Abstract
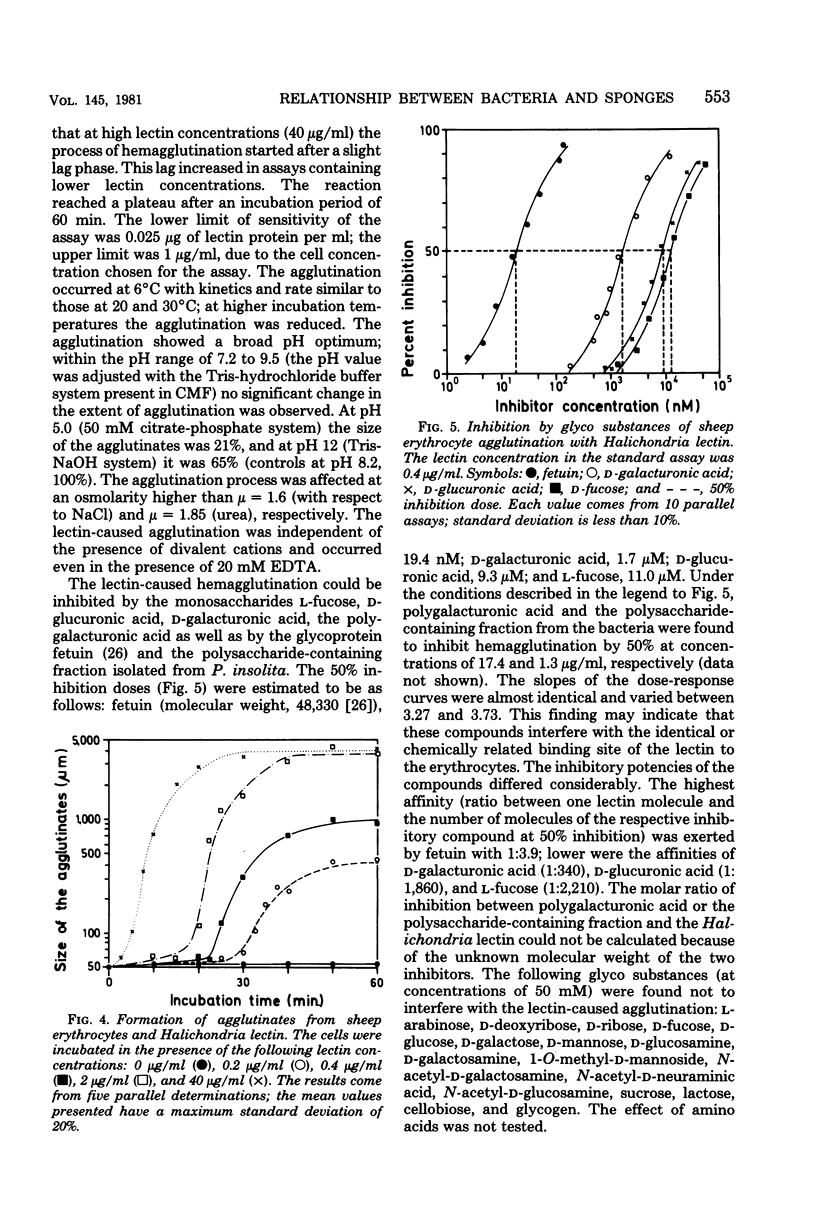
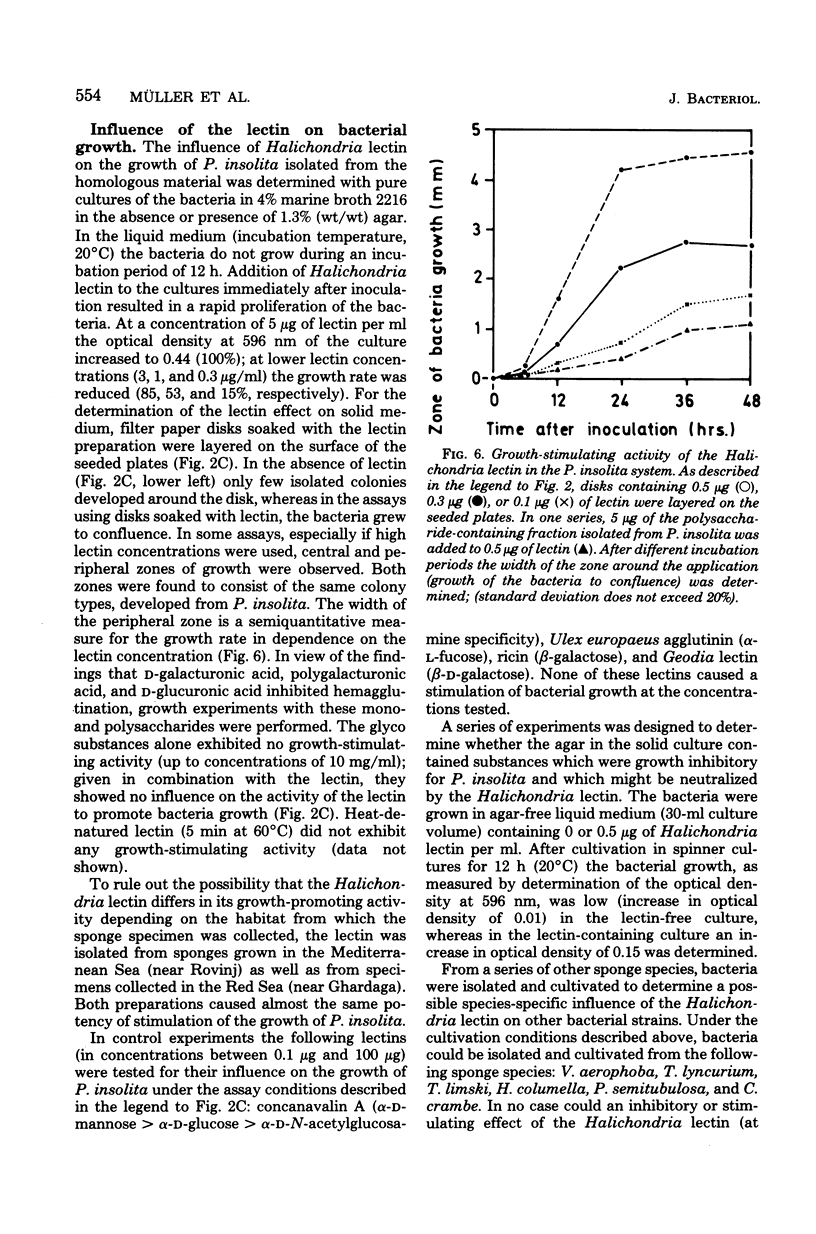
From the marine sponge Halichondria panicea a lectin was isolated and characterized. The homogeneous lectin (composed of protein to 80.7% and of neutral carbohydrates to 14.1%) had a molecular weight of 78,000 (determined by gel filtration) and consisted of four subunits with a molecular weight of 21,000 each (determined by gel electrophoresis in the presence of sodium dodecyl sulfate). The hemagglutinating activity was only slightly dependent upon ionic strength and incubation temperature and did not require divalent cations, but it was inhibited by reagents for thiol groups. The Halichondria lectin was completely inhibited in hemagglutination competition experiments in the presence of fetuin, D-galacturonic acid, D-glucuronic acid, polygalacturonic acid, or L-fucose. The purified Halichondria lectin did not cause reaggregation of dissociated H. panicea cells. From the same sponge species bacteria were isolated and identified as Pseudomonas insolita. These bacteria were cultivated in marine broth 2216. Under these culture conditions the bacteria grew only in the presence of the homologous lectin; the lectin-caused effect was not abolished by D-glucuronic acid or D-galacturonic acid. However, after addition of a polysaccharide-containing fraction isolated from P. insolita, the lectin-caused, growth-promoting effect was abolished. Other lectins were found to exhibit no growth-promoting effect. On the basis of colony counts, P. insolita was the predominant bacterial species in the sponge extract; 1.9 X 10(6) Pseudomonas colonies were measured in extracts isolated from 1 g of sponge. The assumption of an interrelationship between the sponge and the bacterium is supported by the results indicating that the Halichondria lectin has no effect on the growth of such bacteria isolated from six other marine sponge species. Evidence is presented which indicates that the Halichondria lectin is not utilized during growth of the Pseudomonas species. Lectin activity was detected on the surface of mucoid cells from H. panicea. From the data obtained the possibility is discussed that the Halichondria lectin is a basis for a symbiotic relationship between the sponge and the bacterium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigad G. Colorimetric assays for hexuronic acids and some keto sugars. Methods Enzymol. 1975;41:29–31. doi: 10.1016/s0076-6879(75)41007-2. [DOI] [PubMed] [Google Scholar]
- Bretting H., Kabat E. A., Liao J., Pereira M. E. Purification and characterization of the agglutinins from the sponge Aaptos papillata and a study of their combining sites. Biochemistry. 1976 Nov 16;15(23):5029–5038. doi: 10.1021/bi00668a013. [DOI] [PubMed] [Google Scholar]
- Bretting H., Kabat E. A. Purification and characterization of the agglutinins from the sponge Axinella polypoides and a study of their combining sites. Biochemistry. 1976 Jul 27;15(15):3228–3236. doi: 10.1021/bi00660a011. [DOI] [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOWSKA S., NIGRELLI R. F. Antimicrobial substances from sponges. Ann N Y Acad Sci. 1960 Nov 17;90:913–916. doi: 10.1111/j.1749-6632.1960.tb26436.x. [DOI] [PubMed] [Google Scholar]
- John H. A., Campo M. S., Mackenzie A. M., Kemp R. B. Role of different sponge cell types in species specific cell aggregation. Nat New Biol. 1971 Mar 24;230(12):126–128. doi: 10.1038/newbio230126b0. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- LEIFSON E. Staining, shape and arrangement of bacterial flagella. J Bacteriol. 1951 Oct;62(4):377–389. doi: 10.1128/jb.62.4.377-389.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leith A. Role of aggregation factor and cell type in sponge cell adhesion. Biol Bull. 1979 Apr;156(2):212–223. doi: 10.2307/1541044. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Arendes J., Kurelec B., Zahn R. K., Müller I. Species-specific aggregation factor in sponges. Sialyltransferase associated with aggregation factor. J Biol Chem. 1977 Jun 10;252(11):3836–3842. [PubMed] [Google Scholar]
- Müller W. E., Arendes J., Zahn R. K., Schröder H. C. Control of enzymic hydrolysis of polyadenylate segment of messenger RNA: role of polyadenylate-associated proteins. Eur J Biochem. 1978 May;86(1):283–290. doi: 10.1111/j.1432-1033.1978.tb12309.x. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Beyer R., Pondeljak V., Müller I., Zahn R. K. Species-specific aggregation factor in sponges. XIII. Entire and core structure of the large circular proteid particle from Geodia cydonium. Tissue Cell. 1978;10(2):191–199. doi: 10.1016/0040-8166(78)90017-4. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Kurelec B., Zahn R. K., Müller I., Vaith P., Uhlenbruck G. Aggregation of sponge cells. Function of a lectin in its homologous biological system. J Biol Chem. 1979 Aug 25;254(16):7479–7481. [PubMed] [Google Scholar]
- Müller W. E., Müller I., Kurelec B., Zahn R. K. Species-specific aggregation factor in sponges. IV. Inactivation of the aggregation factor by mucoid cells from another species. Exp Cell Res. 1976 Mar 1;98(1):31–40. doi: 10.1016/0014-4827(76)90459-6. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Arendes J., Kurelec B., Steffen R., Müller I. Aggregation of sponge cells. XX. Self-aggregation of the circular proteid particle. Biochim Biophys Acta. 1979 Mar 8;551(2):363–367. doi: 10.1016/0005-2736(89)90012-6. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Kurelec B., Müller I., Uhlenbruck G., Vaith P. Aggregation of sponge cells. A novel mechanism of controlled intercellular adhesion, basing on the interrelation between glycosyltransferases and glycosidases. J Biol Chem. 1979 Feb 25;254(4):1280–1287. [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K. Purification and characterization of a species-specific aggregation factor in sponges. Exp Cell Res. 1973 Jul;80(1):95–104. doi: 10.1016/0014-4827(73)90279-6. [DOI] [PubMed] [Google Scholar]
- Rüegg M., Jaques R. Tribenoside as an inhibitor of chemically induced histamine release. Experientia. 1974 Apr 15;30(4):399–401. doi: 10.1007/BF01921686. [DOI] [PubMed] [Google Scholar]
- Vaith P., Uhlenbruck G., Müller W. E., Holz G. Sponge aggregation factor and sponge hemagglutinin: possible relationships between two different molecules. Dev Comp Immunol. 1979 Summer;3(3):399–416. doi: 10.1016/s0145-305x(79)80037-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]