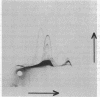
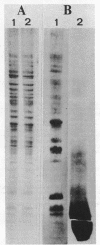
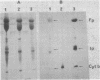
Abstract
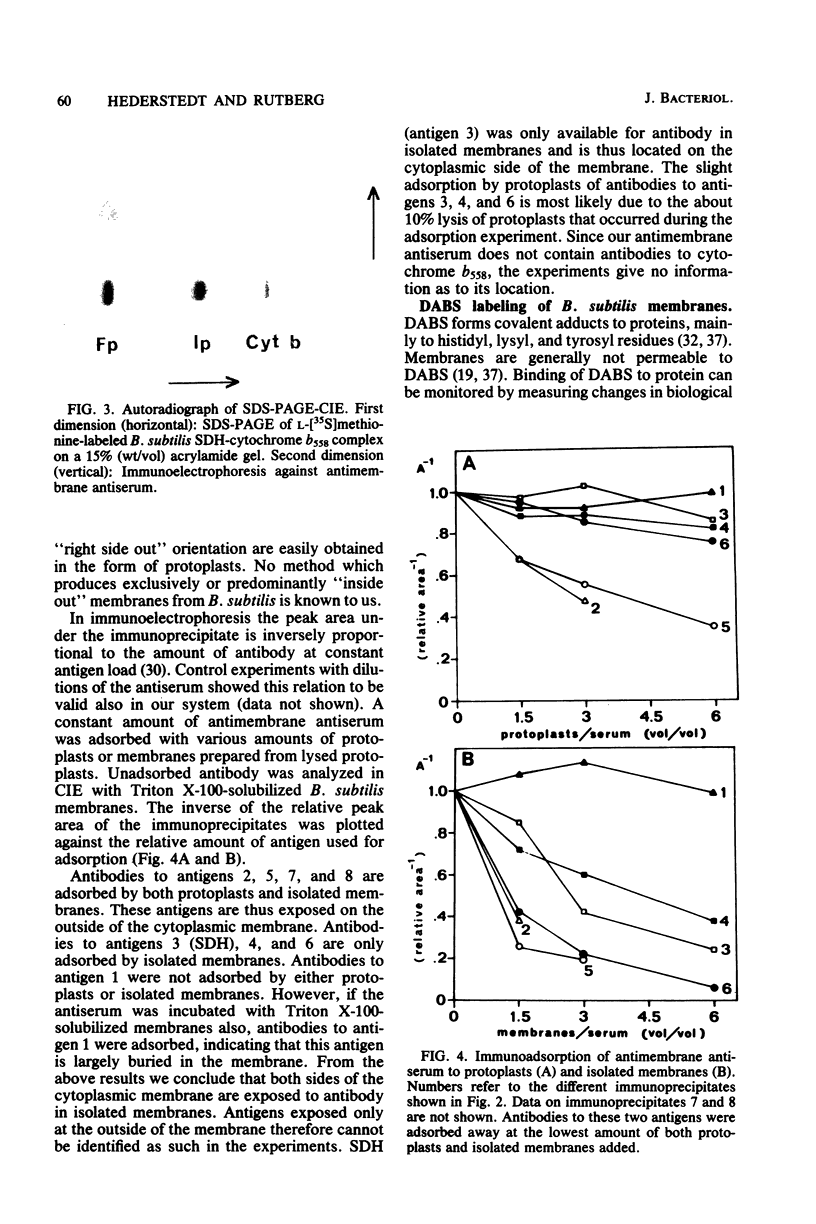
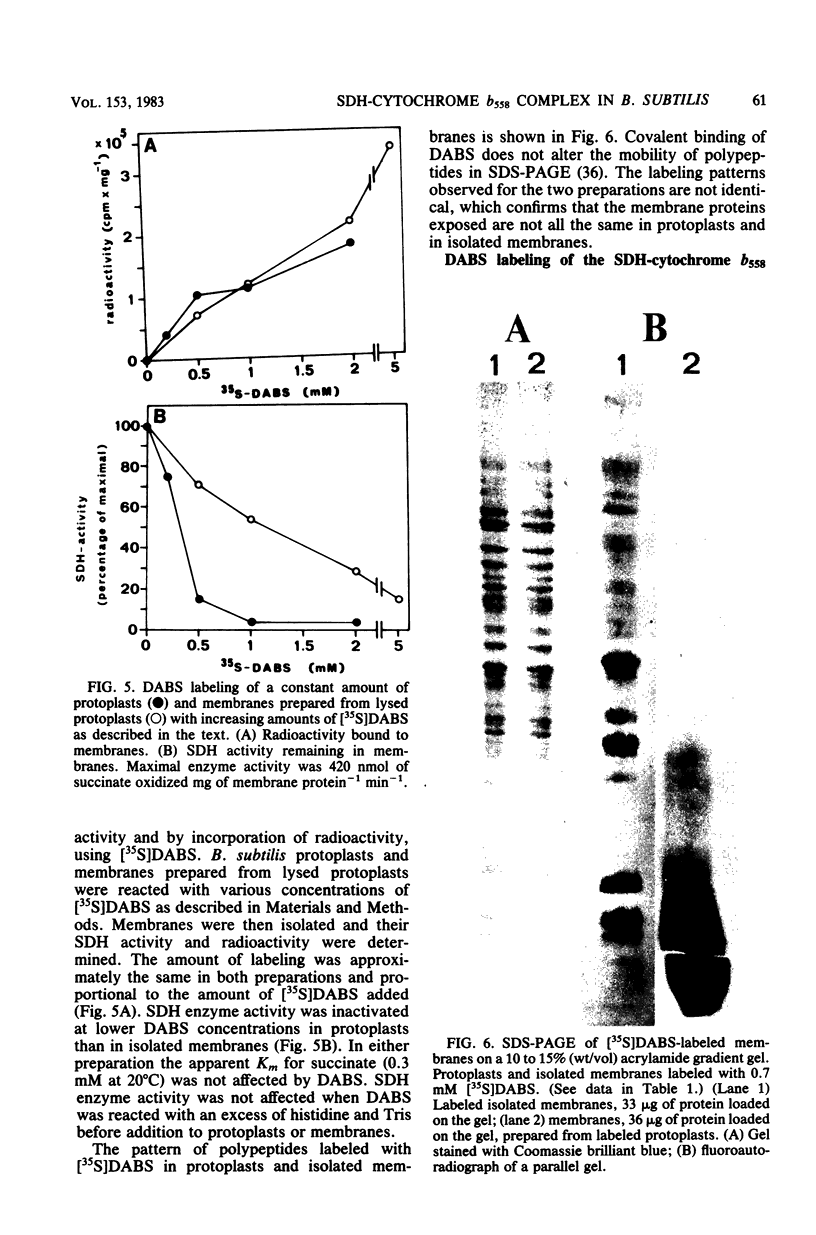
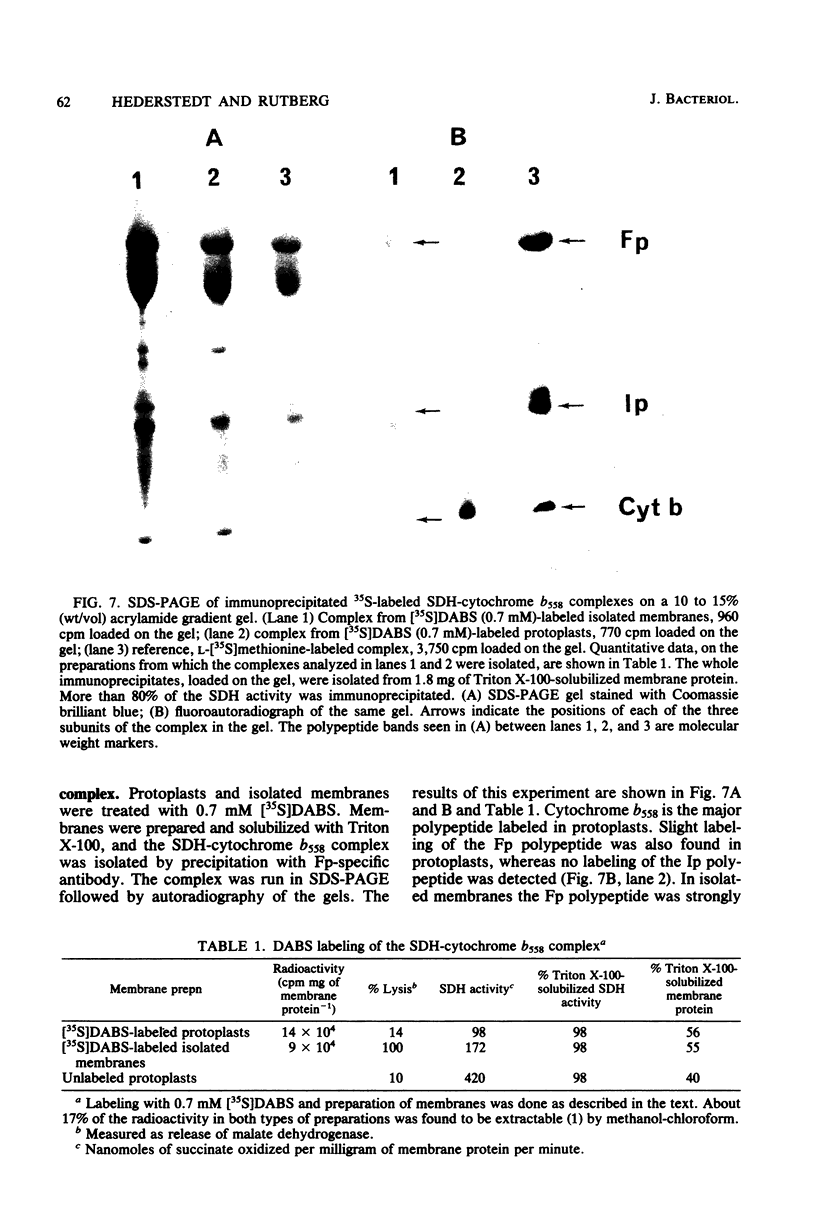
The orientation of the three subunits of the membrane-bound succinate dehydrogenase (SDH)-cytochrome b558 complex in Bacillus subtilis was studied in protoplasts ("right side out") and isolated membranes (random orientation), using immunoadsorption and surface labeling with [35S]diazobenzenesulfonate. Anti-SDH antibodies were adsorbed by isolated membranes but not by protoplasts. The SDH Mr 65,000 flavoprotein subunit was labeled with [35S]diazobenzenesulfonate in isolated membranes but not in protoplasts. The flavoprotein subunit is thus located on the cytoplasmic side of the membrane. The location of the SDH Mr 28,000 iron-protein subunit was not definitely established, but most probably the iron-protein subunit also is located on the cytoplasmic side of the membrane. Antibodies were not obtained to the hydrophobic cytochrome b558. The cytochrome was strongly labeled with [35S]diazobenzenesulfonate in protoplasts, and labeling was also obtained with isolated membranes. Cytochrome b558 is thus exposed on the outside of the membrane. In B. subtilis SDH binds specifically to cytochrome b558, which suggests that the cytochrome is exposed also on the cytoplasmic side of the membrane. The results obtained suggest that the B. subtilis SDH is exclusively located on the cytoplasmic side of the membrane where it is bound to cytochrome b558, which spans the membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma J., Strijker R., Alkema J. Y., Seijen H. G., Konings W. N. NADH dehydrogenase and NADH oxidation in membrane vesicle from Bacillus subtilis. Eur J Biochem. 1981 Dec;120(3):599–606. doi: 10.1111/j.1432-1033.1981.tb05742.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Crawford I. P., Baltscheffsky H. Purification, molecular properties, and amino acid composition of the subunits of Rhodospirillum rubrum succinate dehydrogenase. Arch Biochem Biophys. 1977 Apr 30;180(2):459–464. doi: 10.1016/0003-9861(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdlestone J., Bisson R., Capaldi R. A. Interaction of succinate--ubiquinone reductase (complex II) with (arylazido)phospholipids. Biochemistry. 1981 Jan 6;20(1):152–156. doi: 10.1021/bi00504a025. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Arrangement of respiratory nitrate reductase in the cytoplasmic membrane of Escherichia coli. Location of beta subunit. FEBS Lett. 1980 Apr 21;113(1):15–20. doi: 10.1016/0014-5793(80)80484-4. [DOI] [PubMed] [Google Scholar]
- Gutweniger H., Bisson R., Montecucco C. Membrane topology of beef-heart ubiquinone-cytochrome c reductase (complex III). J Biol Chem. 1981 Nov 10;256(21):11132–11136. [PubMed] [Google Scholar]
- Hederstedt L. Cytochrome b reducible by succinate in an isolated succinate dehydrogenase-cytochrome b complex from Bacillus subtilis membranes. J Bacteriol. 1980 Dec;144(3):933–940. doi: 10.1128/jb.144.3.933-940.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Magnusson K., Rutberg L. Reconstitution of succinate dehydrogenase in Bacillus subtilis by protoplast fusion. J Bacteriol. 1982 Oct;152(1):157–165. doi: 10.1128/jb.152.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Biosynthesis and membrane binding of succinate dehydrogenase in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):941–951. doi: 10.1128/jb.144.3.941-951.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Lamont A., Garland P. B. The mechanism of proton translocation driven by the respiratory nitrate reductase complex of Escherichia coli. Biochem J. 1980 Jul 15;190(1):79–94. doi: 10.1042/bj1900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N. Localization of membrane proteins in membrane vesicles of Bacillus subtilis. Arch Biochem Biophys. 1975 Apr;167(2):570–580. doi: 10.1016/0003-9861(75)90500-7. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dorrer E., Winkler E. The orientation of the substrate sites of formate dehydrogenase and fumarate reductase in the membrane of Vibrio succinogenes. Biochim Biophys Acta. 1980 Jan 4;589(1):118–136. doi: 10.1016/0005-2728(80)90136-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard K., Wingfield P., Arad T., Weiss H. Three-dimensional structure of ubiquinol:cytochrome c reductase from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1981 Jun 25;149(2):259–274. doi: 10.1016/0022-2836(81)90301-6. [DOI] [PubMed] [Google Scholar]
- Merli A., Capaldi R. A., Ackrell B. A., Kearney E. B. Arrangement of complex II (succinate-ubiguinone reductase) in the mitochondrial inner membrane. Biochemistry. 1979 Apr 17;18(8):1393–1400. doi: 10.1021/bi00575a001. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hederstedt L., Holmgren E., Rutberg L. Characterization of succinic dehydrogenase mutants of Bacillus subtilis by crossed immunoelectrophoresis. J Bacteriol. 1978 Oct;136(1):304–311. doi: 10.1128/jb.136.1.304-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem Anal. 1974;22:123–175. doi: 10.1002/9780470110423.ch3. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Melnick R. L., Maguire J., Packer L. Studies on mitochondrial proteins. II. Localization of components in the inner membrane: labeling with diazobenzenesulfonate, a non-penetrating probe. Biochim Biophys Acta. 1974 Apr 12;345(1):118–128. doi: 10.1016/0005-2736(74)90251-x. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Packer L. Chemical modification of mitochondria: surface labeling of inner membranes. Methods Enzymol. 1979;56:613–621. doi: 10.1016/0076-6879(79)56058-3. [DOI] [PubMed] [Google Scholar]
- Unden G., Kröger A. The function of the subunits of the fumarate reductase complex of Vibrio succinogenes. Eur J Biochem. 1981 Dec;120(3):577–584. doi: 10.1111/j.1432-1033.1981.tb05739.x. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Engel W. D. Structure and function of the energy-converting system of mitochondria. Angew Chem Int Ed Engl. 1980;19(9):659–675. doi: 10.1002/anie.198006593. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Sebald W. b-Type cytochromes. Annu Rev Biochem. 1980;49:281–314. doi: 10.1146/annurev.bi.49.070180.001433. [DOI] [PubMed] [Google Scholar]