Abstract
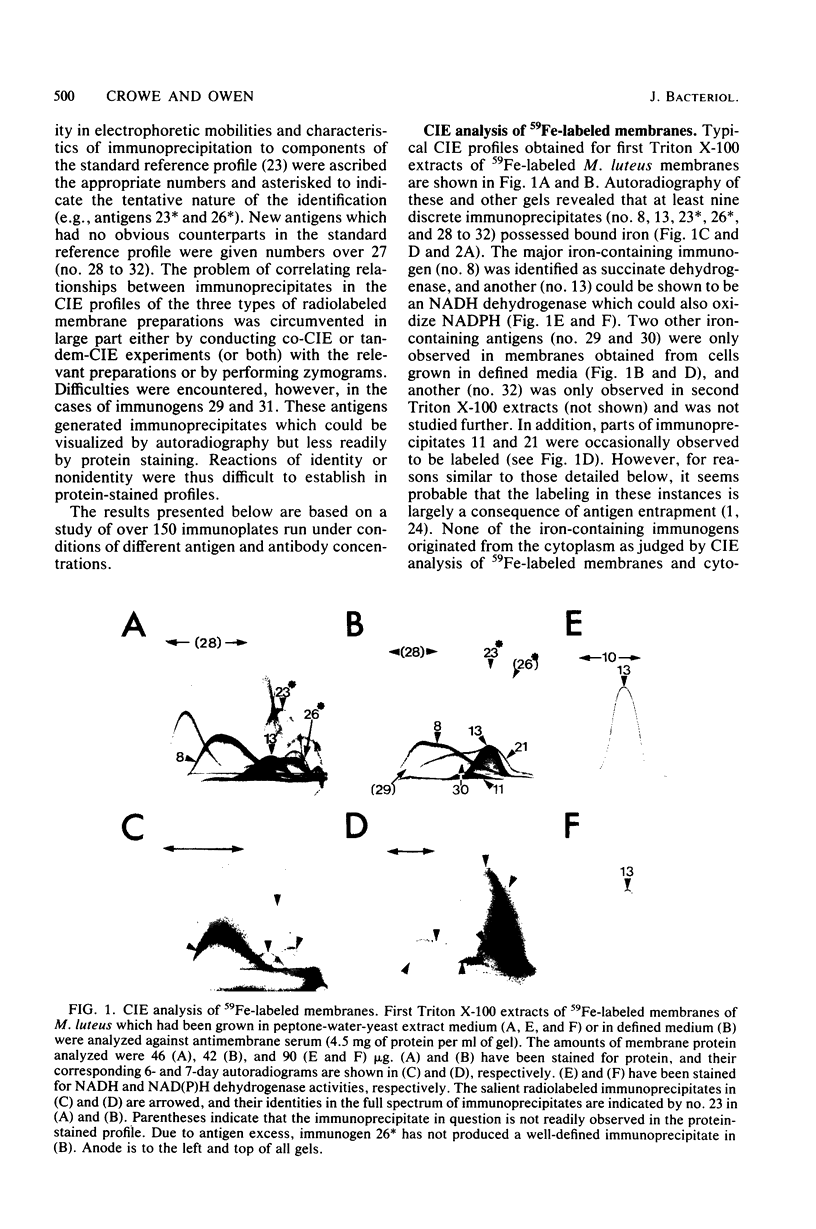
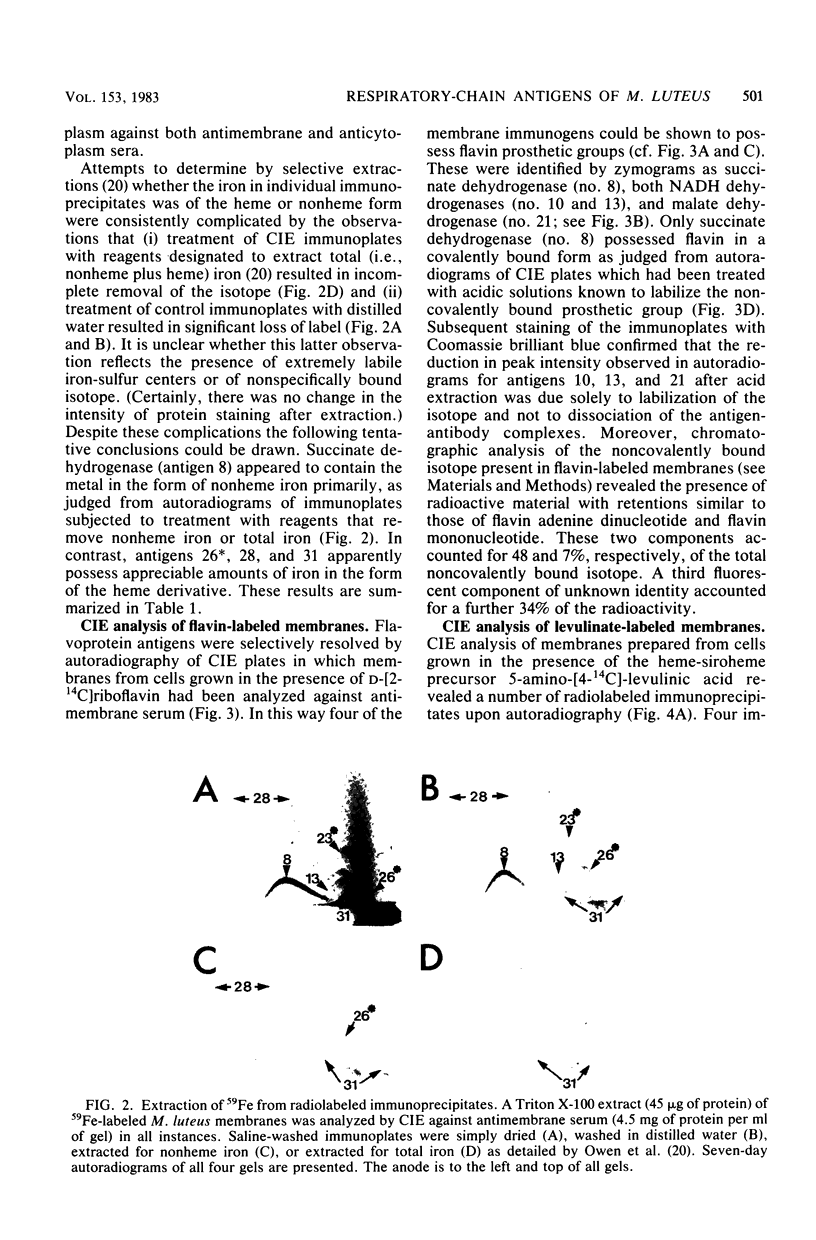
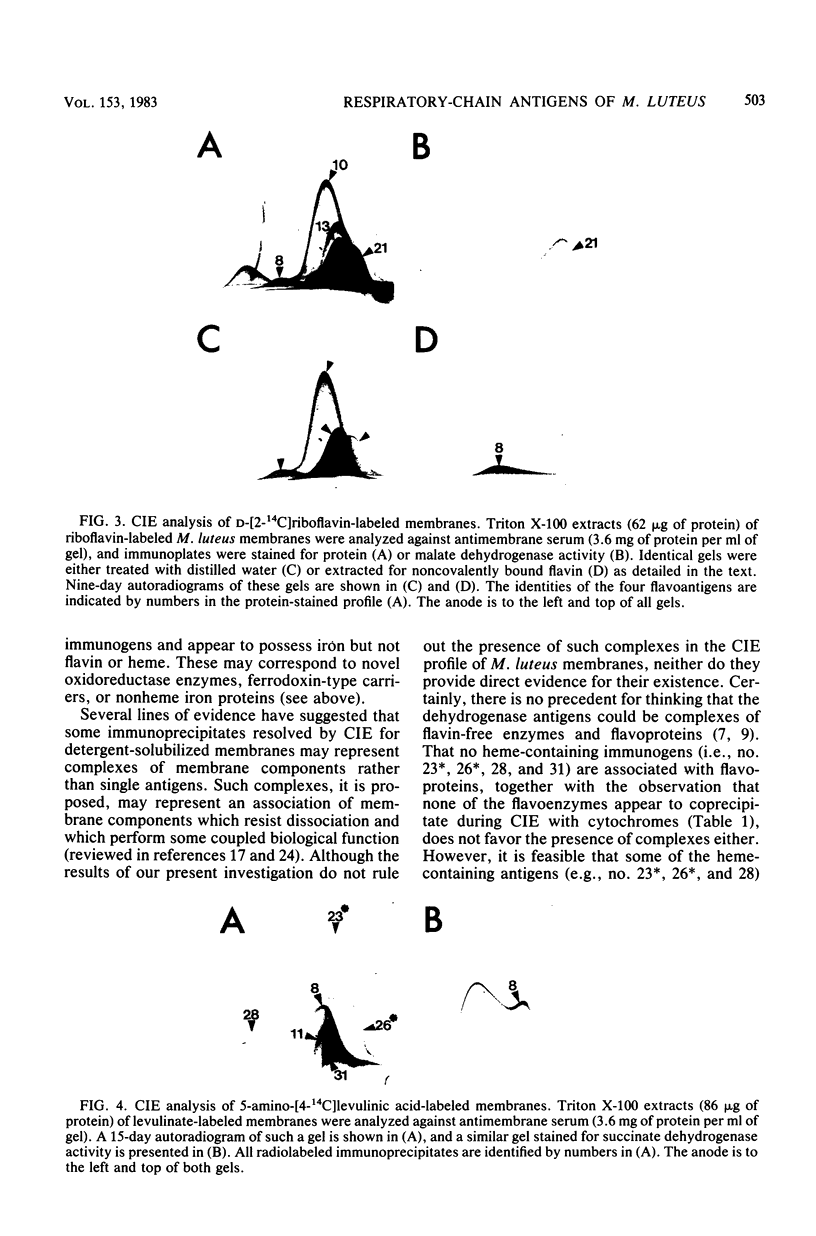
Membrane-bound antigens of the respiratory chain of Micrococcus luteus were analyzed by crossed immunoelectrophoresis after growth of the organism in the presence of 59Fe, the flavin adenine dinucleotide-flavin mononucleotide precursor D-[2-14C]riboflavin, or the heme precursor 5-amino-[4-(14)C]levulinic acid. Using zymograms and procedures of selective extraction in conjunction with autoradiography, it was possible to resolve and partially characterize a number of antigens. Succinate dehydrogenase (EC 1.3.99.1) was shown to possess covalently bound flavin and nonheme iron and was possibly present as a complex with cytochrome. Three other dehydrogenases, namely, NADH dehydrogenase, NAD(P)H dehydrogenase (EC 1.6.99.3), and malate dehydrogenase (EC 1.1.1.37), contained flavin in noncovalent linkage, the NAD(P)H dehydrogenase also possessing nonheme iron. Four other discrete antigens (or antigen complexes) containing both iron and heme centers also resolved, as were two minor immunogens possessing iron as the sole detectable prosthetic group.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J., Revis G. J., Jarrett K. Preparatory electroimmunodiffusion for making precipitins to selected native antigens. Immunol Commun. 1972;1(4):325–336. doi: 10.3109/08820137209022946. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Gel'man N. S., Tikhonova G. V., Simakova I. M., Lukoyanova M. A., Taptykova S. D., Mikelsaar H. M. Fragmentation by detergents of the respiratory chain of Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):321–331. doi: 10.1016/0005-2728(70)90188-x. [DOI] [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder R., Salton M. R. Affinity chromatography of succinate dehydrogenase from the membranes of Micrococcus lysodeikticus. Prep Biochem. 1975;5(4):349–357. doi: 10.1080/00327487508061582. [DOI] [PubMed] [Google Scholar]
- Lukoianova M. A., Petukhova N. M. Vydelenie tsitokhroma b556 iz membran bakterii Micrococcus lysodeiktus. Biokhimiia. 1976 Sep;41(9):1628–1635. [PubMed] [Google Scholar]
- Lukoianova M. A., Taptykova S. D. Tsitokhromy Micrococcus lysodeikticus. Biokhimiia. 1968 Jul-Aug;33(4):888–894. [PubMed] [Google Scholar]
- Norrild B., Bjerrum O. J., Vestergaard B. F. Polypeptide analysis of individual immunoprecipitates from crossed immunoelectrophoresis. Anal Biochem. 1977 Aug;81(2):432–441. doi: 10.1016/0003-2697(77)90714-x. [DOI] [PubMed] [Google Scholar]
- Owen P., Doherty H. Immunochemical analysis of triton X-100-insoluble residues from Micrococcus lysodeikticus membranes. J Bacteriol. 1979 Dec;140(3):881–887. doi: 10.1128/jb.140.3.881-887.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Kaczorowski G. J., Kaback H. R. Resolution and identification of iron-containing antigens in membrane vesicles from Escherichia coli. Biochemistry. 1980 Feb 5;19(3):596–600. doi: 10.1021/bi00544a032. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Isolation and characterization of a mannan from mesosomal membrane vesicles of Micrococcus lysodeikticus. Biochim Biophys Acta. 1975 Oct 6;406(2):214–234. doi: 10.1016/0005-2736(75)90006-1. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Membrane asymmetry and expression of cell surface antigens of Micrococcus lysodeikticus established by crossed immunoelectrophoresis. J Bacteriol. 1977 Dec;132(3):974–978. doi: 10.1128/jb.132.3.974-985.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Tikhonova G. V., Iyelekht L. E., Ostrovskii D. N. Imeet li dykhatel'naia tsep' Micrococcus lysodeikticus vykhody na naruzhnuiu poverkhnost' tsitomembrany? Biokhimiia. 1978 Dec;43(12):2163–2174. [PubMed] [Google Scholar]
- Weiss H., Kolb H. J. Isolation of mitochondrial succinate: ubiquinone reductase, cytochrome c reductase and cytochrome c oxidase from Neurospora crassa using nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):139–149. doi: 10.1111/j.1432-1033.1979.tb13240.x. [DOI] [PubMed] [Google Scholar]
- Zhukova I. G., Shaposhnikov G. L., Ostrovskii D. N. Belki bakterial'nykh membran ochistka NADH-degidrogenazy iz M. lysodeikticus s pomoshch'iu elektrofokusirovki. Biokhimiia. 1976 Oct;41(10):1840–1845. [PubMed] [Google Scholar]