Abstract
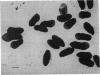
Structures whose morphology is identical to that of bacterial pili have been isolated from spores of Bacillus cereus. The structures are absent from log-phase and sporulating cells. The pili are 6.8 nm in diameter, are of variable length, and appear to emanate randomly from the exosporium. Examination of spores from 12 Bacillus species showed that only those from B. cereus and B. thuringiensis have pili. Although isolated spore pili were shown to be composed of protein, their subunit nature was not discernible due to the extreme insolubility of the structure. An antiserum to spore pili was labeled with ferritin and used to examine the distribution of pilus antigen on the outer spore surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Date T., Inuzuka M., Tomoeda M. Purification and characterization of F pili from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5579–5585. doi: 10.1021/bi00644a030. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977 Jul;131(1):259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., RIBI E. ULTRASTRUCTURE OF THE EXOSPORIUM ENVELOPING SPORES OF BACILLUS CEREUS. J Bacteriol. 1964 Dec;88:1774–1789. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- Kondo M., Foster J. W. Chemical and electron microscope studies on fractions prepared from coats of Bacillus spores. J Gen Microbiol. 1967 May;47(2):257–271. doi: 10.1099/00221287-47-2-257. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter crescentus pili: structure and stage-specific expression. J Bacteriol. 1977 Jul;131(1):340–346. doi: 10.1128/jb.131.1.340-346.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W., Bulla L. A., Jr Physiology of sporeforming bacteria associated with insects: minimal nutritional requirements for growth, sporulation, and parasporal crystal formation of Bacillus thuringiensis. Appl Microbiol. 1974 Jul;28(1):124–128. doi: 10.1128/am.28.1.124-128.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Pope L., Yolton D. P., Rode L. J. Appendages of Clostridium bifermentans spores. J Bacteriol. 1967 Oct;94(4):1206–1215. doi: 10.1128/jb.94.4.1206-1215.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. N., Vincent P., Ward M. E. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977 Sep;102(1):169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Rode L. J., Crawford M. A., Williams M. G. Clostridium spores with ribbon-like appendages. J Bacteriol. 1967 Mar;93(3):1160–1173. doi: 10.1128/jb.93.3.1160-1173.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER S. J. Preparation of an electron-dense antibody conjugate. Nature. 1959 May 30;183(4674):1523–1524. doi: 10.1038/1831523a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yanagawa R., Otsuki K. Some properties of the pili of Corynebacterium renale. J Bacteriol. 1970 Mar;101(3):1063–1069. doi: 10.1128/jb.101.3.1063-1069.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]