or for a downstream bHLH repressor HES1 (Jensen et al., 2000b), which possibly controls ngn3 (Tanabe and Jessell, 1996), all show premature endocrine differentiation. Therefore, we ectopically expressed ngn3 in adult duct cells to assess its role as a switch activating the expression of other developmental transcription factors and Delta-Notch proteins and consequently resulting in the appearance of endocrine differentiation markers, in particular insulin.
Results
Absence of regulators of embryonic endocrine differentiation in postnatal human pancreatic duct cells
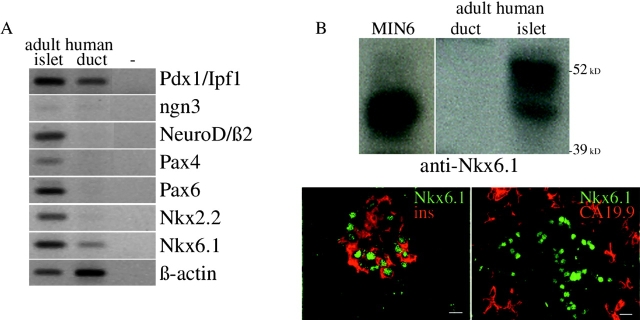
Adult human β-cell preparations express a series of transcription factors that are crucial for embryonic development of mouse endocrine pancreas (Fig. 1 A). Transcripts encoding Pdx1/Ipf1, NeuroD/β2, Pax4, Pax6, Nkx2.2, and Nkx6.1 were abundant in adult human islets. The expression of Pax4 in human islets is at variance with its absence in postnatal mouse islets (Smith et al., 1999) or rat-purified β-cells (see Fig. 4 A). A parallel analysis of adult human duct cell transcripts shows the presence of relatively high levels Pdx1/Ipf1 and Nkx6.1 transcripts (Fig. 1 A). Pdx1/Ipf1 is also expressed in adult human duct cells at the protein level (Heimberg et al., 2000), but this is not the case for Nkx6.1 (Fig. 1 B), suggesting it is subject to posttranscriptional regulation. Sections of adult human pancreas with both β-cells and duct cells clearly indicated that Nkx6.1-positive nuclei were associated with insulin-containing cells and not with cells that expressed the ductal cell marker CA19.9 (Bouwens and Pipeleers, 1998) (Fig. 1 B). Ngn3 mRNA level was low in both islets and duct cells. Compared with islet cells, the duct cell levels of transcripts coding for Notch 1, 2, and 3 receptors were higher, those for Jagged 1 and 2 ligands were similar, and those for Dll1 and Dll4 ligands were much lower (see Fig. 3 C).
Figure 1.
Endogenous expression of key developmental transcription factors in adult human pancreatic duct cells. (A) RT-PCR analysis of RNA extracted from adult human duct cells compared with adult human islet cells. (B) Nkx6.1 protein in adult human pancreas as determined by immunoblot of extracts from enriched duct or islet cells (MIN6 were control cells), and immunostaining on sections of human donor pancreas. Bars, 10 μm.
Figure 4.
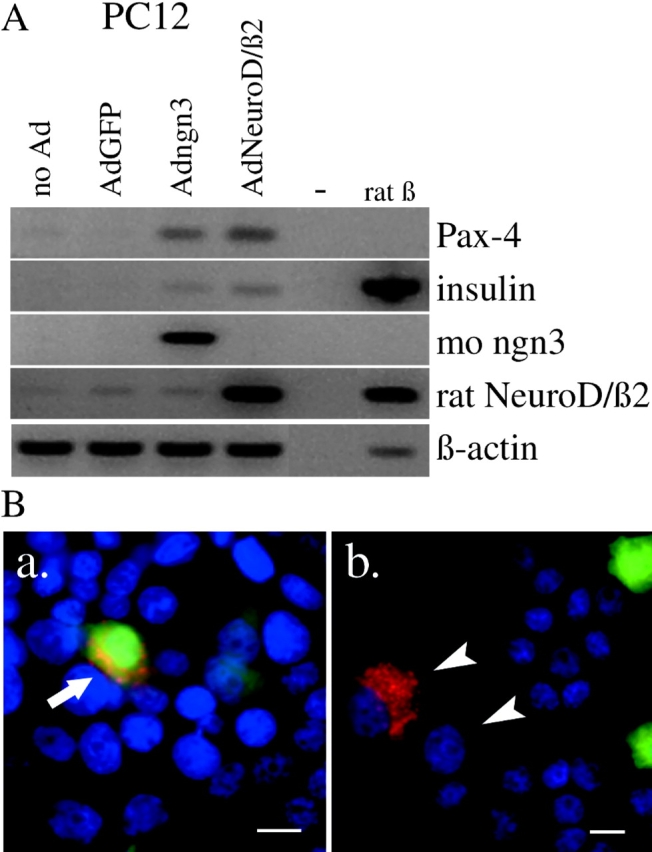
Effect of adenovirus-mediated expression of ngn3 or NeuroD/β2 in the neuroendocrine cell line PC12. (A) RT-PCR analysis of RNA encoding Pax4 and insulin from control and virus-infected PC12 cells and purified rat β-cells. (B) Immunostaining of insulin in PC12 cells that were infected at low MOI with Adngn3. Arrow points to insulin-positive PC12 cell that still contains lots of active GFP; arrowheads point to PC12 cells that express insulin but contain no active GFP or ngn3 anymore. Bars, 10 μm.
Figure 3.
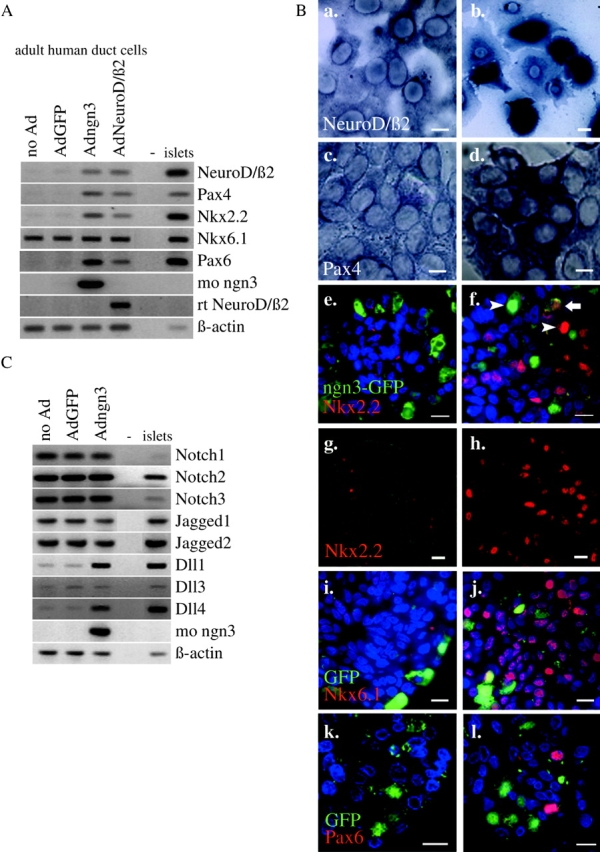
Effect of adenovirus-mediated ectopic expression of ngn3 or NeuroD/β2 on key transcription factors and signal transduction proteins in adult human duct cells. (A) RT-PCR analysis of RNA encoding key developmental transcription factors from control and virus-infected duct cells and islets. Adngn3-expressed mouse ngn3 and AdNeuroD/β2-expressed rat NeuroD/β2. (B) Analysis of the effects of ngn3 (b, d, f, h, j, and l) compared with AdGFP-infected control cells (a, c, e, g, i, and k) at the cellular level by in situ hybridization of NeuroD/β2 (a and b) and Pax4 (c and d) mRNA and immunofluorescence for Nkx2.2 (e–h), ngn3 (e and f), Nkx6.1 (i and j) and Pax6 (k and l). Sections for immunocytochemistry underwent short (1 h) fixation to preserve GFP fluorescence. Arrowheads in panel f point to cells expressing either Nkx2.2 or ngn3 (intense red, respectively, green nuclear staining), and arrow points to a cell expressing Nkx2.2 and still containing GFP (weak green fluorescence in cytoplasm combined with weak red staining in the nucleus) without high level expression of ngn3. Panels g and h represent a combination of phase–contrast and fluorescence microscopy (Nkx2.2 immunostaining), emphasizing the massive effect of ngn3. Bars, 10 μm. (C) RT-PCR analysis of RNA encoding Notch, Jagged, and Delta isoforms from control and virus-infected duct cells and human islets.
Ngn3 induces expression of regulators of embryonic endocrine differentiation in postnatal human pancreatic duct cells
We ectopically expressed ngn3 and its downstream target NeuroD/β2 in adult human duct cells. Common transfection methods were unsuccessful, but infection with recombinant adenoviruses (Ad) resulted in efficient expression of the transgenes that were under control of a cytomegalovirus (CMV) promotor (Fig. 2). Both Adngn3 and AdNeuroD/β2 coexpressed GFP as a reporter, and AdGFP served as control for nonspecific viral effects. A multiplicity of infection (MOI) of 50 gave a favorable balance between infection efficiency (30–40% GFP expression) (Fig. 2) and cell survival (>85% living cells).
Figure 2.
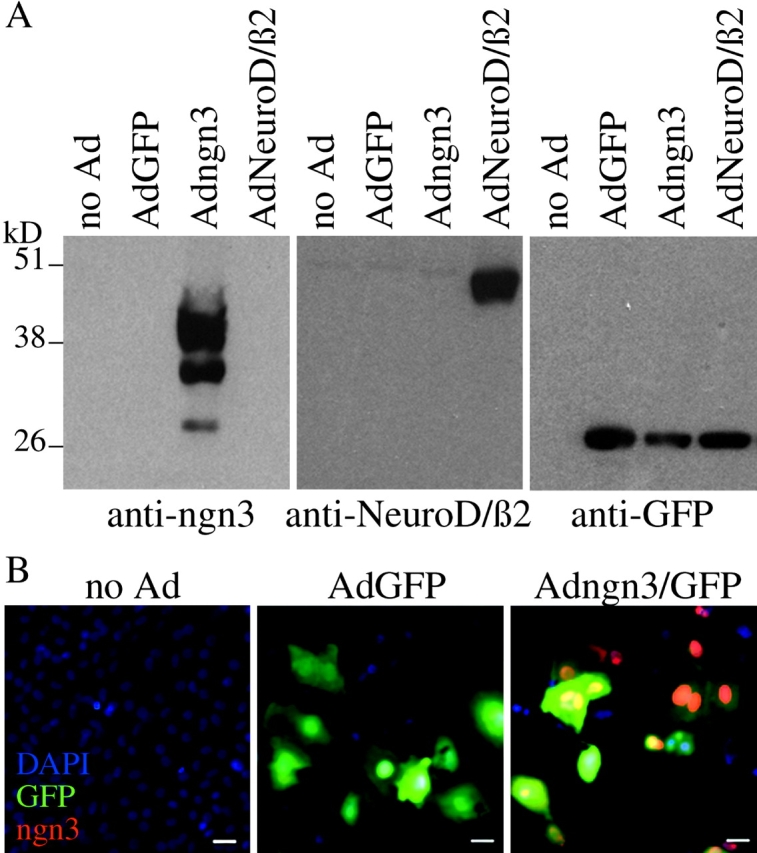
Specificity and efficiency of adenovirus-mediated transgene expression. Immunoblotted protein extracts (2 μg total protein) (A) and immunostained monolayers (B) of noninfected and virally infected adult human pancreatic duct cells. Bars, 10 μm.
1 d after ngn3 infection, Pax4 gene expression was activated (Fig. 3 A) together with Dll1 and Dll4, the latter two to similar levels as in control islet preparations (Fig. 3 C). 2 d later, NeuroD/β2 was induced, followed by Nkx2.2 and Pax6 (Fig. 3 A). The Pdx1/Ipf1 gene remained silent or constant in, respectively, monolayer or suspension cultures of Adngn3-infected duct cells (unpublished data). These effects were confirmed by in situ hybridization and immunocytochemistry. 10 d after infection, many cells expressed either Nkx2.2 or ngn3 (Fig. 3 B, f, arrowheads) but rarely both. However, some Nkx2.2-expressing cells still contained GFP (Fig. 3 B, f, arrow). There was no significant increase in the level of Nkx6.1-encoding mRNA, but the protein appeared abundant (Fig. 3 B, j). Nuclear protein extracts from Adngn3-infected duct cells showed gel retardation of E box-1 and E box-3 sequences from the NeuroD/β2 promotor (unpublished data). Compared with ngn3, NeuroD/β2-induced changes in gene expression were similar but appeared with a several days delay (unpublished data). The same recombinant Ad constructs were used to infect the clonal neuroendocrine cell line PC12 and the unrelated HeLa cell line. In PC12 cells, which endogenously express NeuroD/β2 and Nkx2.2, both ngn3 and NeuroD/β2 induced Pax4 but not Nkx6.1 (Fig. 4 A). In HeLa cells, none of these endocrinogenic transcription factors were induced after infection with Adngn3 or AdNeuroD/β2.
Ngn3 induces expression of neuroendocrine markers, in particular insulin, in postnatal human pancreatic duct cells
Control duct cell preparations were negative by RT-PCR analysis for the endocrine cell markers synaptophysin, chromogranin A, and prohormone convertases (PC1/3 and PC2), and for the islet cell markers insulin, somatostatin, glucagon, glucose transporter type II, and glucokinase. Transcripts encoding these proteins were clearly present in the human islet cell fraction (Fig. 5 A; not depicted). 10 d after infection with Adngn3 or AdNeuroD/β2, duct cell preparations exhibited a strong activation of synaptophysin, chromogranin A, and PC1/3 gene expression, and a weaker increase for the glucokinase and insulin genes (Fig. 5 A). No signals were detected for Glut2, somatostatin, or glucagon transcripts. These effects of ectopic ngn3 expression were also reflected at the protein level (Fig. 5 B). The fraction of cells that were immunopositive for insulin and synaptophysin increased from 1% in control preparations to, respectively, 13 and 22% 10 d after Adngn3 infection. The percentage of glucagon- or somatostatin-positive cells remained under 2% (unpublished data). However, although ectopic ngn3 did not affect the number of glucagon-containing cells, it increased the percentage of somatostatin-positive cells 3.5-fold (0.2 ± 0.1% versus 0.7 ± 0.1%, P < 0.05; n = 4). A similar effect was observed after infection with AdNeuroD/β2. Interestingly, most cells that expressed these endocrine markers were negative for ngn3 (Fig. 5 B, b, c, and g, arrowheads point to single positive cells). At this time point, the percentage of ngn3-positive cells was markedly lower than at day 3. Furthermore, the rare ngn3-positive cells that coexpressed endocrine markers exhibited a much lower ngn3 fluorescence intensity than those that did not (Fig. 5 B, b and g, arrows).
Figure 5.
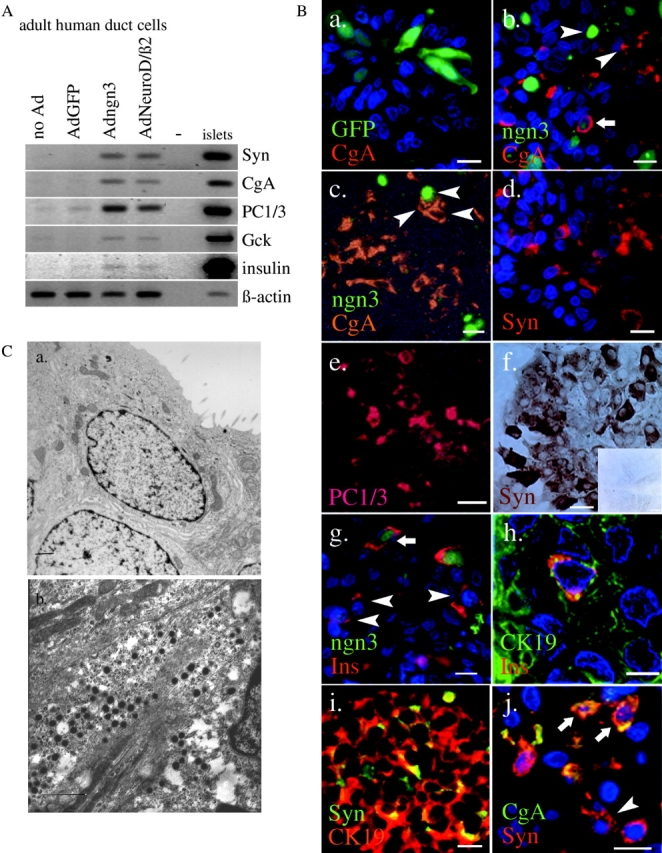
Effect of adenovirus-mediated ectopic expression of ngn3 or NeuroD/β2 on markers of the differentiated endocrine phenotype in adult human duct cells. (A) RT-PCR analysis of RNA encoding endocrine marker proteins in adult human duct cells and islet cells. (B) Immunostaining of control (AdGFP-) (a and f, inset) and Adngn3- (b and j) infected duct cells. Nuclei stained blue by DAPI (a, b, d, g, h, and j). All immunoreactions were labeled with a fluorescent secondary antibody except for panel f which was immunochemically stained (ABC peroxidase). Panel j represents a double labeling for CgA (FITC) and Syn (Cy3). Panels c and e represent a combination of phase– contrast and fluorescence microscopy. Noninfected and control-infected duct cells contained a low number of endocrine cells (<1% insulin positivity and <2% synaptophysin positivity). Endocrine marker proteins were chromogranin A (CgA), synaptophysin (Syn), prohormone convertase 1/3 (PC1/3), and insulin (Ins); duct cell marker was CK19. Noninfected and GFP control- infected duct cells contained none of the endocrine proteins under study as determined by costaining for the duct cell markers CK19 or CA19.9. Bars, 10 μm. Sections for immunocytochemistry underwent overnight fixation which abolished GFP fluorescence. Arrowheads, single positive cells on panels b and c (either ngn3 or CgA), g (insulin), and j (Syn); arrows, cells coexpressing CgA and ngn3 (b), insulin and ngn3 (g), or CgA and Syn (j). (C) Electron micrograph of control (a) and transdifferentiating (b) adult human pancreatic duct cells 10 d postinfection with AdGFP (a) or Adngn3 (b). Adngn3-infected cells display 180-nm secretory granules with a homogenous, electron dense matrix. Bars, 1 μm.
10 d after Adngn3 infection, many but not all synaptophysin-positive cells expressed chromogranin A (Fig. 5 B, k and l). More than 90% of the synaptophysin-expressing cells were still positive for the duct cell marker cytokeratin (CK)19 (Fig. 5 B, i); however, this was the case for only part of the insulin- or somatostatin-positive cells (Fig. 5 B, h and j; not depicted). Electronmicrographs confirmed the presence of a large percentage of ngn3-infected cells with secretory granules (Table I) that were, however, smaller than the characteristic large granules of fully differentiated endocrine islet cells (Fig. 5 C).
Table I.
Effect of ngn3 or NeuroD/β2 on the fraction of granulated and synaptophysin- or insulin-positive cells
| Granulation+ n = 3 | Synaptophysin+ n = 3 | Insulin+ n = 3 | |
|---|---|---|---|
| % | % | % | |
| AdGFP | < 2 | < 2 | < 2 |
| Adngn3 | 10 ± 2a | 22 ± 4a | 13 ± 3a |
| AdNeuroD/β2 | 8 ± 4a | 10 ± 1a | 8 ± 2a |
Granulation was analyzed by EM and synaptophysin and insulin positivity by immunostaining. Results are the average ± standard error of the mean of n independent duct cell preparations infected with AdGFP, Adngn3, or AdNeuroD/β2 expressed as a percentage of total cells.
P < 0.05 versus AdGFP-infected duct cells as determined by the paired Student's t test.
Ectopic ngn3 or NeuroD/β2 expression in postnatal human pancreatic duct cells resulted in a threefold increase in the insulin content and insulin release of the preparations (Table II). In view of the 10-fold increase in the percentage of insulin-positive cells, these data suggest that the newly formed insulin-positive cells have a low insulin content.
Table II.
Effect of ngn3 or NeuroD/β2 on the relative concentration of insulin in duct cells and duct cell culture medium
| Insulin content n = 4 |
Insulin release n = 3 |
|
|---|---|---|
| AdGFP | 100 | 100 |
| Adngn3 | 250 ± 60a | 340 ± 20a |
| AdNeuroD/β2 | 310 ± 50a | 330 ± 20a |
Insulin release was measured as the amount of insulin that accumulated in the medium during 48 h. Results are the average ± standard error of the mean of n independent duct cell preparations infected with AdGFP, Adngn3, or AdNeuroD/β2 expressed as percentage of AdGFP-infected control cells.
P < 0.05 versus AdGFP-infected duct cells as determined by the paired Student's t test.
In PC12 cells, ngn3 and NeuroD/β2 induced insulin expression, both at the transcript and protein level (Fig. 4) but failed to induce glucokinase, Glut2, somatostatin or glucagon. No ngn3-induced gene expression was seen in HeLa cells.
Discussion
The present study demonstrates that adenovirus-mediated delivery of ngn3, a key transcription factor for the generation of endocrine islet cells in mouse embryos, shifts adult human pancreatic duct cells into a neuroendocrine phenotype with expression of insulin in a significant fraction of transdifferentiated cells. It is unlikely that this effect requires the participation of Pdx1/Ipf1. This transcription factor is expressed in adult human pancreatic duct cell suspensions, be it at lower abundance and with a different phosphorylation status and DNA-binding activity compared with mature human islet cells (Heimberg et al., 2000); however, the ngn3-induced (neuro)endocrine differentiation was also achieved in duct cell monolayers, which are negative for Pdx1/Ipf1 (unpublished data). Of the known developmental transcription factors that operate downstream of Pdx1/Ipf1 to specifically control embryonic pancreas formation, ngn3 comes first in sequence. Ngn3 is a major regulator of lateral inhibition that controls endocrinogenesis in the embryonic mouse pancreas (Apelqvist et al., 1999). It has been proposed as a marker for pancreatic islet progenitor cells during embryogenesis and in adult mice (Jensen et al., 2000a; Gu et al., 2002). The amplified signal for ngn3 transcript in duct cells was similar to islets and suggests very low but specific expression in this cell fraction. Adenovirus-mediated overexpression of ngn3 in adult human pancreatic duct cells was found to activate expression of neuroendocrine differentiation markers and of β-cell–specific genes Pax4 and insulin. Despite their independence of Pdx1/Ipf1, the requirement for ngn3 and the induction of Pax4 and somatostatin expression suggest that the ngn3-transdifferentiated (neuro)endocrine cells resemble cells of the second rather than the first wave of pancreatic endocrinogenesis. The described effects are cell type restricted, since they were reproduced in the PC12 neuroendocrine cell line but not in HeLa cells.
It is unknown presently whether this forced transdifferentiation is restricted to a subpopulation of duct cells. A major proportion of ngn3-infected cells expressed the (neuro)endocrine markers, but only a fraction was insulin positive. The sequential activation of a comprehensive set of (neuro)endocrine-specific genes rather than the existence of (sub)populations of cells expressing only few individual markers is characteristic for the induction of a coordinated differentiation program. Based on its rapid nature and independence of cell proliferation (unpublished data), this process likely represents immediate transdifferentiation of the duct cells without the need for an intermediate cellular state (Shen et al., 2000; Slack and Tosh, 2001). Transdifferentiated cells are characterized by coexpression of the duct cell marker CK19 and the neuroendocrine marker synaptophysin. Such double positive cells are virtually absent in noninfected duct cell preparations. The expression levels of both markers changed gradually and reciprocally with time after infection. Only little cytokeratin positivity was left in the cells that finally expressed insulin. A combination of the transient nature of the adenoviral expression system and the elimination of fluorescence by GFP after extended fixation allowed simple tracing of cell fate. A direct relation between the ngn3-infected duct cells and the cells that became positive for endocrine markers was observed. Rare cells coexpressed endocrine marker proteins and traces of ngn3, suggesting a causal relationship between expression of ngn3 and the endocrine protein. Furthermore, several endocrine cells still contained the stable green fluorescent protein that remains present for days after its transcript, and consequently the mRNA encoding the less stable ngn3 protein, have disappeared (Corish and Tyler-Smith, 1999).
The mechanism whereby ngn3 induces duct cell differentiation into endocrine β-cells seems to involve activation of the Pax4 and the NeuroD/β2 promotor. Ngn3 is known to activate NeuroD/β2 expression in chicken embryos (Grapin-Botton et al., 2001), Xenopus embryos, and (neuro)endocrine cell lines (Huang et al., 2000). This is also the case in HeLa cells that had been transfected with E47, a class A bHLH heterodimerization partner of ngn3 (Huang et al., 2000). Adult human duct cells contain high endogenous levels of E47 (unpublished data), allowing E-box binding of the ectopically expressed ngn3 and activation of the NeuroD/β2 promotor. The delayed induction of NeuroD/β2 by ngn3 suggests the existence of intermediate transcription factors. Moreover, the delay in NeuroD/β2-induced Pax4 activation compared with ngn3 uncovers that both Pax4 and NeuroD/β2 are ngn3 targets, instead of Pax4 being downstream of NeuroD/β2. The present study thus supplements the hierarchy model of transcription factors involved in the formation of embryonic β-cells (Schwitzgebel et al., 2000). It also demonstrates that the embryological program in mice can be recapitulated in postnatal human duct cells, leading to formation of insulin, and to a minor extent somatostatin-expressing cells. In experimental terms, adult duct cells infected with adenoviruses expressing recombinant transcription factors are a simple in vitro model for studying the molecular biology of endocrine transdifferentiation.
Ectopic expression of ngn3 or NeuroD/β2 in isolated adult duct cells activates several (neuro)endocrine-specific genes, such as insulin and somatostatin, but not glucagon. In mouse or chicken embryonic endoderm cells in vivo, ectopic ngn3 induces glucagon but not insulin (Grapin-Botton et al., 2001). Activation of β-cell–specific Pax4 in duct cells might be responsible for the absence of glucagon expression (Smith et al., 1999; Petersen et al., 2000). The role of Nkx2.2 and Nkx6.1 in this transdifferentiation process is unclear: Nkx2.2 was stimulated by ngn3 at the transcriptional level, and Nkx6.1 was stimulated at the posttranscriptional level. Both transcription factors appear essential during embryonic development of β-cells, with Nkx2.2 acting upstream of Nkx6.1 (Sander et al., 2000). Adngn3-infected duct cells failed to generate glucose-induced insulin release within the limits of the present study, i.e., 10 d after infection (unpublished data). Our study can thus not be taken as evidence for the ability to produce functionally mature β-cells from pancreatic duct cells. Nevertheless, the data are indicative for a selective ability of adult duct cells to differentiate toward (neuro)endocrine and islet cells. Ngn3 induced moderate to high expression of synaptophysin, chromogranin A, PC1/3, and glucokinase, but the degree of insulin gene activation is low and so is the cellular insulin content. Transdifferentiated pancreatic duct cells exhibit the ultrastructural characteristics of immature endocrine cells, which is consistent with the absence of a glucose-regulated secretory activity. The absence of Glut2 induction is compatible with the earlier report that Glut2 is poorly expressed in human β-cells compared with human liver cells or rodent β-cells (De Vos et al., 1995).
The incomplete differentiation of this particular phenotype might be caused by, or related to, a variety of factors. (a) Although Pax4 is subject to autoregulation in mouse islet cell lines (Smith et al., 2000), ngn3-induced activation of Pax4 in human duct cells was not. This allows the relatively high levels of Pax4 to exert their inhibitory action on insulin gene expression (Qiu et al., 1998; Smith et al., 1999). Low levels of Pax4 also occurred in normal adult human islet cells in contrast to its restriction to the embryonic mice pancreas (Sosa-Pineda et al., 1997; Smith et al., 1999, 2000). It is likely that in mature islet β-cells a yet unknown factor overrules the Pax4 repressor activity. Given the transient presence of ngn3 and Pax4 in the embryonic mouse pancreas, it needs to be investigated whether a closer simulation of the embryonic situation by conditional expression of ngn3 in adult human duct cells would augment the insulin levels. (b) The state of the Delta-Notch pathway probably also has its influence on the degree of (neuro)endocrinogenesis. In uninfected duct cells, high levels of Notch1, 2, and 3 but not of Dll1, 3, and 4 were found. Ngn3 infection activated transcription of Dll1 and Dll4 and thus increased Notch signaling in neighboring pairs, which is expected to limit overall endocrine differentiation. It may thus be useful to add antimorphic forms of Delta that antagonize Notch signaling (Jen et al., 1997) or to introduce ngn3 targets that bypass stimulation of Delta genes in order to specifically drive the formation of endocrine cells. (c) The transdifferentiated cells likely are dependent on extracellular factors to promote their maturation. (d) Finally, repression of specific proneural regulators induced by ngn3 may be necessary to allow full endocrine differentiation. Although the transdifferentiated duct cells did not express genes that label differentiated neuronal cells (N-Cam, neurofilament, peripherin, and class III β-tubulin [unpublished data]), major overlaps exist between the expression profile of endocrine pancreas and neurons (Atouf et al., 1997; unpublished data). These similarities were key to design conditions that drive embryonic stem cells to insulin production (Lumelsky et al., 2001). Moreover, insulin-producing cells are present in primary cell cultures from mammalian fetal brain (Clarke et al., 1986), and insulin-producing neurons in Drosophila brain show remarkable similarities with β-cells in mammalian islets of Langerhans (Rulifson et al., 2002). All these data are highly suggestive for a common ancestral insulin-producing cell of neural origin, which complicates the identification of specific proneural factors that are nonessential for islet cell differentiation.
In more general terms, the present findings extend the role of lateral inhibition in cell differentiation from embryonic, poorly differentiated tissues (Artavanis-Tsakonas et al., 1999) to postnatal differentiated cell populations. Adult human duct cells were shown to transdifferentiate into neuroendocrine and into insulin-producing cells after ectopic expression of ngn3. Pancreatic duct and islet cells have a common embryonic progenitor, but according to a recent lineage tracing study ngn3 is never expressed in duct cells or their progenitors (Gu et al., 2002). However, our current study shows that ngn3 might serve as a master switch that drives transdifferentiation to a (neuro)endocrine phenotype when misexpressed in adult duct cells. It is still unknown whether ngn3 can be activated by environmental factors in normal duct cells of regenerating postnatal pancreas and thus result in the formation of new β-cells. Current efforts focus on finding ways to differentiate embryonic stem cells into insulin-producing cells with the purpose of producing sufficient cells for transplantation in diabetes (Assady et al., 2001; Lumelsky et al., 2001). Our observations have indicated mechanisms through which adult duct cells could be forced to differentiate into insulin-producing cells. The abundance of duct cells and the ease of their in vitro manipulation support further attempts to explore their potential as a source for new β-cells.
Materials and methods
Production of recombinant adenoviruses
Adenoviral plasmid pAdEasy-1 and shuttle vector pAdTrack-CMV were made available by T.-C. He and B. Vogelstein (Johns Hopkins Oncology Center, Baltimore, MD). Coding sequences of hemagglutinin-tagged mouse ngn3 or rat NeuroD/β2 were subcloned in the shuttle vector and constitutively expressed under control of the CMV promotor. pAdTrack-CMV contained the eGFP cDNA downstream of a separate CMV promotor. Recombinant, replication-deficient adenoviruses expressing GFP (AdGFP), ngn3 in combination with GFP (Adngn3), or NeuroD/β2 in combination with GFP (AdNeuroD/β2) were generated following the standard protocol as described by He et al. (1998).
Cell isolation and cell culture
The duct cells in this study were obtained from heart-beating cadaveric nondiabetic donors as the discarded fraction of an islet cell isolation to prepare grafts for transplantation in type I diabetes patients. Human donor pancreases were procured at European hospitals and made available to the β-Cell Bank in Brussels through the intermediate of Eurotransplant Foundation (Leiden, The Netherlands). The endocrine preparations contained >60% hormone-positive cells (Keymeulen et al., 1998). In the nonendocrine fraction, <2% expressed islet cell markers and >90% expressed the duct cell–specific phenotypic markers cytokeratin 19 and carbohydrate antigen 19.9 when cultured for at least 4 d (Bouwens et al., 1994). The nonendocrine cell preparation was cultured in suspension in Ham's F10 (Bio-Whittaker), 0.5% BSA (Boehringer Mannheim), 7.5 mM glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM l-glutamine at 37°C in a humidified atmosphere of 5% CO2. 24 h after isolation, the cells were washed, and the medium was renewed. On day 4 of culture, cells were counted and were infected and further cultured in suspension. Alternatively, day 4 cells were plated to form monolayers in the presence of 5% FBS (Life Technologies) and infected 6 d later. Under all conditions, cell culture medium was renewed every other day.
PC12 cells were cultured in suspension in RPMI 1640 with Glutamax (Life Technologies), 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Rat β-cells were prepared as described before (Heimberg et al., 1996).
Viral infection of isolated exocrine cells and PC12 cells
Cells were either infected with control virus (AdGFP) or with Adngn3 or AdNeuroD/β2 as described previously (Heimberg et al., 2001). Adult human duct cells were infected at an MOI of 50 for 4 h at 37°C. PC12 cells were infected at an MOI 20 for 4 h at 37°C.
Protein analysis
Immunohistochemical analysis was performed on 4-μm-thick paraffin sections by indirect immunofluorescence as described (Heimberg et al., 2000). Similar methods were applied to cells from suspension cultures after fixation for 1 h in 4% paraformaldehyde and pelleting in 2% agarose before paraffin embedding. Before incubation with the first antibody, sections for CK19 staining were trypsin treated. Rabbit polyclonal PC1/3 antiserum was from I. Lindberg (Louisiana State University Health Sciences Center, New Orleans, LA) (Vindrola and Lindberg, 1992), rabbit polyclonal anti-ngn3 was from M. German (University of California San Francisco, San Francisco, CA), rabbit polyclonal Pax6 was from S. Saule (Institut Curie, Paris, France), and guinea pig polyclonal insulin was from C. Van Schravendijck (Brussels Free University). The mouse monoclonal anti-Nkx2.2 was developed by T. Jessell (Columbia University, New York, NY) and obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Rabbit polyclonal anti-Nkx6.1 has been described before (Oster et al., 1998). Rabbit polyclonal antisomatostatin was from J. DeMey (Brussels Free University), mouse monoclonal anti–chromogranin A was from Biogenex, rabbit polyclonal antisynaptophysin was from Novocastra, mouse monoclonal anti–cytokeratin 19 was from Dako, mouse monoclonal anti–CA19–9 from Biomed, and mouse monoclonal anti-HA was from Boehringer. Secondary antibodies were Cy3- or FITC-labeled anti–rabbit, anti–mouse, and anti–guinea pig (Jackson ImmunoResearch Laboratories). Immunoblot analysis was done as described before (Heimberg et al., 2000).
mRNA analysis
Total RNA was isolated using RNEasy columns (QIAGEN) and reverse transcription followed by PCR were performed and analyzed as described (Heimberg et al., 1996). Primers specifically amplified human Pdx1/Ipf1 (277 bp), 5′-CTGCCTTTCCCATGGATGAA-3′ (forward) and 5′-CGCTTCTTGTCCTCCTCCTTT-3′ (reverse), human ngn3 (286 bp), 5′-AGACGACGCGAAGCTCACC-3′ (forward) and 5′-AAGCCAGACTGCCTGGGCT-3′ (reverse), human NeuroD/β2 (439 bp), 5′-ATCCCAACCCACCACCAACC-3′ (forward) and 5′-CAGCGGTGCCTGAGAAGATT-3′ (reverse), human Pax4 (496 bp), 5′-AGGAGGACCAGGGACTACCGT-3′ (forward) and 5′-TTTAGGTGGGGTGTCACTCAG-3′ (reverse), human Pax6 (301 bp), 5′-CAAAAGTCCAAGTGCTGGACAA-3′ (forward) and 5′-CCCATCTGTTGCTTTTCGCT-3′ (reverse), human Nkx2.2 (329 bp), 5′-TGCAGCACATGCAGTACAACG-3′ (forward) and 5′-TCCCAAGGTTCAGAAGGAGAGG-3′ (reverse), human Nkx6.1 (284 bp), 5′-TCTTCTGGCCCGGGGTGATG-3′ (forward) and 5′-AGCCGCGTGCTTCTTCCTCC-3′ (reverse), human Notch1 (160 bp), 5′-GAATCCAACCCTTGTGTCAAC-3′ (forward) and 5′-GCAACGTCGTCAATACACGTG-3′ (reverse), human Notch2 (231 bp), 5′-CGCTGCATTGACCTGGTCAAT-3′ (forward) and 5′-TACATGTTGCACCCTTGCGA-3′ (reverse), mouse Notch3 (240 bp), 5′-GGCATTGCTAGCTTCTCGTGT-3′ (forward) and 5′-CATAACGGTTGATGCCATCAC-3′ (reverse), human Jagged1 (417 bp), 5′-ATCTGTCCACCTGGCTATGCAG-3′ (forward) and 5′-ATTTGCCTCCCGACTGACTCTT-3′ (reverse), human Jagged2 (338 bp), 5′-GGAAGCCATGCCTTAACGCTT-3′ (forward) and 5′-GCTCACAAAGGTCGACATCCA-3′ (reverse), human Delta1 (309 bp), 5′-CCTGATGACCTCGCAACAGAA-3′ (forward) and 5′-CATGCTGCTCATCACATCCAG-3′ (reverse), human Delta4 (273 bp), 5′-ACCACTTCGGCCACTATGTGT-3′ (forward) and 5′-TCTTGGTCACAAAACAGGCCT-3′ (reverse), human synaptophysin (214 bp), 5′-GCCACATGCGGCAGCTACAG-3′ (forward) and 5′-ACACGGCCACGGTGACAAAG-3′ (reverse), human chromogranin A (286 bp), 5′-CCGCTGTCCTGGCTCTTCT-3′ (forward) and 5′-CCGCTGTGTTTCTTCTGCTG-3′ (reverse), human/rat insulin (438 bp), 5′-GCAGCCTTTGTGAACCAACA-3′ (forward) and 5′-TCTGCGGTCATCAAATGAGG-3′ (reverse), human glucagon (221 bp), 5′-CCCAAGATTTTGTGCAGTGGTT-3′ (forward) and 5′-GCGGCCAAGTTCTTCAACAAT-3′ (reverse), human glucokinase (607 bp), 5′-CTGGACGACAGAGCCAGGAT-3′ (forward) and 5′-TCACCATTGCCACCACATCCAT-3′ (reverse), human PC1/3 (355 bp), 5′-CAAGATACCAGGATGACGGCA-3′ (forward) and 5′-GCCTCAATAGCATCCGTCACA-3′ (reverse), mouse ngn3 (288 bp), 5′-CCGGATGACGCCAAACTTACA-3′ (forward) and 5′-ACACCAGTGCTCCCGGGAG-3′ (reverse), rat NeuroD/Beta2 (300 bp), 5′-GGACTTTCTTGCCTGAGCAGA-3′ (forward) and 5′-AACTCGGTGGATGGTTCGTGT-3′ (reverse), rat Pax4 (224 bp), 5′-ATGCGACCCTGTGACATCTCA-3′ (forward) and 5′-AAGCCCTTCAGCACAAAGCTG-3′ (reverse), rat Nkx2.2 (209 bp), 5′-CATGTCGCTGACCAACACAAAG-3′ (forward) and 5′-TCGCTGCTGTCGTAGAAAGGA-3′ (reverse), and rat/human β-actin (361 bp), 5′-AGAGCTATGAGCTGCCTGAC-3′ (forward) and 5′-CTGATCCACATCTGCTGGAA-3′ (reverse). For in situ hybridization, human-specific NeuroD/β2, Pax4, and ngn3 PCR products were subcloned into the pGEM-T Easy vector (Promega) and SalI linearized (Life Technologies). Digoxigenin-labeled transcripts were produced according to manufacturer's instructions (MAXI Script; Ambion). RNA in situ hybridization was performed as described (Gradwohl et al., 2000; Petersen et al., 2000). Detection of hybridized probes made use of an alkaline phosphatase-labeled antidigoxigenin antibody (Boehringer) and the substrate BM-Purple (Boehringer).
Electron microscopy
Cell preparations were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), postfixed in 1% osmium tetroxide, stained with 2% uranyl acetate, embedded in Spurr's resin, and ultra thin plastic sections were examined with a ZEISS EM 9S2 electron microscope.
Data analysis
Results obtained from infected cells were compared with uninfected and/or control virus-infected cells and statistically analyzed using the paired Student's t test.
Acknowledgments
Bert Vogelstein, Mike German, Simon Saule, Iris Lindberg, and Chris Van Schravendijck are acknowledged for viral vectors and antisera. We are grateful to Steven De Vos, Jan De Jonge, Karen Sterck, Veerle Laurysens, Mette Jorgensen, Marjorie Jenny, Luc Bouwens, and Jorge Ferrer for technical help, discussions, and critical advice.
The authors are members of the Juvenile Diabetes Research Foundation Center for β-Cell Therapy in Europe. Harry Heimberg is recipient of a Career Development Award from the Juvenile Diabetes Research Foundation and a Post-Doctoral Research Fellowship from the Fund for Scientific Research (Flanders, Belgium).
Footnotes
Abbreviations used in this paper: Ad, adenovirus; bHLH, basic helix-loop-helix; CK, cytokeratin; CMV, cytomegalovirus; MOI, multiplicity of infection; ngn3, neurogenin 3.
References
- Apelqvist, A., H. Li, L. Sommer, P. Beatus, D.J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signalling controls pancreatic cell differentiation. Nature. 400:877–881. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. [DOI] [PubMed] [Google Scholar]
- Assady, S., G. Maor, M. Amit, J. Itskovitz-Eldor, K.L. Skorecki, and M. Tzukerman. 2001. Insulin production by human embryonic stem cells. Diabetes. 50:1691–1697. [DOI] [PubMed] [Google Scholar]
- Atouf, F., P. Czernichow, and R. Scharfmann. 1997. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J. Biol. Chem. 272:1929–1934. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir, S., M. Taneja, G.C. Weir, K. Tatarkiewicz, K.H. Song, A. Sharma, and J.J. O'Neil. 2000. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA. 97:7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens, L., and D.G. Pipeleers. 1998. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia. 41:629–633. [DOI] [PubMed] [Google Scholar]
- Bouwens, L., R.N. Wang, E. De Blay, D.G. Pipeleers, and G. Kloppel. 1994. Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes. 43:1279–1283. [DOI] [PubMed] [Google Scholar]
- Clarke, D.W., L. Mudd, F.T. Boyd, Jr., M. Fields, and M.K. Raizada. 1986. Insulin is released from rat brain neuronal cells in culture. J. Neurochem. 47:831–836. [DOI] [PubMed] [Google Scholar]
- Corish, P., and C. Tyler-Smith. 1999. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 12:1035–1040. [DOI] [PubMed] [Google Scholar]
- De Vos, A., H. Heimberg, E. Quartier, P. Huypens, L. Bouwens, D. Pipeleers, and F. Schuit. 1995. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J. Clin. Invest. 96:2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund, H. 1999. Pancreas: how to get there from the gut? Curr. Opin. Cell Biol. 11:663–668. [DOI] [PubMed] [Google Scholar]
- Edlund, H. 2001. Factors controlling pancreatic cell differentiation and function. Diabetologia. 44:1071–1079. [DOI] [PubMed] [Google Scholar]
- Gradwohl, G., A. Dierich, M. LeMeur, and F. Guillemot. 2000. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA. 97:1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton, A., A.R. Majithia, and D.A. Melton. 2001. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 15:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, G., J. Dubauskaite, and D.A. Melton. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 129:2447–2457. [DOI] [PubMed] [Google Scholar]
- He, T.C., S. Zhou, L.T. da Costa, J. Yu, K.W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg, H., A. De Vos, K. Moens, E. Quartier, L. Bouwens, D. Pipeleers, E. Van Schaftingen, O. Madsen, and F. Schuit. 1996. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc. Natl. Acad. Sci. USA. 93:7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg, H., L. Bouwens, Y. Heremans, M. Van De Casteele, V. Lefebvre, and D. Pipeleers. 2000. Adult human pancreatic duct and islet cells exhibit similarities in expression and differences in phosphorylation and complex formation of the homeodomain protein Ipf-1. Diabetes. 49:571–579. [DOI] [PubMed] [Google Scholar]
- Heimberg, H., Y. Heremans, C. Jobin, R. Leemans, A.K. Cardozo, M. Darville, and D.L. Eizirik. 2001. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes. 50:2219–2224. [DOI] [PubMed] [Google Scholar]
- Huang, H.P., M. Liu, H.M. El-Hodiri, K. Chu, M. Jamrich, and M.J. Tsai. 2000. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol. Cell. Biol. 20:3292–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen, W.C., D. Wettstein, D. Turner, A. Chitnis, and C. Kintner. 1997. The Notch ligand, X-Delta-2, mediates segmentation of the paraxial mesoderm in Xenopus embryos. Development. 124:1169–1178. [DOI] [PubMed] [Google Scholar]
- Jensen, J., R.S. Heller, T. Funder-Nielsen, E.E. Pedersen, C. Lindsell, G. Weinmaster, O.D. Madsen, and P. Serup. 2000. a. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 49:163–176. [DOI] [PubMed] [Google Scholar]
- Jensen, J., E.E. Pedersen, P. Galante, J. Hald, R.S. Heller, M. Ishibashi, R. Kageyama, F. Guillemot, P. Serup, and O.D. Madsen. 2000. b. Control of endodermal endocrine development by Hes-1. Nat. Genet. 24:36–44. [DOI] [PubMed] [Google Scholar]
- Keymeulen, B., Z. Ling, F.K. Gorus, G. Delvaux, L. Bouwens, A. Grupping, C. Hendrieckx, M. Pipeleers-Marichal, C. Van Schravendijk, K. Salmela, and D.G. Pipeleers. 1998. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia. 41:452–459. [DOI] [PubMed] [Google Scholar]
- Lumelsky, N., O. Blondel, P. Laeng, I. Velasco, R. Ravin, and R. McKay. 2001. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 292:1389–1394. [DOI] [PubMed] [Google Scholar]
- Oster, A., J. Jensen, H. Edlund, and L.I. Larsson. 1998. Homeobox gene product Nkx 6.1 immunoreactivity in nuclei of endocrine cells of rat and mouse stomach. J. Histochem. Cytochem. 46:717–721. [DOI] [PubMed] [Google Scholar]
- Petersen, H.V., M.C. Jorgensen, F.G. Andersen, J. Jensen, T.F. Nielsen, R. Jorgensen, O.D. Madsen, and P. Serup. 2000. Pax4 represses pancreatic glucagon gene expression. Mol. Cell. Biol. Res. Commun. 3:249–254. [DOI] [PubMed] [Google Scholar]
- Qiu, C., M.B. De Young, A. Finn, and D.A. Dichek. 1998. Cationic liposomes enhance adenovirus entry via a pathway independent of the fiber receptor and alpha(v)-integrins. Hum. Gene Ther. 9:507–520. [DOI] [PubMed] [Google Scholar]
- Rooman, I., Y. Heremans, H. Heimberg, and L. Bouwens. 2000. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia. 43:907–914. [DOI] [PubMed] [Google Scholar]
- Rulifson, E.J., S.K. Kim, and R. Nusse. 2002. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 296:1118–1120. [DOI] [PubMed] [Google Scholar]
- Sander, M., and M.S. German. 1997. The beta cell transcription factors and development of the pancreas. J. Mol. Med. 75:327–340. [DOI] [PubMed] [Google Scholar]
- Sander, M., L. Sussel, J. Conners, D. Scheel, J. Kalamaras, F. Dela Cruz, V. Schwitzgebel, A. Hayes-Jordan, and M. German. 2000. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 127:5533–5540. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel, V.M., D.W. Scheel, J.R. Conners, J. Kalamaras, J.E. Lee, D.J. Anderson, L. Sussel, J.D. Johnson, and M.S. German. 2000. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 127:3533–3542. [DOI] [PubMed] [Google Scholar]
- Shapiro, A.M., J.R. Lakey, E.A. Ryan, G.S. Korbutt, E. Toth, G.L. Warnock, N.M. Kneteman, and R.V. Rajotte. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343:230–238. [DOI] [PubMed] [Google Scholar]
- Shen, C.N., J.M. Slack, and D. Tosh. 2000. Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2:879–887. [DOI] [PubMed] [Google Scholar]
- Slack, J.M. 1995. Developmental biology of the pancreas. Development. 121:1569–1580. [DOI] [PubMed] [Google Scholar]
- Slack, J.M., and D. Tosh. 2001. Transdifferentiation and metaplasia–switching cell types. Curr. Opin. Genet. Dev. 11:581–586. [DOI] [PubMed] [Google Scholar]
- Smith, S.B., H.C. Ee, J.R. Conners, and M.S. German. 1999. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol. Cell. Biol. 19:8272–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S.B., H. Watada, D.W. Scheel, C. Mrejen, and M.S. German. 2000. Autoregulation and maturity onset diabetes of the young transcription factors control the human PAX4 promoter. J. Biol. Chem. 275:36910–36919. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda, B., K. Chowdhury, M. Torres, G. Oliver, and P. Gruss. 1997. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 386:399–402. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y., and T.M. Jessell. 1996. Diversity and pattern in the developing spinal cord. Science. 274:1115–1123. [DOI] [PubMed] [Google Scholar]
- Vindrola, O., and I. Lindberg. 1992. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol. Endocrinol. 6:1088–1094. [DOI] [PubMed] [Google Scholar]