Abstract
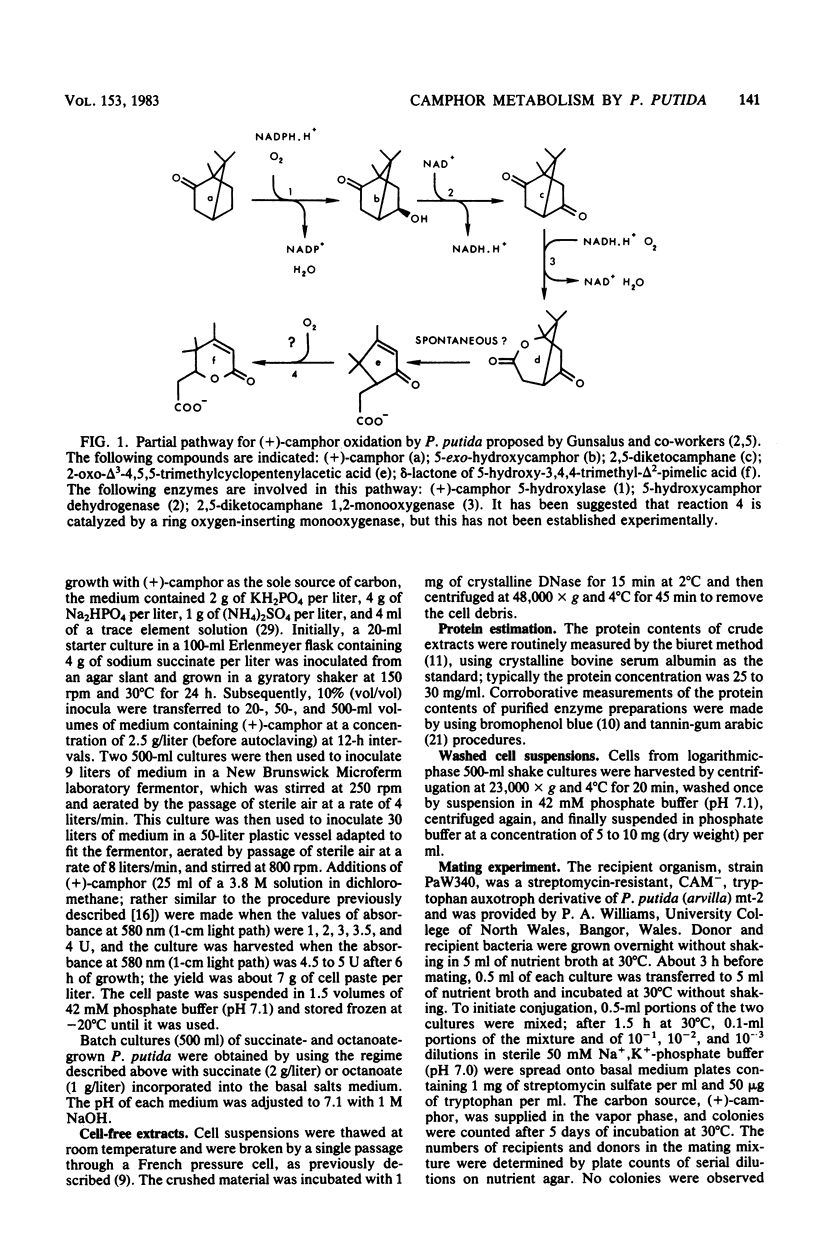
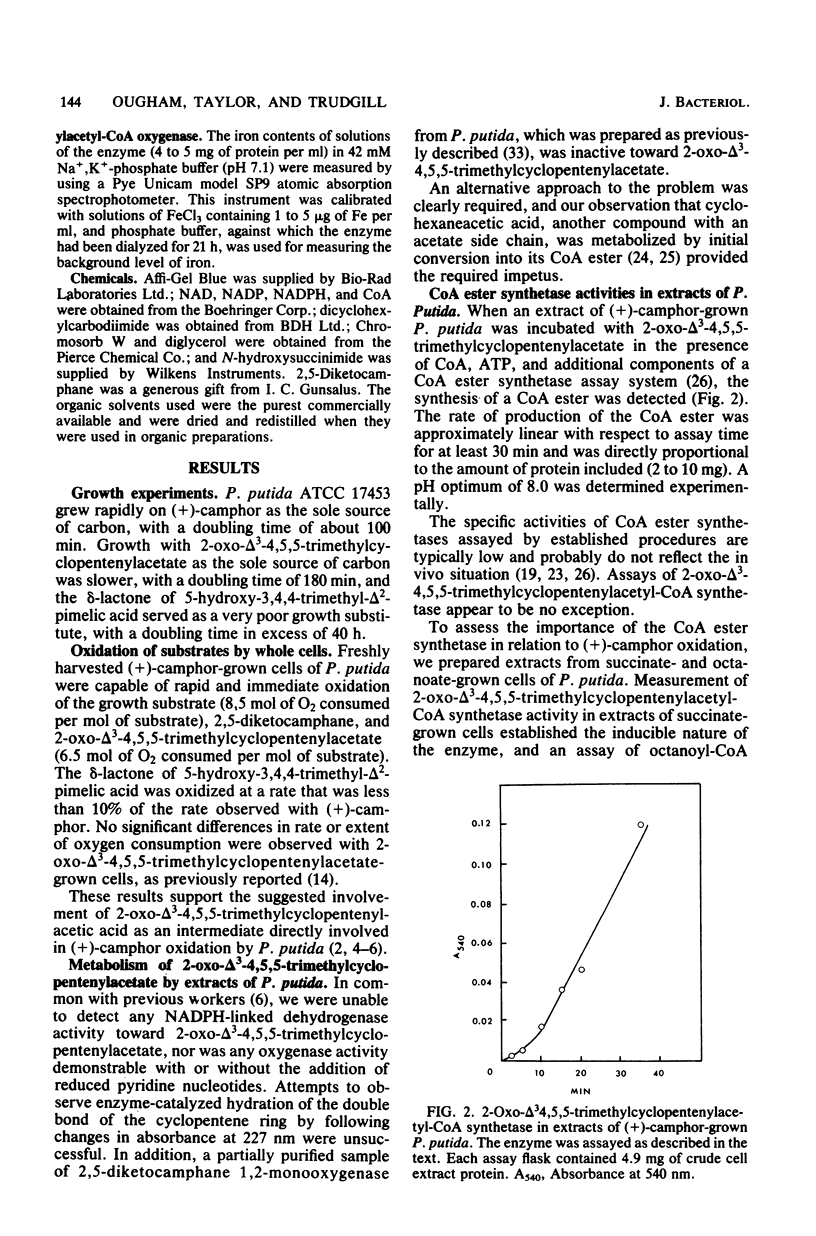
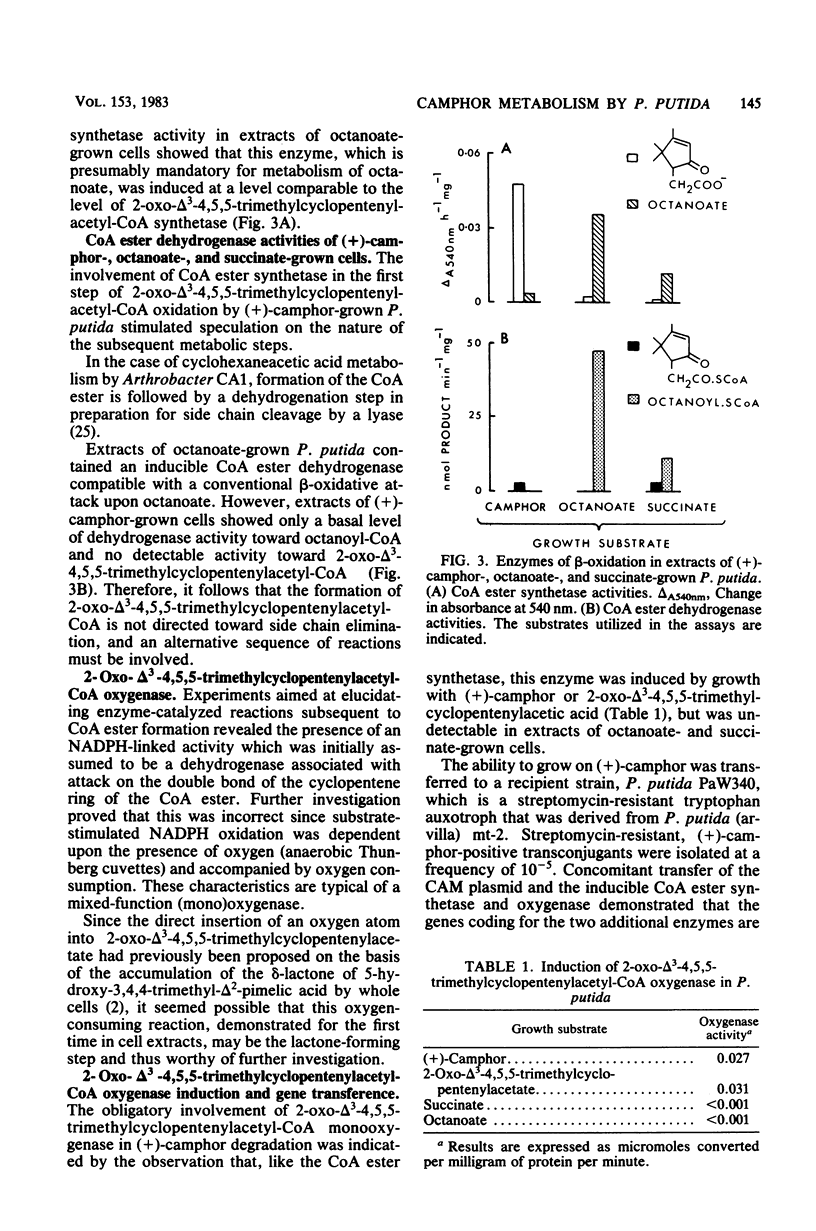
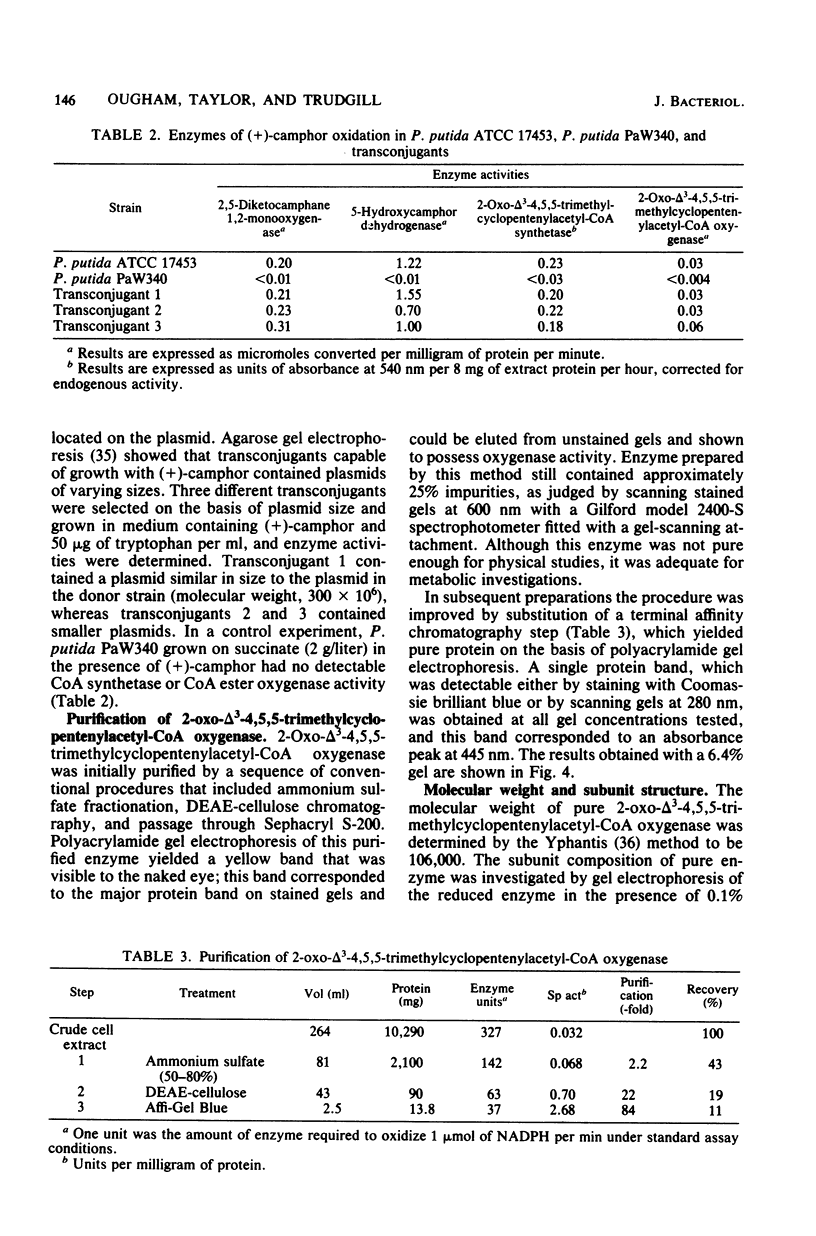
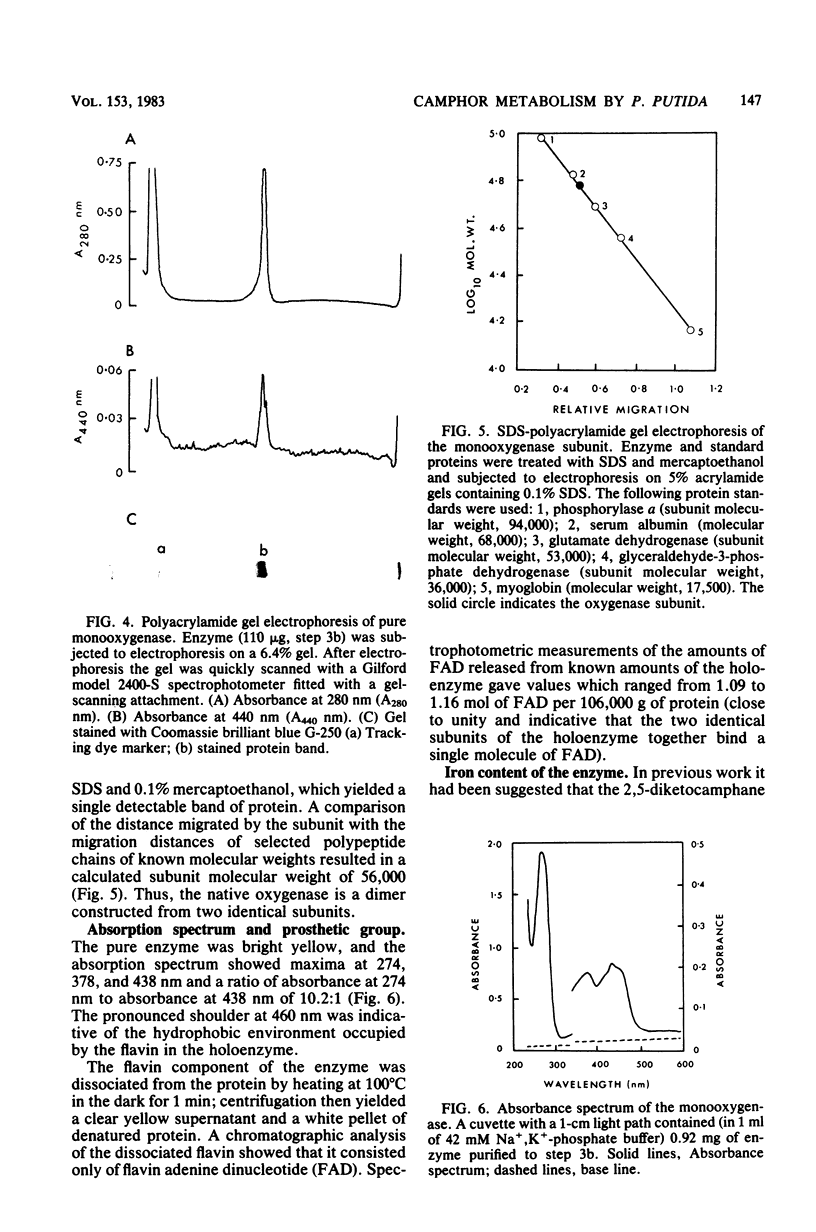
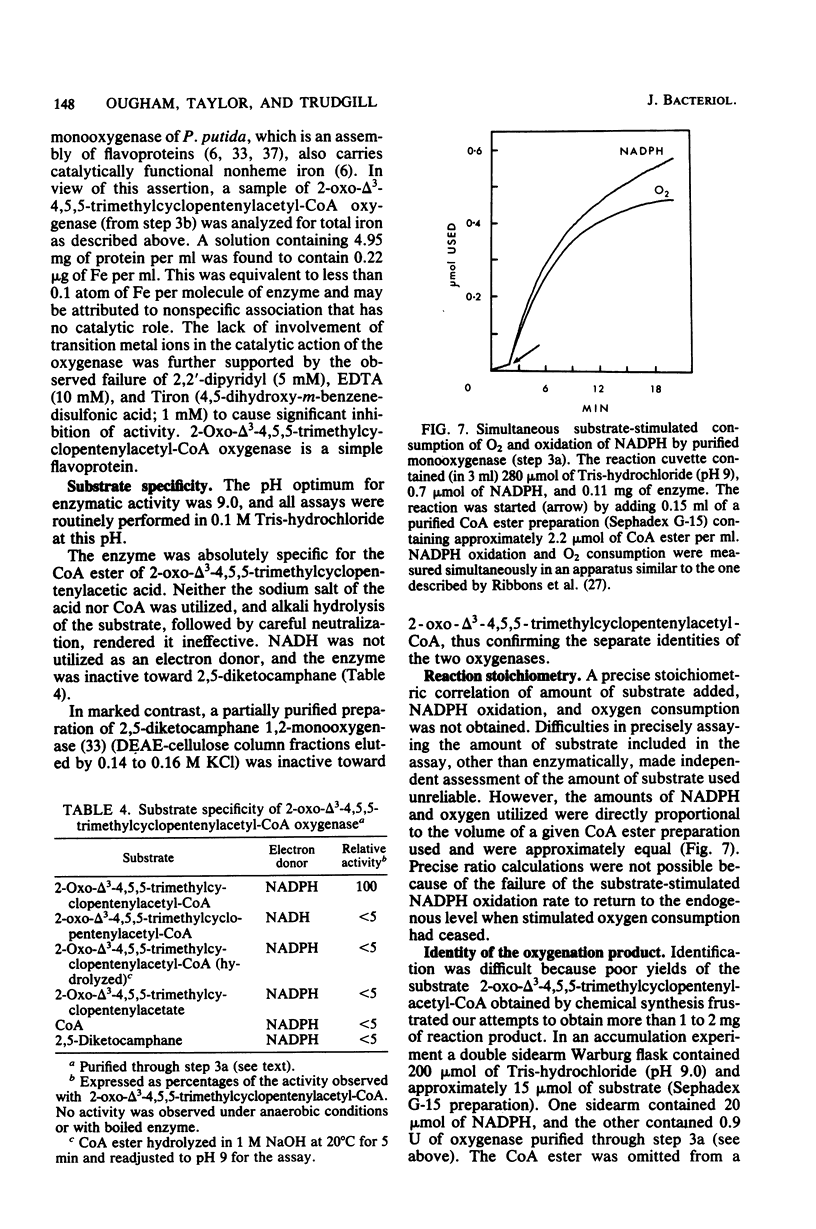
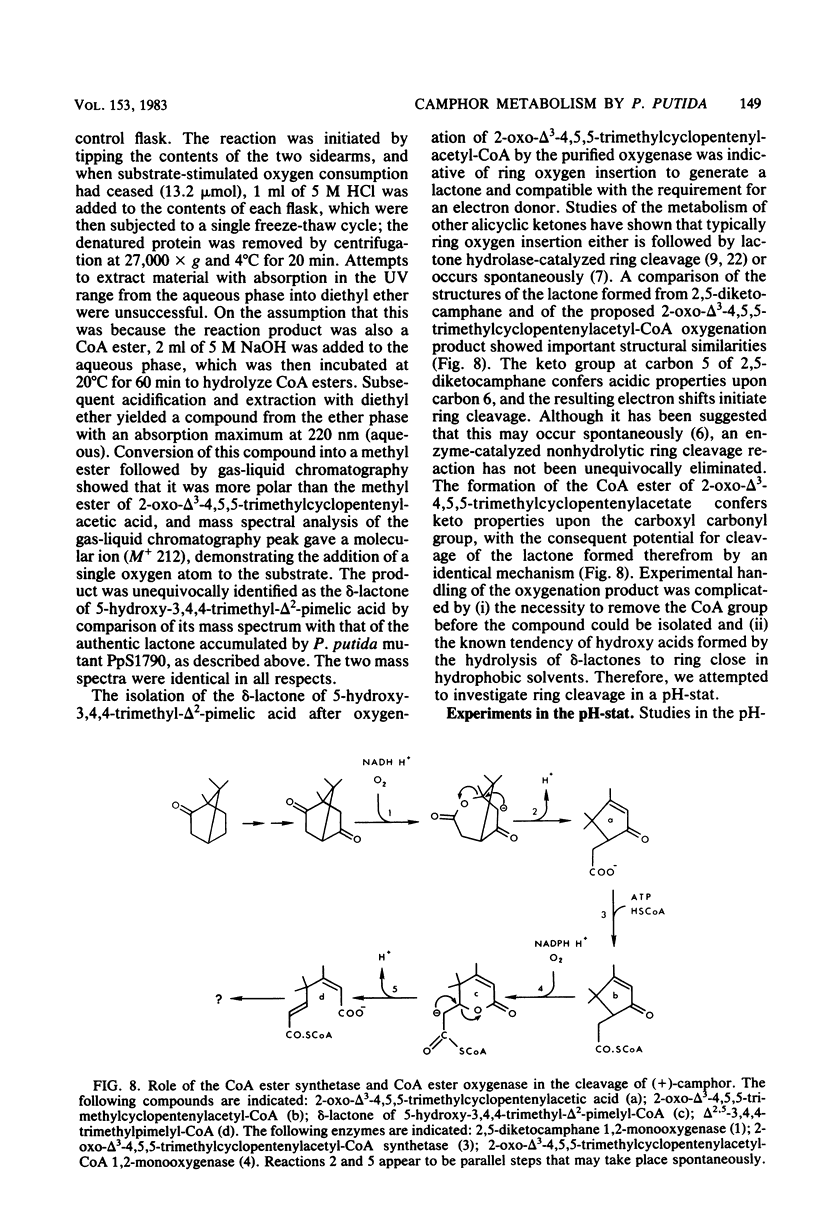
Previously, Pseudomonas putida was shown to degrade (+)-camphor, and cleavage of the first ring of the bicyclic structure involved two monooxygenases (a hydroxylase and a ring oxygen-inserting enzyme), a dehydrogenase, and spontaneous cleavage of an unstable oxygenation product (lactone). Cleavage of the second ring was not demonstrated but was assumed also to occur by ring oxygen insertion, since the predicted oxygenation product was extracted from whole-cell incubation systems. Our investigation established that metabolism of the first ring cleavage intermediate, 2-oxo-delta 3-4,5,5-trimethylcyclopentenylacetic acid, occurred through the sequential action of two inducible enzymes, a coenzyme A ester synthetase and an oxygenase. The oxygenase was purified to homogeneity and had a molecular weight of 106,000. This enzyme carried a single molecule of flavin adenine dinucleotide and consisted of two identical subunits. Iron was not present at a significant level. The oxygenase was specific for NADPH as the electron donor and absolutely specific for the coenzyme A ester of 2-oxo-delta 3-4,5,5-trimethylcyclopentenylacetic acid as the substrate. The reaction stoichiometry was compatible with this enzyme being a monooxygenase, and a mass spectral analysis of the methyl ester of the product confirmed the insertion of a single oxygen atom. The enzyme appeared to be analogous to, although distinct from. 2,5-diketocamphane 1,2-monooxygenase in catalyzing a "biological Baeyer-Villiger" reaction with the formation of a lactone. Structural analogy suggested that this lactone, like the first, was also unstable and susceptible to spontaneous ring opening, although this was not experimentally established.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Arif A., Blecher M. Synthesis of fatty acyl CoA and other thiol esters using N-hydroxysuccinimide esters of fatty acids. J Lipid Res. 1969 May;10(3):344–345. [PubMed] [Google Scholar]
- CAIN R. B. The metabolism of protocatechuic acid by a vibrio. Biochem J. 1961 May;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., GUNSALUS I. C. An enzyme system for cyclic ketone lactonization. Biochem Biophys Res Commun. 1961 Nov 29;6:293–297. doi: 10.1016/0006-291x(61)90382-5. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., NAMTVEDT M. J., GUNSALUS I. C. MIXED FUNCTION OXIDATION. II. SEPARATION AND PROPERTIES OF THE ENZYMES CATALYZING CAMPHOR LACTONIZATION. J Biol Chem. 1965 Jan;240:495–503. [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus C. F., Gunsalus I. C. Transduction and the clustering of genes in fluorescent Pseudomonads. Proc Natl Acad Sci U S A. 1968 May;60(1):168–175. doi: 10.1073/pnas.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davey J. F., Trudgill P. W. The metabolism of trans-cyclohexan-1,2-diol by an Acinetobacter species. Eur J Biochem. 1977 Mar 15;74(1):115–127. doi: 10.1111/j.1432-1033.1977.tb11373.x. [DOI] [PubMed] [Google Scholar]
- Donoghue N. A., Trudgill P. W. The metabolism of cyclohexanol by Acinetobacter NCIB 9871. Eur J Biochem. 1975 Dec 1;60(1):1–7. doi: 10.1111/j.1432-1033.1975.tb20968.x. [DOI] [PubMed] [Google Scholar]
- Flores R. A rapid and reproducible assay for quantitative estimation of proteins using bromophenol blue. Anal Biochem. 1978 Aug 1;88(2):605–611. doi: 10.1016/0003-2697(78)90462-1. [DOI] [PubMed] [Google Scholar]
- Griffin M., Trudgill P. W. The metabolism of cyclopentanol by Pseudomonas N.C.I.B. 9872. Biochem J. 1972 Sep;129(3):595–603. doi: 10.1042/bj1290595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Bertland A. U., 2nd, Jacobson L. A. Enzyme induction and repression in anabolic and catabolic pathways. Arch Mikrobiol. 1967;59(1):113–122. doi: 10.1007/BF00406322. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Haeffner E. W. Sephadex G-15 column separation of [9,10-3H] stearyl coenzyme A. J Chromatogr. 1970 Jul 8;50(1):140–141. doi: 10.1016/s0021-9673(00)97930-5. [DOI] [PubMed] [Google Scholar]
- Hartline R. A., Gunsalus I. C. Induction specificity and catabolite repression of the early enzymes in camphor degradation by Pseudomonas putida. J Bacteriol. 1971 May;106(2):468–478. doi: 10.1128/jb.106.2.468-478.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda K., Nunn W. D. Purification and characterization of acyl coenzyme A synthetase from Escherichia coli. J Biol Chem. 1981 Jun 10;256(11):5702–5707. [PubMed] [Google Scholar]
- Lapidot Y., Rappoport S., Wolman Y. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J Lipid Res. 1967 Mar;8(2):142–145. [PubMed] [Google Scholar]
- Norris D. B., Trudgill P. W. The metabolism of cyclohexanol by Nocardia globerula CL1. Biochem J. 1971 Feb;121(3):363–370. doi: 10.1042/bj1210363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougham H. J., Trudgill P. W. Metabolism of cyclohexaneacetic acid and cyclohexanebutyric acid by Arthrobacter sp. strain CA1. J Bacteriol. 1982 Jun;150(3):1172–1182. doi: 10.1128/jb.150.3.1172-1182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougham H. J., Trudgill P. W. The microbial metabolism of cyclohexylacetic acid [proceedings]. Biochem Soc Trans. 1978;6(6):1324–1326. doi: 10.1042/bst0061324. [DOI] [PubMed] [Google Scholar]
- ROGERS L. J. A SIMPLE APPARATUS FOR DISC ELECTROPHORESIS. Biochim Biophys Acta. 1965 Mar 29;94:324–329. doi: 10.1016/0926-6585(65)90041-5. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Trudgill P. W., DuBus R., Gunsalus I. C. Mixed function oxidation. VI. Purification of a tightly coupled electron transport complex in camphor lactonization. J Biol Chem. 1966 Sep 25;241(18):4288–4290. [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Gunsalus I. C. Monoxygenases. VII. Camphor ketolactonase I and the role of three protein components. J Biol Chem. 1969 Nov 25;244(22):6149–6152. [PubMed] [Google Scholar]