Abstract
Myelin-associated glycoprotein (MAG) is a potent inhibitor of neurite outgrowth from a variety of neurons. The receptor for MAG or signals that elicit morphological changes in neurons remained to be established. Here we show that the neurotrophin receptor p75 (p75NTR) is the signal transducing element for MAG. Adult dorsal root ganglion neurons or postnatal cerebellar neurons from mice carrying a mutation in the p75NTR gene are insensitive to MAG with regard to neurite outgrowth. MAG activates small GTPase RhoA, leading to retarded outgrowth when p75NTR is present. Colocalization of p75NTR and MAG binding is seen in neurons. Ganglioside GT1b, which is one of the binding partners of MAG, specifically associates with p75NTR. Thus, p75NTR and GT1b may form a receptor complex for MAG to transmit the inhibitory signals in neurons.
Keywords: p75; neurotrophin; myelin-associated glycoprotein; Rho; ganglioside
Introduction
The neurotrophin receptor p75 (p75NTR)* is a membrane glycoprotein that binds to all neurotrophins identified so far. Motor neurons in the spinal cord, most sympathetic and sensory neurons in the peripheral nervous system, and cerebellar Purkinje cells and retinal ganglion cells all express p75NTR at high levels during the outgrowth of axons (Buck et al., 1987; Ernfors et al., 1988; Yan and Johnson, 1988; Large et al., 1989; von Bartheld et al., 1991). In dendrite-bearing neurons, p75NTR is also expressed during the time of dendritic arborization. Some neurons markedly up-regulate p75NTR after lesion or seizure (Ernfors et al., 1989; Roux et al., 1999). It was shown recently that the neurotrophin binding to p75NTR promoted axonal outgrowth of neurons and inactivated small GTPase RhoA (Yamashita et al., 1999). The link between p75NTR and RhoA seems to be functionally relevant, since the modulation of neurite outgrowth in p75NTR-expressing neurons by neurotrophins mimics the results obtained after RhoA inactivation.
Myelin-associated glycoprotein (MAG) is a well-characterized component of both the central nervous system and the peripheral nervous system myelin and is a potent inhibitor of axonal regeneration (McKerracher et al., 1994; Mukhopadhyay et al., 1994). It inhibits axonal outgrowth from adult dorsal root ganglion (DRG) and in postnatal ages of cerebellar, retinal, spinal, hippocampal, and superior cervical ganglion neurons. Although the molecular mechanism of action of MAG on neurons or the receptor that transmits the signals remained to be established, neurite growth inhibition by MAG is blocked if neurons are exposed to neurotrophins before encountering the inhibitor (Cai et al., 1999). We attempted to ask if p75NTR had some roles in transducing the inhibitory signals of MAG in neurons. The results we obtained were surprising, since p75NTR itself turned out to be a signal transducing element for MAG and neurotrophins. Association of p75NTR with ganglioside GT1b is also shown to provide a possible link between MAG and p75NTR.
Results and discussion
Inhibition of neurite outgrowth is dependent on p75NTR
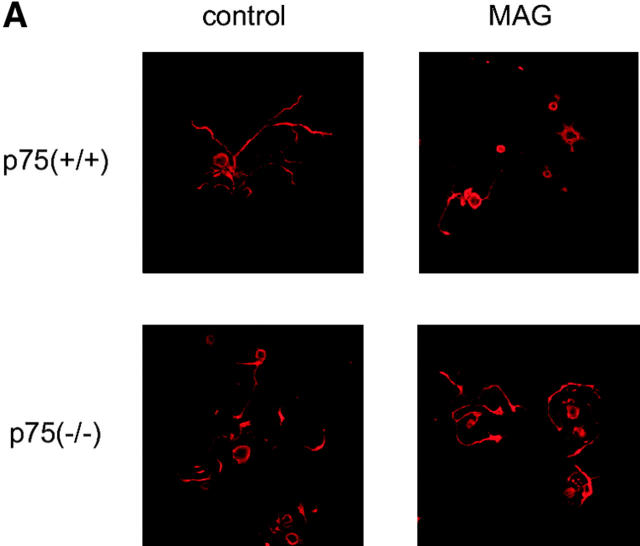
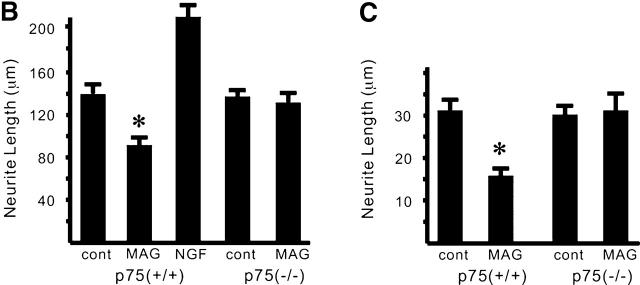
We first asked if p75NTR was associated with the effects of MAG on neurons. Neurite outgrowth of adult DRG neurons from mice carrying a mutation in the p75NTR gene (Lee et al., 1992) or wild-type mice was examined. A soluble chimeric form of MAG, consisting of the extracellular domain of MAG fused to the Fc region of human IgG (MAG–Fc), was used. It was shown that soluble MAG, released in abundance from myelin and found in vivo, and MAG–Fc could potently inhibit axonal growth (Tang et al., 1997a,b). We compared the neurite length between MAG-treated and MAG-untreated neurons. MAG–Fc at the concentration of 25 μg/ml inhibited neurite outgrowth of DRG neurons from adult wild-type mice (Fig. 1, A and B). Fc had no effect on the neurons (unpublished data). Interestingly, the inhibitory effect of MAG could not be observed in DRG neurons from adult mice carrying a mutation in the p75NTR gene. Exactly the same results were obtained whether total process outgrowth or length of the longest neurite was measured (unpublished data).
Figure 1.
The effects of MAG on neurons are dependent on p75 NTR . (A) Dissociated DRG neurons were incubated for 24 h with or without MAG–Fc and then immunostained with monoclonal antibody (TuJ1) recognizing the neuron-specific β–tubulin III protein. p75(+/+), wild type; p75(−/−), mice carrying a mutation in the p75NTR gene. (B) Mean length of the longest neurite per neuron. Data are mean ± SEM. An asterisk indicates statistical significance (*p < 0.01, Student's t test). (C) Mean length of the longest neurite per neuron. Dissociated cerebellar neurons were incubated for 24 h with or without MAG–Fc.
Similar experiments with postnatal cerebellar neurons were performed. At a concentration of 25 μg/ml of MAG–Fc, neurite growth was significantly inhibited when cerebellar neurons from P9 wild-type mice were used (Fig. 1 C). Again, no inhibition by MAG was observed in the neurons from P9 mice carrying a mutation in the p75NTR gene. These results suggest that MAG inhibits neurite outgrowth by a p75NTR-dependent mechanism.
p75NTR has been shown to be required for the inhibition of axonal growth and target innervation of peripheral neurons in vivo and in vitro (Kimpinski et al., 1999; Kohn et al., 1999) and for suppression of hyperinnervation of cholinergic neurons in vivo (Yeo et al. 1997). It was reported recently that the growth of sympathetic axons within the myelinated portions of the cerebellum was greater in NGF transgenic mice lacking expression of p75NTR compared with those expressing p75NTR in vivo (Walsh et al., 1999). It may be a relevant finding supporting our data, since neurons carrying a mutation in the p75NTR gene are suggested to be refractory to inhibitory factors.
Signaling mechanisms of MAG on the neurons
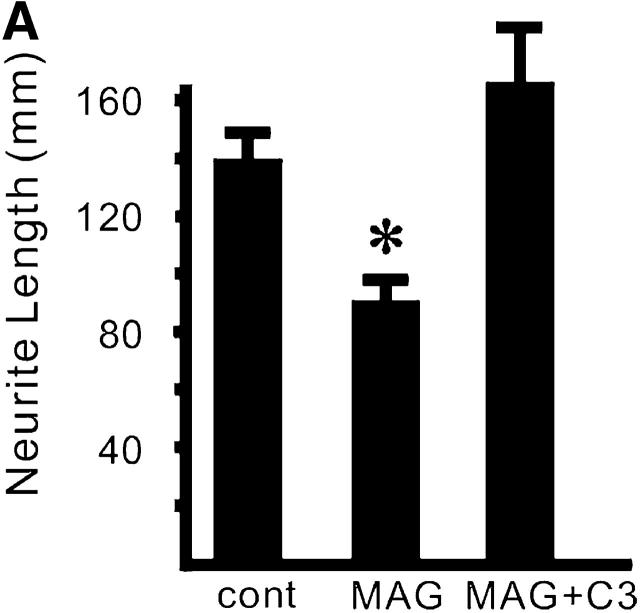
Some neurons extend neurites rapidly when RhoA is inactivated, and neurite retraction occurs when RhoA is active (Davies, 2000). Previous study shows that inactivation of RhoA promoted axonal regeneration in vivo (Lehmann et al., 1999). Thus, to examine if activation of RhoA is necessary for modification of neurite outgrowth by MAG in our system, we employed the exoenzyme C3 transferase from Clostridium botulinum, which ADP ribosylates RhoA. The recombinant C3 transferase was introduced into the cytoplasm of DRG neurons by trituration. The C3 transferase completely abolished the effect of MAG on DRG neurons from wild-type mice (Fig. 2 A). These data are consistent with the previous report, suggesting RhoA is on the pathway of MAG signaling (Lehmann et al., 1999).
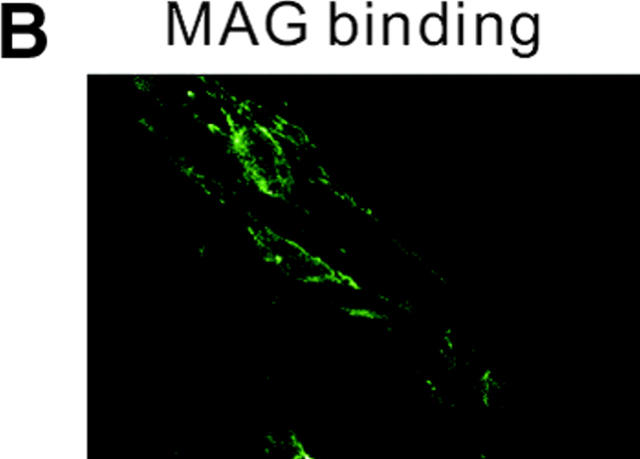
Figure 2.
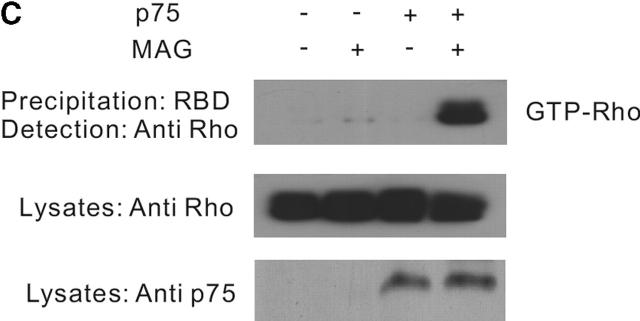
MAG activates RhoA through a p75 NTR -dependent mechanism. (A) The effect of C3 transferase on MAG-treated DRG neurons from wild-type mice. Mean length of the longest neurite per neuron. Data are mean ± SEM. Asterisks indicate statistical significance (*p < 0.01, Student's t test). (B) Binding of MAG–Fc to 293 cells was visualized by incubation with an FITC-tagged anti–human IgG. (C) Affinity precipitation of RhoA in transfected 293 cells. MAG–Fc (25 μg/ml) elicits activation of RhoA only when 293 cells express p75NTR.
The next hypothesis tested was that MAG regulates RhoA activity by a p75NTR-dependent mechanism. 293 cells, which express no p75NTR endogenously, were used as binding of MAG–Fc to the cell surface was diffusely observed (Fig. 2 B). Using the RhoA-binding domain of the effector protein Rhotekin (Ren et al., 1999), the GTP-bound form of RhoA can be affinity precipitated. The direct measurement of RhoA activity in the cells can be done using this method. The assay revealed that within 30 min after the addition of soluble MAG (25 μg/ml) extracts of 293 cells transfected with p75NTR and RhoA contained dramatically increased amounts of GTP–RhoA compared with the control (Fig. 2 C), though no change in the activity was observed by the addition of Fc (unpublished data). However, no increase in GTP–RhoA content was observed in the cells untransfected with p75NTR by the addition of MAG–Fc (Fig. 2 C).
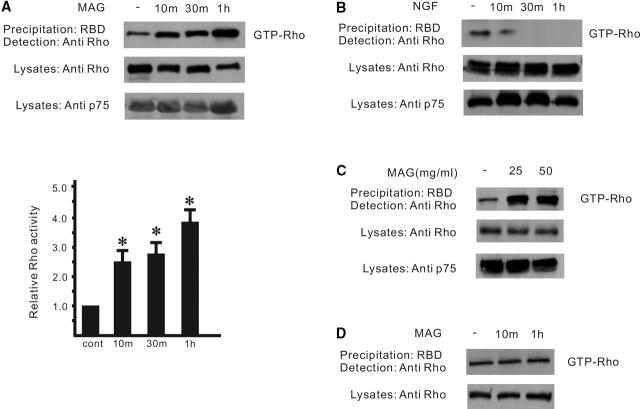
Regulation of RhoA activity when the proteins are artificially expressed may be difficult to detect in natural cells. Therefore, to see if RhoA activity is regulated by MAG in the cells expressing endogenous p75NTR postnatal cerebellar granule neurons were used, since these neurons also are sensitive to MAG with regard to neurite outgrowth. Consistent with the observation in transfected 293 cells, MAG–Fc activates RhoA in cerebellar granule neurons from wild-type mice (P9), which express abundant p75NTR (Fig. 3 A). This rapid activation was in contrast with the effect of NGF on the neurons, which is also mediated by p75NTR, since they don't express trkA (Fig. 3 B). RhoA activity (Fig. 3 C) and the effect of MAG on neurite outgrowth (unpublished data) seem to be saturated by MAG at the concentration of 25 μg/ml. Activation of RhoA by MAG was lost in the neurons from mice carrying a mutation in the p75NTR gene (Fig. 3 D). These data demonstrate that MAG activates RhoA by a p75NTR-dependent mechanism, thus inhibiting neurite outgrowth of postnatal cerebellar granule neurons.
Figure 3.
Affinity precipitation of RhoA in postnatal cerebellar neurons. (A) RhoA activity was increased after the addition of MAG–Fc (25 μg/ml). RhoA activity is indicated by the amount of RBD-bound RhoA normalized to the amount of RhoA in the lysates. Values represent RhoA activity relative to the cells at time 0. Results are means ± SE from three experiments. Asterisks indicate statistical significance (*p < 0.01, Student's t test). (B) NGF rapidly inhibits RhoA activity (∼10 min). (C) Dose response. (D) The activation was lost in the neurons from mice carrying a mutation in the p75NTR gene.
Only the wild type of RhoA, which is predominantly in a GDP-bound form, but not the constitutive active form of RhoA, interacts with p75NTR (Yamashita et al., 1999). In transfected cells, overexpression of p75NTR activated RhoA in a neurotrophin-independent manner. Therefore, the GDP-bound form of RhoA may interact with the p75NTR helical domain to be activated after exposure to MAG. More detailed structure-function analyses of p75NTR should help to elucidate the precise mechanism of regulation of RhoA activity by p75NTR.
Colocalization of p75NTR and MAG binding
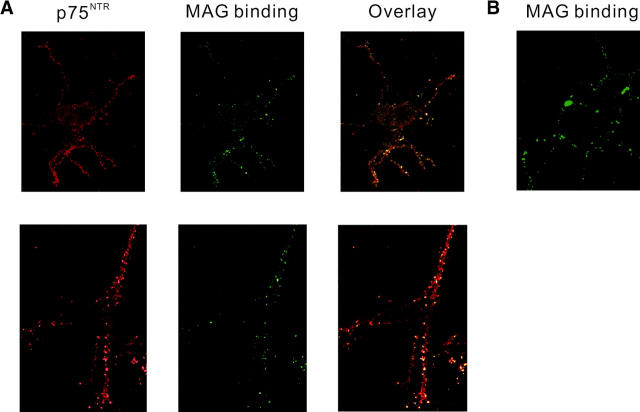
MAG binds to neurons in a sialic acid–dependent manner, but MAG's sialic acid binding site is distinct from its neurite inhibitory activity. The sialic acid–dependent binding to MAG is not sufficient or necessary for MAG's inhibitory effect (Tang et al., 1997a). Therefore, it is possible that the binding partner and the signal transducing element for MAG may form a receptor complex. We assumed that the binding partner for MAG and p75NTR might interact in a cis manner. To test this hypothesis, the localization of p75NTR and MAG binding was assessed on the subcellular level. Binding of MAG–Fc was visualized by incubation with a fluorescent-tagged anti–human IgG. Fig. 4 shows binding of MAG–Fc to adult DRG neurons using confocal laser microscopy. MAG–Fc binding appears punctate. The same cells were stained with an anti-p75NTR antibody, and the distribution was assessed. p75NTR expression on the cell body was rather diffuse but that on the neurites showed fine speckled staining (Fig. 4 A, top). The vast majority of puncta for p75NTR immunoreactivity was colocalized with MAG binding. At high magnification, the colocalization was evident by the similar distribution of hot spots on the neuritic plasma membrane (Fig. 4 A, bottom). Binding of MAG–Fc was still observed in DRG neurons from mice carrying a mutation in the p75NTR gene (Fig. 4 B). These data demonstrate that the p75NTR and MAG binding colocalize.
Figure 4.
Colocalization of p75 NTR and MAG binding. (A) DRG neurons were stained with the anti-p75NTR antibody and an Alexa fluor™ 568–conjugated secondary antibody. Binding of MAG–Fc was visualized by incubation with the FITC-tagged anti–human IgG. Confocal microscopy was performed on a ZEISS LSM-510 laser scanning microscope. Representative single optical sections for p75NTR (left), MAG binding (middle), and overlay images (right) are shown. Close association of these markers on the neurites was seen in almost all of the neurons with p75NTR immunoreactivity. (B) Binding of MAG–Fc to DRG neurons from mice carrying a mutation in the p75NTR gene.
p75NTR binds to ganglioside GT1b
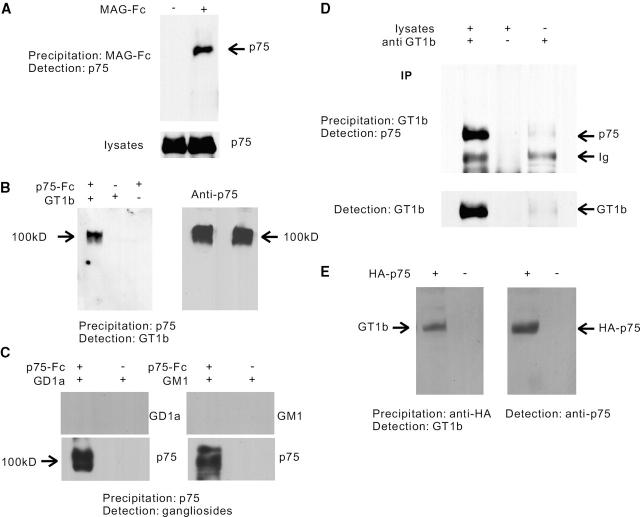
We next examined interaction of endogenous p75NTR and MAG using lysates prepared from postnatal cerebellum from mice. In the MAG–Fc precipitates, the anti-p75NTR antibody revealed the presence of a protein corresponding to p75NTR (Fig. 5 A). However, since MAG–Fc did not precipitate recombinant p75NTR protein in our preliminary experiments (unpublished data) these data suggest indirect interaction of MAG with p75NTR. Thus, p75NTR may not be the binding partner but it may be signal-transducing element.
Figure 5.
Association of MAG, p75 NTR , and GT1b. (A) Coprecipitation of p75NTR and MAG–Fc using lysates prepared from P9 cerebellum. In the MAG–Fc precipitates, the anti-p75NTR antibody revealed the presence of a protein corresponding to p75NTR. (B) Coprecipitation of recombinant p75NTR and GT1b. Association was examined by Western blot analysis of the precipitates produced with protein A sepharose and Fc-fused protein of p75NTR. The anti-GT1b antibody revealed the presence of a 100-kD protein (left), which was shown to be p75NTR by the anti-p75NTR antibody (right). (C) Coprecipitation of recombinant p75NTR and other gangliosides. (D) Coimmunoprecipitation of p75NTR and GT1b using lysates prepared from P9 cerebellum. In the GT1b immunoprecipitates, the anti-p75NTR antibody revealed the presence of a protein corresponding to p75NTR. The bottom bands correspond to the Ig of the antibodies used. (E) Coimmunoprecipitation of p75NTR and GT1b using transfected 293 cells. In the p75NTR immunoprecipitates, the anti-GT1b antibody revealed the presence of a protein (left), which was shown to be p75NTR by the anti-p75NTR antibody (right).
MAG binds to specific sialylated glycans and gangliosides present on the cell surface of neurons. The ability of MAG to bind specific gangliosides bearing terminal α-2–3–linked sialic acid has been well documented (Yang et al., 1996). MAG was shown to bind GT1b and GD1a and the α-series gangliosides, and antibody cross-linking of cell surface GT1b, but not GD1a, mimics the effect of MAG (Vinson et al., 2001). The pathological features of the nervous system of the complex ganglioside knockout mice closely resemble those reported in mice with a disrupted gene for MAG (Sheikh et al., 1999). These data prompted us to examine association of p75NTR with these gangliosides, assuming that p75NTR and gangliosides form a receptor complex for MAG. Recombinant p75NTR extracellular domain fused to Fc purified from Sf21 cells was used to precipitate gangliosides. In the p75NTR precipitates, the anti-GT1b antibody revealed the presence of an ∼100-kD band (Fig. 5 B, left). To confirm the positive band to be p75NTR, anti-GT1b antibody was stripped from the membrane, and the membrane was reprobed with the anti-p75NTR antibody. The results showed the positive band to be p75NTR (Fig. 5 B, right). It is not a nonspecific interaction of GT1b, since association of extracellular domain of EGF receptor and GT1b was not observed (unpublished data). Thus, GT1b binds to p75NTR in a manner that is SDS resistant. Though GD1a was also shown to associate with MAG (Vinson et al., 2001), we did not see any interaction of p75NTR with GD1a (Fig. 5 C). Also, no interaction of p75NTR with GM1 was found (Fig. 5 C), demonstrating specific interaction of p75NTR with GT1b. Using an anti-GT1b antibody, we examined interaction of endogenous p75NTR and GT1b using lysates prepared from postnatal cerebellum from mice. Immunocytochemistry using the antibody confirmed the expression of GT1b on the surface of these neurons. In the GT1b immunoprecipitates, the anti- p75NTR antibody revealed the presence of a protein corresponding to p75NTR (Fig. 5 D). Preincubation of anti-GT1b antibody with synthetic GT1b abolished the detection of p75NTR (unpublished data). Finally, we assessed interaction of p75NTR with GT1b using transfected 293 cells, which express abundant GT1b on the cell surface (unpublished data). As expected, immunoprecipitated p75NTR was complexed with GT1b in a SDS-resistant manner (Fig. 5 E). These data suggest that GT1b and p75NTR form a receptor complex for MAG.
Dual signals elicited by p75NTR
p75NTR has been shown to bind more than just neurotrophins, such as CRNF (Fainzilber et al., 1996) or rabies virus glycoprotein (Tuffereau et al., 1998), but it is not known if these ligands trigger any signals through p75NTR. Thus, our findings demonstrating that p75NTR is a signal transducer not only for neurotrophins but also for MAG is intriguing. More interestingly, neurotrophins binding to p75NTR promote axonal outgrowth of neurons presumably by inhibiting RhoA activity (Yamashita et al., 1999), but MAG elicits the opposite effect via p75NTR on neurons by activating RhoA. This implies that p75NTR has dual signals as a transducing element. It is also important to note that essentially all adult neurons are sensitive to inhibition by MAG, whereas p75NTR has a restricted distribution. The identification and characterization of MAG signals have shed light on a previously unrecognized mechanism by which neurons respond to extracellular inhibitory molecules.
Materials and methods
Animals
The strain of mice bearing a targeted disruption of the third exon of the p75NTR gene (Lee et al., 1992) that was originally obtained from Jackson ImmunoResearch Laboratories on a C57BL/6J background was used.
Neurite outgrowth assay
DRGs were removed from adult mice and dissociated into single cells by incubation with 0.025% trypsin and 0.15% collagenase type I (Sigma-Aldrich) for 30 min at 37°C. For cerebellar neurons, the cerebella from two animals were combined in 5 ml of 0.025% trypsin, triturated, and incubated for 10 min at 37°C. DME containing 10% FCS was added, and the cells were centrifuged at 800 rpm. Neurons were plated in Sato medium (Cai et al., 1999) on poly-l-lysine–coated chamber slides. For outgrowth assays, plated cells were incubated for 24 h, fixed in 4% (wt/vol) paraformaldehyde, and immunostained with a monoclonal antibody (TuJ1) recognizing the neuron-specific β–tubulin III protein. Then, the length of the longest neurite or the total process outgrowth for each β–tubulin III–positive neuron was determined. Where indicated, recombinant rat MAG–Fc chimera (R&D Systems) was added to the medium after plating. The recombinant C3 transferase was introduced into the cytoplasm of the neurons before plating by trituration as described previously (Borasio et al., 1989).
Affinity precipitation of GTP–RhoA
293 cells were transfected with pcDNA3 vectors containing wild-type NH2-terminally HA-tagged RhoA (Yamashita et al., 1999) and/or full-length human p75NTR by lipofection using Lipofectamine 2000 (GIBCO BRL). Cerebellar neurons from P9 mice were isolated as described previously (Cai et al., 1999). Cells were lysed in 50 mM Tris, pH 7.5, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, and 10 mM MgCl2 with leupeptin and aprotinin each at 10 μg/ml. Cell lysates were clarified by centrifugation at 13,000 g at 4°C for 10 min, and the supernatants were incubated with the 20 μg of GST-Rho binding domain of Rhotekin beads (Upstate Biotechnology) at 4°C for 45 min. The beads were washed four times with washing buffer (50 mM Tris, pH 7.5, containing 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2,10 μg/ml each of leupeptin and aprotinin). Bound Rho proteins were detected by Western blotting using a monoclonal antibody against RhoA (Santa Cruz Biotechnology).
MAG–Fc binding and immunocytochemistry
DRG neuron cultures were fixed in 1% paraformaldehyde in PBS for 30 min. They were then blocked in PBS containing 2% FCS. To localize the MAG binding molecule, MAG–Fc (5 μg/ml) and anti–human IgG (1μg/ml) were precomplexed for 30 min at room temperature before being added to the fixed and blocked DRG neurons (Turnley and Bartlett, 1999). To identify p75NTR, cells were permeabilized with 0.2% Triton X-100/PBS and then incubated overnight with polyclonal antibody to p75NTR (Promega) followed by an Alexa fluor™ 568–labeled anti–rabbit IgG (Molecular Probes) for 1 h. The specificity of the antibodies was assessed by Western blot analysis of cells expressing the proteins, and control experiments of immunocytochemistry were performed by leaving out the primary antibodies.
Coprecipitation of recombinant p75NTR and GT1b
Recombinant human p75–Fc chimera (1 μg; Genzyme-Techne) and 1 μg of purified ganglioside GT1b (>98% purity; Seikagaku Co.) were incubated in 200 μl 0.025% Tween 20/PBS for 2 h, and p75NTR was precipitated using protein A sepharose (Amersham Pharmacia Biotech). The resultant precipitates were electrophoretically transferred to polyvinylidene difluoride membranes after SDS-PAGE with 7% gels and were immunoblotted with anti-GT1b antibody (IgM; Seikagaku Co.) or anti-p75NTR antibody.
Coimmunoprecipitation experiments
Cells were lysed on ice for 20 min with lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 25 μg/ml leupeptin, and 25 μg/ml aprotinin). The lysates were centrifuged at 13,000 g for 20 min, and the supernatants were collected. They were then incubated with the anti-GT1b antibody or an anti-HA antibody (for transfected HA-p75NTR) overnight followed by incubation with the anti–mouse IgM antibody (for GT1b). Immunocomplex or MAG–Fc was collected with protein A sepharose (Amersham Pharmacia Biotech). The suspension was centrifuged at 1,000 g for 5 min. The pellets were washed four times with lysis buffer and subjected to SDS-PAGE followed by immunoblot analysis.
Acknowledgments
This work was supported by a Grant-in-Aid from Senri Life Science Foundation.
Footnotes
Abbreviations used in this paper: DRG, dorsal root ganglion; MAG, myelin-associated glycoprotein; p75NTR, the neurotrophin receptor p75.
References
- Borasio, G.D., J. John, A. Wittinghofer, Y.A. Barde, M. Sendtner, and R. Heumann. 1989. ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurons. Neuron. 2:1087–1096. [DOI] [PubMed] [Google Scholar]
- Buck, C.R., H.J. Martinez, I.B. Black, and M.V. Chao. 1987. Developmentally regulated expression of the nerve growth factor receptor gene in the periphery and brain. Proc. Natl. Acad. Sci. USA. 84:3060–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, D., Y. Shen, M. De Bellard, S. Tang, and M.T. Filbin. 1999. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 22:89–101. [DOI] [PubMed] [Google Scholar]
- Davies, A.M. 2000. Neurotrophins: neurotrophic modulation of neurite growth. Curr. Biol. 10:R198–R200. [DOI] [PubMed] [Google Scholar]
- Ernfors, P., F. Hallbook, T. Ebendal, E.M. Shooter, M.J. Radeke, T.P. Misko, and H. Persson. 1988. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1:983–996. [DOI] [PubMed] [Google Scholar]
- Ernfors, P., A. Henschen, L. Olson, and H. Persson. 1989. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 2:1605–1613. [DOI] [PubMed] [Google Scholar]
- Fainzilber, M., A.B. Smit, N.I. Syed, W.C. Wildering, P..M. Hermann, R.C. van der Schors, C. Jimenez, K.W. Li, J. van Minnen, A.G. Bulloch, C.F. Ibanez, and W.P. Geraerts. 1996. CRNF, a molluscan neurotrophic factor that interacts with the p75 neurotrophin receptor. Science. 274:1540–1543. [DOI] [PubMed] [Google Scholar]
- Kimpinski, K., K.S. Jelinski, and K. Mearow. 1999. The anti-p75 antibody, MC192, and brain-derived neurotrophic factor inhibit nerve growth factor-dependent neurite growth from adult sensory neurons. Neuroscience. 93:253–263. [DOI] [PubMed] [Google Scholar]
- Kohn, J., R.S. Aloyz, J.G. Toma, M. Haak-Frendscho, and F.D. Miller. 1999. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J. Neurosci. 19:5393–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large, T.H., G. Weskamp, J.C. Helder, M.J. Radeke, T.P. Misko, E.M. Shooter, and L.F. Reichardt. 1989. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 2:1123–1134. [DOI] [PubMed] [Google Scholar]
- Lee, K.F., E. Li, L.J. Huber, S.C. Landis, A.H. Sharpe, M.V. Chao, and R. Jaenisch. 1992. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 69:737–749. [DOI] [PubMed] [Google Scholar]
- Lehmann, M., A. Fournier, I. Selles-Navarro, P. Dergham, A. Sebok, N. Leclerc, G. Tigyi, and L. McKerracher. 1999. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 19:7537–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher, L., S. David, D.L. Jackson, V. Kottis, R.J. Dunn, and P.E. Braun. 1994. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 13:805–811. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, G., P. Doherty, F.S. Walsh, P.R. Crocker, and M.T. Filbin. 1994. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 13:757–767. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., W.B. Kiosses, and M.A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskelton. EMBO J. 18:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, P.P., M.A. Colicos, P.A. Barker, and T.E. Kennedy. 1999. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J. Neurosci. 19:6887–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, K.A., J. Sun, Y. Liu, H. Kawai, T.O. Crawford, R.L. Proia, J.W. Griffin, and R.L. Schnaar. 1999. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc. Natl. Acad. Sci. USA. 96:7532–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S., Y.J. Shen, M.E. DeBellard, G. Mukhopadhyay, J.L. Salzer, P.R. Crocker, and M.T. Filbin. 1997. a. Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J. Cell Biol. 138:1355–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S., R.W. Woodhall, Y.J. Shen, M.E. deBellard, J.L. Saffell, P. Doherty, F.S. Walsh, and M.T. Filbin. 1997. b. Soluble myelin-associated glycoprotein found in vivo inhibits axonal regeneration. Mol. Cell. Neurosci. 9:333–346. [DOI] [PubMed] [Google Scholar]
- Tuffereau, C., J. Benejean, D. Blondel, B. Kieffer, and A. Flamand. 1998. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO J. 17:7250–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley, A.M., and P.F. Bartlett. 1999. Nerve growth factor modulates myelin-associated glycoprotein binding to sensory neurons. Int. J. Dev. Neurosci. 17:109–119. [DOI] [PubMed] [Google Scholar]
- Vinson, M., P.J. Strijbos, A. Rowles, L. Facci, S.E. Moore, D.L. Simmons, and F.S. Walsh. 2001. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J. Biol. Chem. 276:20280–20285. [DOI] [PubMed] [Google Scholar]
- von Bartheld, C.S., J.G. Heuer, and M. Bothwell. 1991. Expression of nerve growth factor (NGF) receptors in the brain and retina of chick embryos: comparison with cholinergic development. J. Comp. Neurol. 310:103–129. [DOI] [PubMed] [Google Scholar]
- Walsh, G.S., K.M. Krol, K.A. Crutcher, and M.D. Kawaja. 1999. Enhanced neurotrophin-induced axon growth in myelinated portions of the CNS in mice lacking the p75 neurotrophin receptor. J. Neurosci. 19:4155–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, T., K.L. Tucker, and Y.A. Barde. 1999. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 24:585–593. [DOI] [PubMed] [Google Scholar]
- Yan, Q., and E.M. Johnson, Jr. 1988. An immunohistochemical study of the nerve growth factor receptor in developing rats. J. Neurosci. 8:3481–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L.J., C.B. Zeller, N.L. Shaper, M. Kiso, A. Hasegawa, R.E. Shapiro, and R.L. Schnaar. 1996. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Natl. Acad. Sci. USA. 93:814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, T.T., J. Chua-Couzens, L.L. Butcher, D.E. Bredesen, J.D. Cooper, J.S. Valletta, W.C. Mobley, and F.M. Longo. 1997. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J. Neurosci. 17:7594–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]