Abstract
An NADH-linked disulfide reductase specific for disulfides containing pantethine 4',4"-diphosphate moieties was purified 23,000-fold to homogeneity from spores of Bacillus megaterium. The enzyme had a native molecular weight of 122,000 with two apparently identical subunits, contained one molecule of flavin adenine dinucleotide per subunit, and was inhibited by the vicinal dithiol reagent arsenite. The enzyme was active only on disulfides containing pantethine 4',4"-diphosphate moieties, including pantethine 4',4"-diphosphate, oxidized coenzyme A, and coenzyme A in disulfide linkage to acyl carrier protein. However, the Km values for pantethine 4',4"-diphosphate and oxidized coenzyme A were 0.65 and 7.4 mM, respectively. The enzyme was at a low level in log-phase cells but increased up to 10-fold early in the stationary phase and had a similar specific activity in both the mother cell and the forespore compartment; the enzyme activity fell only slowly during spore germination and outgrowth. The enzyme was not detected in several eucaryotic sources and was present in at most a low level in a number of gram-negative bacteria. Surprisingly, the specific activity of this enzyme varied more than 200-fold in extracts from different Bacillus species, with values in B. subtilis being 5- to 6-fold lower and values in B. cereus and B. sphaericus being 8- and 35-fold higher, respectively, than the maximum value in B. megaterium. However, the high specific activity in B. sphaericus did not represent more enzyme protein than in B. megaterium. The possible function of this newly discovered enzyme is discussed.
Full text
PDF


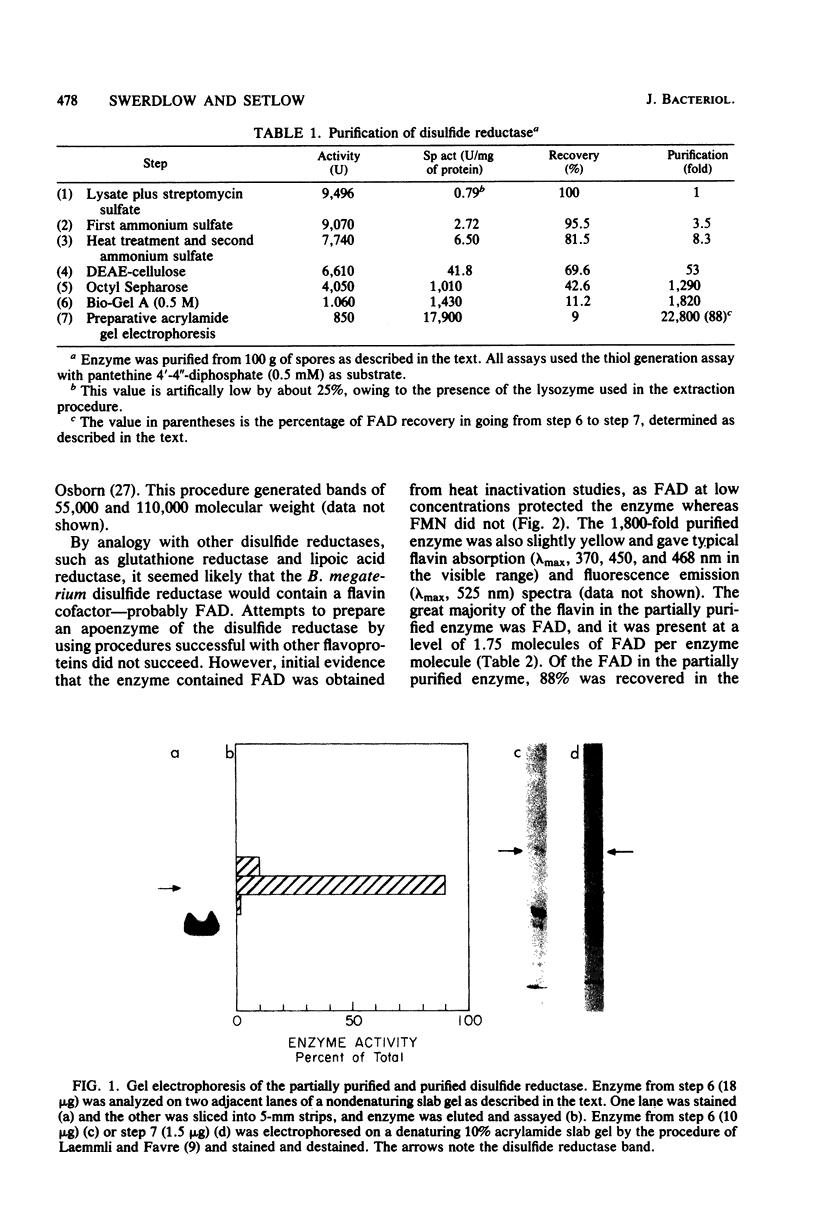
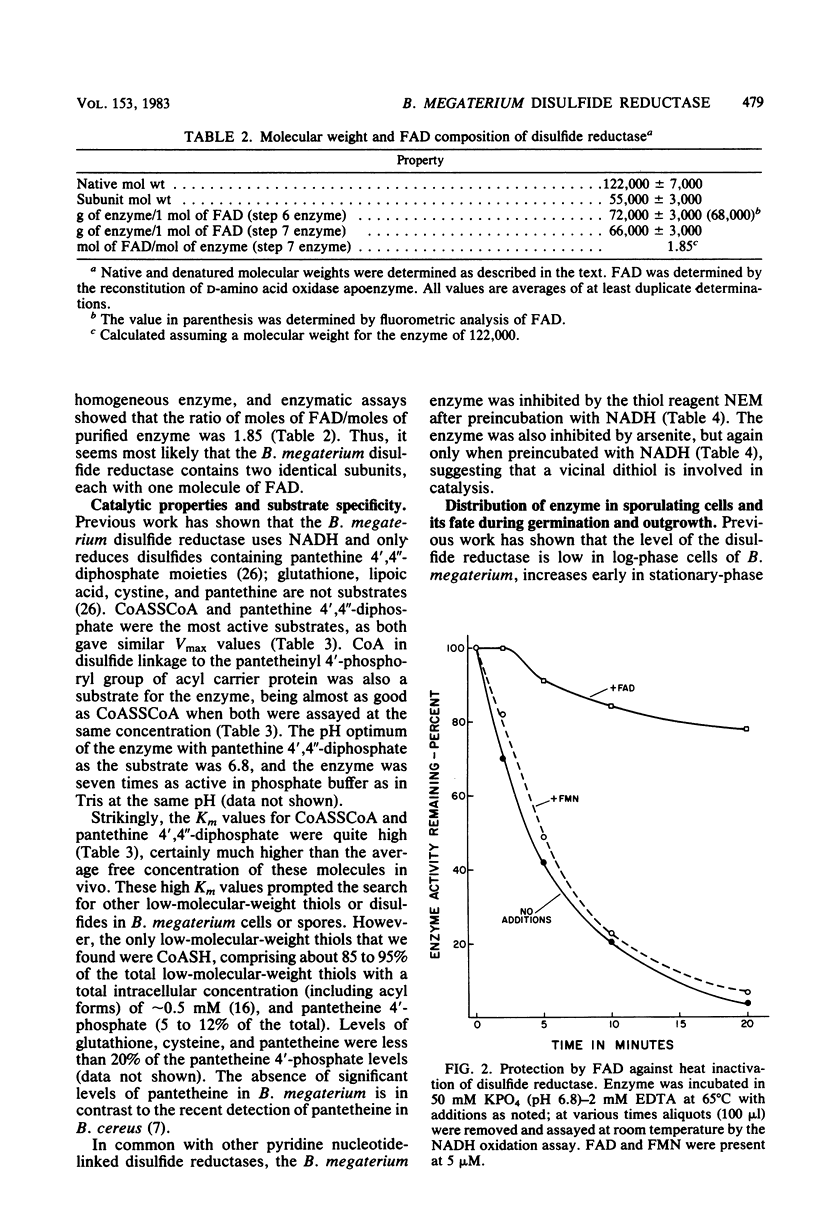
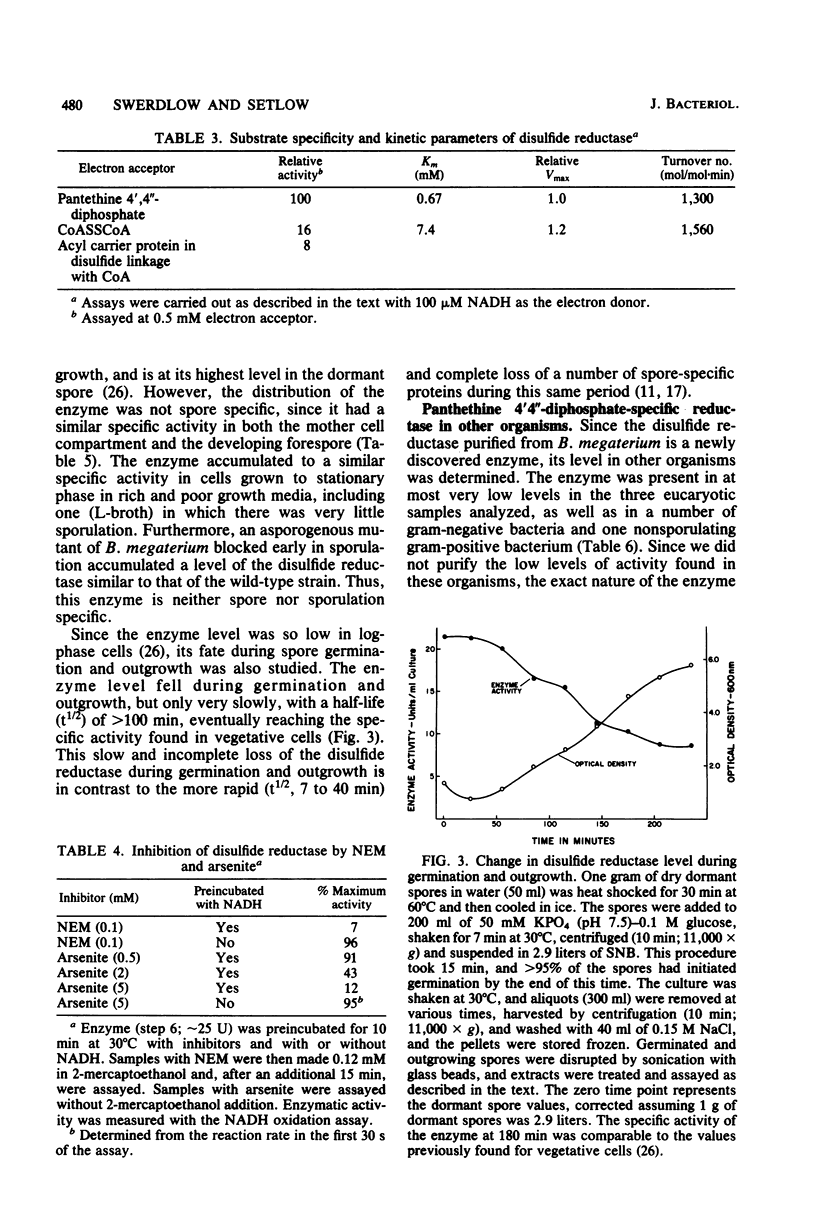
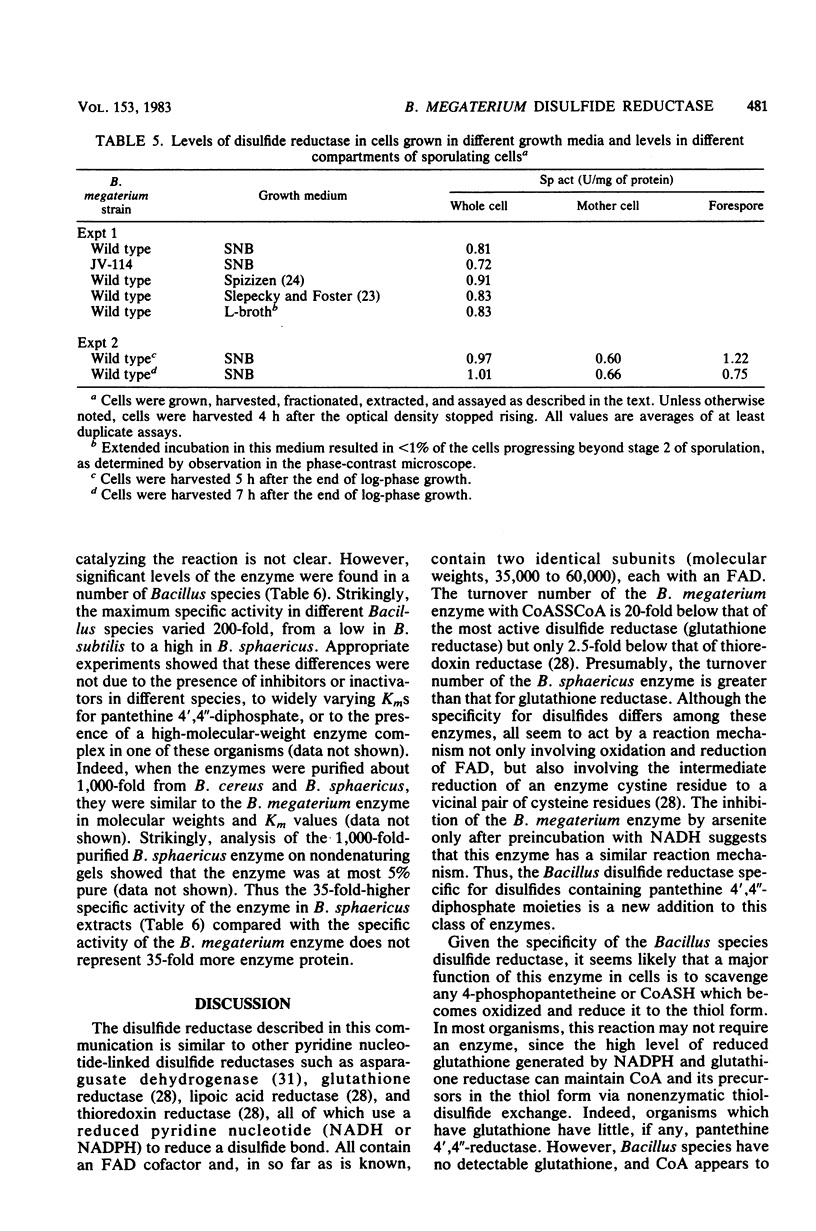


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonsen P. P., Spudich J. A., Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XII. A sulfonic acid as a major sulfur compound of Bacillus subtilis spores. J Bacteriol. 1969 Apr;98(1):62–68. doi: 10.1128/jb.98.1.62-68.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography by thiol-disulfide interchange. Methods Enzymol. 1974;34:531–544. doi: 10.1016/s0076-6879(74)34069-4. [DOI] [PubMed] [Google Scholar]
- DELUCA C., KAPLAN N. O., WEBER M. M. A specific spectrophotometric assay for flavin adenine dinucleotide. J Biol Chem. 1956 Nov;223(1):559–567. [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brown W. C., Adams W. B., Worsham M. B. Occurrence of glutathione in bacteria. J Bacteriol. 1978 Mar;133(3):1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Newton G. L., Dorian R., Kosower E. M. Analysis of biological thiols: derivatization with monobromotrimethylammoniobimane and characterization by electrophoresis and chromatography. Anal Biochem. 1980 Sep 1;107(1):1–10. doi: 10.1016/0003-2697(80)90483-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Loshon C. A., Setlow P. Bacillus megaterium spore protease: purification, radioimmunoassay, and analysis of antigen level and localization during growth, sporulation, and spore germination. J Bacteriol. 1982 Apr;150(1):303–311. doi: 10.1128/jb.150.1.303-311.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Massey V., Curti B. A new method of preparation of D-amino acid oxidase apoprotein and a conformational change after its combination with flavin adenine dinucleotide. J Biol Chem. 1966 Jul 25;241(14):3417–3423. [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of acetyl coenzyme A, reduced and oxidized coenzyme A, and coenzyme A in disulfide linkage to protein in dormant and germinated spores and growing and sporulating cells of Bacillus megaterium. J Bacteriol. 1977 Nov;132(2):444–452. doi: 10.1128/jb.132.2.444-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Waites W. M. Identification of several unique, low-molecular-weight basic proteins in dormant spores of clastridium bifermentans and their degradation during spore germination. J Bacteriol. 1976 Aug;127(2):1015–1017. doi: 10.1128/jb.127.2.1015-1017.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. D., Green C. L., Setlow B., Setlow P. Identification of an NADH-linked disulfide reductase from Bacillus megaterium specific for disulfides containing pantethine 4',4''-diphosphate moieties. J Biol Chem. 1979 Aug 10;254(15):6835–6837. [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yanagawa H., Egami F. Asparagusate dehydrogenases and lipoyl dehydrogenase from asparagus mitochondria. Physical, chemical, and enzymatic properties. J Biol Chem. 1976 Jun 25;251(12):3637–3644. [PubMed] [Google Scholar]
- Zahler W. L., Cleland W. W. A specific and sensitive assay for disulfides. J Biol Chem. 1968 Feb 25;243(4):716–719. [PubMed] [Google Scholar]



