Abstract
SEC2 is an essential gene required for polarized growth of the yeast Saccharomyces cerevisiae. It encodes a protein of 759 amino acids that functions as a guanine nucleotide exchange factor for the small GTPase Sec4p, a regulator of Golgi to plasma membrane transport. Activation of Sec4p by Sec2p is needed for polarized transport of vesicles to exocytic sites. Temperature-sensitive (ts) mutations in sec2 and sec4 result in a tight block in secretion and the accumulation of secretory vesicles randomly distributed in the cell. The proper localization of Sec2p to secretory vesicles is essential for its function and is largely independent of Sec4p. Although the ts mutation sec2-78 does not affect nucleotide exchange activity, the protein is mislocalized. Here we present evidence that Ypt31/32p, members of Rab family of GTPases, regulate Sec2p function. First, YPT31/YPT32 suppress the sec2-78 mutation. Second, overexpression of Ypt31/32p restores localization of Sec2-78p. Third, Ypt32p and Sec2p interact biochemically, but Sec2p has no exchange activity on Ypt32p. We propose that Ypt32p and Sec4p act as part of a signaling cascade in which Ypt32p recruits Sec2p to secretory vesicles; once on the vesicle, Sec2p activates Sec4p, enabling the polarized transport of vesicles to the plasma membrane.
Keywords: membrane traffic; Ypt31/32; exchange factor; Rab; yeast
Introduction
Eukaryotic cells use vesicular transport to effect processes as diverse as synaptic transmission and the polarized growth of the yeast Saccharomyces cerevisiae. The secretory pathway consists of a series of stages in which proteins in one organelle are selectively packaged into vesicles that are then transported through the cytoplasm to fuse with the next organelle along the pathway. This process requires a complex cascade of cellular events that together ensure the fidelity of each stage of transport. Rab proteins, members of the Ras superfamily of small GTPases, stand out as some of the key regulators of membrane traffic. There are 11 yeast Rab proteins, mostly termed Ypt proteins, and >60 mammalian Rab proteins. Like all small GTPases, Rab proteins cycle between active (GTP) and inactive (GDP) conformations. This cycle is regulated by guanine nucleotide exchange factors (GEFs),* which exchange GDP for GTP, and GTPase activating proteins (GAPs), which stimulate the rate of GTP hydrolysis. Proteins that bind to Rab-GTP are considered effectors and mediate the different steps of membrane traffic (Pfeffer, 2001).
Transport of vesicles from the Golgi apparatus to the plasma membrane has been studied in many cell types. In yeast, Golgi apparatus–derived vesicles are transported to sites of polarized growth, including tips of small buds and mother–daughter necks. This process relies on the actin cytoskeleton and Myo2p, a class V myosin (Novick and Botstein, 1985; Govindan et al., 1995; Pruyne et al., 1998; Schott et al., 1999; Karpova et al., 2000). Cells treated with the actin polymerization inhibitor latrunculin A or harboring mutations that affect either actin function (act1-1) or Myo2p function (myo2-66) accumulate secretory vesicles randomly throughout the cell. The polarized delivery of post-Golgi vesicles also depends on the functions of Sec4p and Sec2p. Sec4p is the Rab protein that regulates this stage of the secretory pathway and Sec2p is the GEF that activates Sec4p (Salminen and Novick, 1987; Walch-Solimena et al., 1997). Sec2p and Sec4p are found in association with secretory vesicles and therefore localize to sites of polarized secretion. Temperature-sensitive (ts) mutants defective in most of the genes required for Golgi to plasma membrane transport accumulate a cluster of secretory vesicles adjacent to normal exocytic sites at the restrictive temperature, indicating that vesicle delivery was not blocked. However, ts mutations in sec2 cause an accumulation of vesicles randomly distributed throughout the cell, similar to that seen in act1-1, myo2-66, and latrunculin A–treated cells. This suggests that activation of Sec4p by Sec2p is necessary for the vectorial transport or retention of vesicles at sites of secretion. There are several examples in which Rab proteins interact with components of the actin or microtubule cytoskeleton to regulate membrane traffic (Echard et al., 1998; Hume et al., 2001; Lapierre et al., 2001). However, the only known effector for Sec4p is Sec15p, a component of the exocyst complex, which is necessary for tethering secretory vesicles to exocytic sites (Guo et al., 1999). This implies that there may yet be an unidentified effector of Sec4p that mediates the polarized transport of vesicles.
The mechanism by which Sec2p binds to secretory vesicles is not understood. Sec2p does not contain transmembrane domains or lipid modifications that would anchor it to the vesicle membrane and, moreover, the association of Sec2p with secretory vesicles is partially independent of its interaction with Sec4p (Elkind et al., 2000). Sec2p contains a stretch of 58 amino acids (aa 450–508) that is necessary but not sufficient for its association with secretory vesicles. Mutations or truncations of this domain give rise to ts alleles that disrupt the ability of Sec2p to bind secretory vesicles, but do not affect its exchange activity. Based on this evidence, we hypothesize that a Sec2p receptor must exist on secretory vesicles.
Here we report the results of a high copy suppressor screen for either effectors of Sec4p involved in polarized transport or membrane receptors for Sec2p. We screened for genes whose overexpression can suppress the growth defect of sec2-78 at 37°C. The sec2-78 allele has a point mutation changing amino acid 483 from a cysteine to a tyrosine within the 58–amino acid stretch that is critical for proper localization of the protein. Two of the suppressors identified in this screen, YPT31 and YPT32, encode functionally redundant Rab proteins implicated in intra-Golgi transport and budding of secretory vesicles from the Golgi apparatus (Benli et al., 1996; Jedd et al., 1997). We demonstrate that overexpression of Ypt32p restores the localization of two mutant Sec2 proteins to exocytic sites. We also show through biochemical studies that Ypt32p binds to Sec2p preferentially in its GTP-bound state. Based on these results, we propose that Ypt32p and Sec4p together through their interaction with Sec2p regulate delivery of post-Golgi vesicles to the plasma membrane.
Results
YPT31/32 suppress the sec2-78 ts mutation
To find factors involved in Sec2p function and in mediating its association with secretory vesicles, we performed a genetic screen for high copy number suppressors of the ts mutation sec2-78. Five genes with suppression activity were identified: SEC4, HYP2, PAK1, YPT31, and YPT32. SEC4 is a common suppressor of many late-acting sec mutants, and probably suppresses the sec2-78 allele by increasing the concentration of the active form, Sec4p-GTP. Pak1p is a Ser-Thr protein kinase that was isolated as a suppressor for a DNA polymerase α mutation (cdc17-1). Sec2p is phosphorylated at the COOH terminus, so it is possible that Pak1p plays a role in the regulation of Sec2p function and/or localization (Hovland et al., 1997; Elkind et al., 2000). Hyp2p is a translation initiation factor (Wohl et al., 1993). Overexpression of Hyp2p could cause upregulation of components of Golgi to plasma membrane transport. However, it does not upregulate expression of Sec2p or Sec4p (unpublished data). Here we will discuss two suppressors, YPT31/32; the others will be discussed elsewhere. YPT31/32 encode small GTPases of the Ypt/Rab family. They have been implicated in intra-Golgi transport, the budding of vesicles from the trans-Golgi apparatus, and the recycling of vesicles from the plasma membrane back to the Golgi complex (Benli et al., 1996; Jedd et al., 1997). They share 80% sequence identity and are functionally redundant.
To confirm that overexpression of Ypt31p and Ypt32p suppress the sec2-78 mutation, cells were transformed with a plasmid containing YPT31 and hemagglutinin (HA) epitope–tagged YPT32 under the control of the GAL1 promoter. Overproduction of Ypt31p and Ypt32p was confirmed by Western blotting with antibodies against Ypt32p (Fig. 1 A). We also transformed the cells with HA–YPT1 and HA–SEC4 under the control of the GAL1 promoter. Fig. 1 B shows a Western blot using an anti-HA antibody (12CA5). The constructs were expressed at levels similar to HA–YPT32. We then grew the cells on YP plates containing either glucose (YPD) or a mixture of galactose and raffinose (YPGR), at different temperatures (25°C, 34°C, and 37°C). As shown in Fig. 1 C, wild-type cells grow well at all temperatures, wheras sec2-78 cells were unable to grow at 37°C on YPD or YPGR. However, when Ypt31p, Ypt32p, or Sec4p were overexpressed, growth at the restrictive temperature (37°C) was restored. Ypt1p was unable to suppress the sec2-78 growth defect. We also tested whether overexpression of these proteins was able to suppress another sec2 allele, sec2-59. This mutant lacks most of the COOH terminus and is unable to grow at 34°C or 37°C. Overexpression of Ypt31p and Ypt32p was able to restore growth of sec2-59 at 34°C but not at 37°C (Fig. 1 C; unpublished data). However, under these conditions, Ypt1p and Sec4p were unable to suppress the growth defect of sec2-59 at either temperature.
Figure 1.
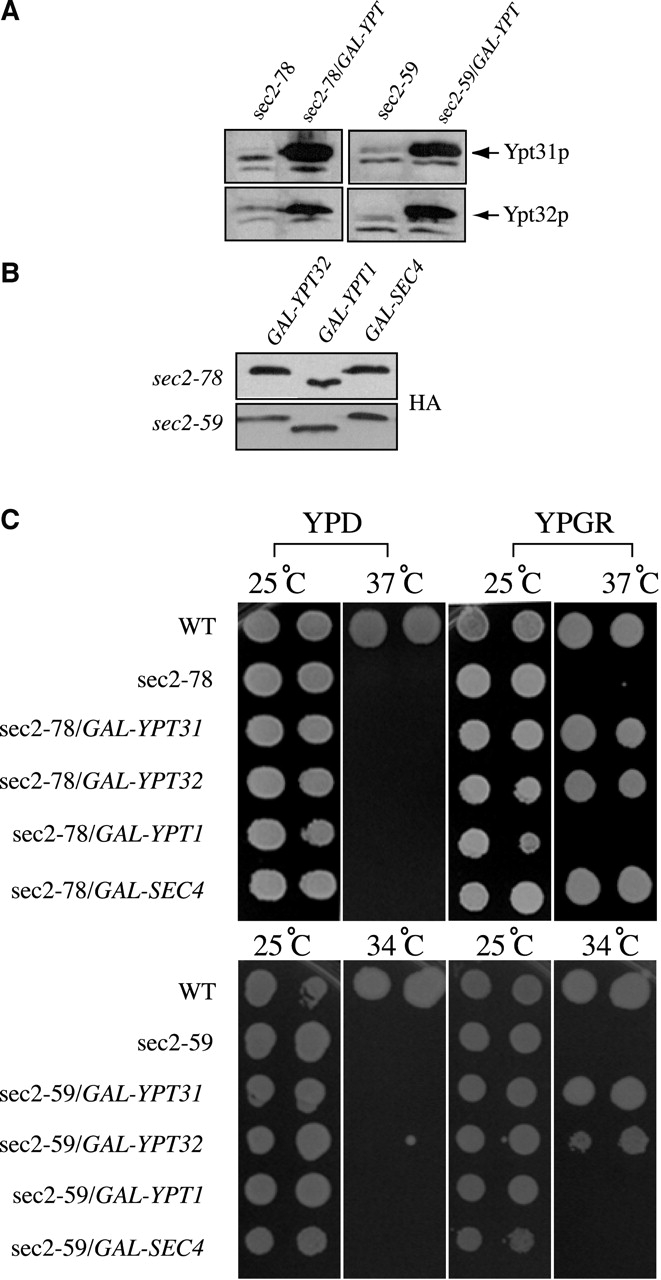
Overexpression of Ypt31p and Ypt32p suppresses the growth defect of sec2-78 and sec2-59. (A) Ypt31p and HA–Ypt32p are overexpressed in NY2426 (sec2-59, GAL-YPT32), NY2427 (sec2-78, GAL-YPT32), NY2444 (sec2–59, GAL-YPT31), and NY2445 (sec2-78, GAL-YPT31) when they are grown on YPGR compared with NY26 (sec2-59) and NY2179 (sec2-78). Western blots were probed with αYpt32p antibody. (B) HA–Ypt1p and HA–Sec4p are expressed in NY2440 (sec2-78, GAL-YPT1), NY2441 (sec2-78, GAL-SEC4), NY2442 (sec2-59, GAL-YPT1), and NY2443 (sec2-59, GAL-SEC4). All cells were grown on YPGR and Western blots were probed with αHA antibody (12CA5). (C) NY10 (WT), NY26 (sec2-59), NY2179 (sec2-78), NY2426 (sec2–59, GAL-YPT32), NY2427 (sec2-78, GAL-YPT32), NY2440 (sec2-78, GAL-YPT1), NY2441 (sec2-78, GAL-SEC4), NY2442 (sec2-59, GAL-YPT1), NY2443 (sec2-59, GAL-SEC4), NY2444 (sec2-59, GAL-YPT31), and NY2445 (sec2-78, GAL-YPT31) were spotted on either YPD or YPGR plates and incubated at 25°C, 34°C, and 37°C. Ypt31p, Ypt32p, and Sec4p suppress the growth defect of sec2-78 at 37°C, whereas Ypt31p and Ypt32p suppress sec2-59 at 34°C.
We examined secretory pathway function in wild-type and sec2-78 cells with and without overexpression of Ypt32p by monitoring invertase secretion. Synthesis of the secreted form of invertase was derepressed when cells were grown in low concentrations of glucose. In wild type, virtually all invertase was secreted after 1 h of derepression at 37°C. In contrast, sec2-78 cells accumulated significant amounts of invertase. Overexpression of Ypt32p in sec2-78 increased secretion of invertase by at least 10%. This partial restoration of secretory function appeared to be sufficient to restore growth at 37°C (unpublished data).
Overexpression of Ypt32p restores the localization of Sec2-78p–GFP
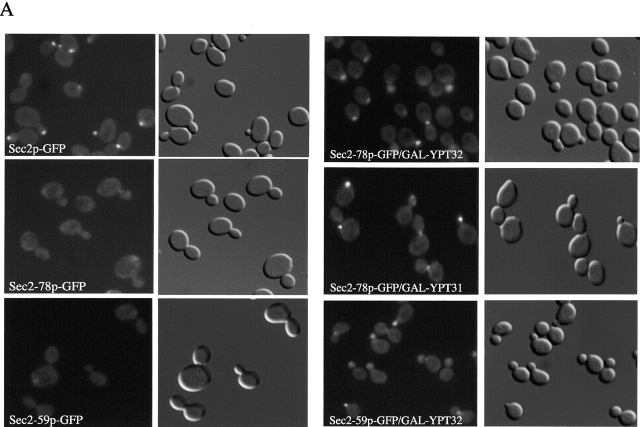
In wild-type yeast, Sec2p associates with secretory vesicles. As a result, Sec2p–green fluorescent protein (GFP) localizes to exocytic sites, bud tips, and mother–daughter necks. Mutations or truncations in the COOH terminus, such as Sec2-78p and Sec2-59p, cause the protein to dissociate from secretory vesicles, resulting in a diffuse distribution throughout the cytoplasm (Walch-Solimena et al., 1997; Elkind et al., 2000).
To study the effects of Ypt31p and Ypt32p overexepression on the localization of Sec2-78p–GFP, we used strains NY2429 (sec2-78–GFP, GAL-YPT32) and 2430 (sec2-78–GFP, GAL-YPT31). All strains in this experiment were grown at 25°C on YPgalactose (YPGal) to induce expression of the GAL1 constructs. This was confirmed by Western blotting using anti-Ypt31/32p antibodies (unpublished data). Wild-type Sec2p–GFP showed characteristic localization to bud tips and necks, whereas Sec2-78p– and Sec2–59p–GFP cells exhibited diffuse cytoplasmic staining as previously described (Elkind et al., 2000; Fig. 2 A). When Ypt31p and Ypt32p were overproduced, the localization of Sec2-78p–GFP was substantially restored to bud tips and necks. Localization of Sec2-59p–GFP was also restored to sites of secretion upon overproduction of Ypt32p (Fig. 2 A). When these experiments were performed at 37°C, some loss of polarized localization was observed even in wild-type cells and no restoration of Sec2-78p–GFP was seen by Ypt32p overexpression (unpublished data). Previous studies showed that only small amounts of full-length Sec2p are sufficient to suppress the growth defect of various sec2 alleles (Nair et al., 1990). It is possible then that at 37°C, only small amounts of protein are correctly localized to bud tips and necks and can't be detected by fluorescence. However, these small amounts of protein would be enough to support growth.
Figure 2.
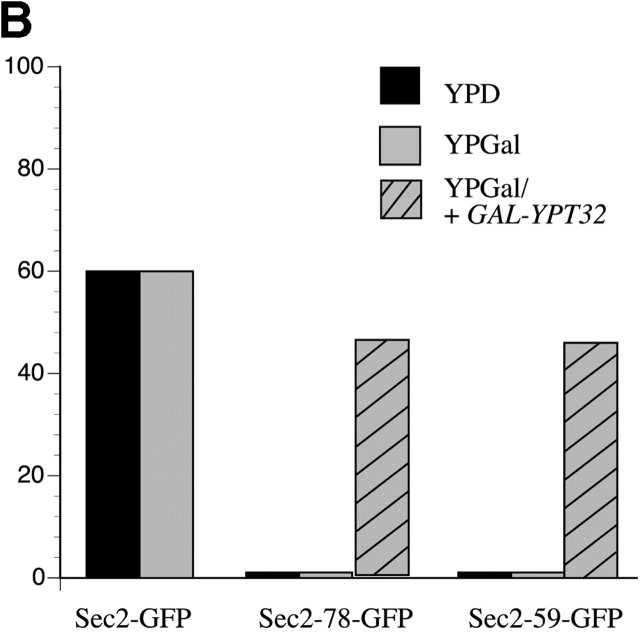
Overexpression of Ypt32p restores the localization of Sec2-78p–GFP and Sec2-59p–GFP. Yeast were grown from stationary cultures overnight at 25°C to log phase on YPGal. For GFP visualization, cells were harvested, fixed in methanol, permeabilized, washed with PBS, and directly observed. (A) Representative wild-type Sec2p–GFP (NY2146), Sec2-59p–GFP (NY2147), and Sec2-78p–GFP (NY2152) fields are shown. Sec2-78p–GFP/GAL-YPT32 (NY2429), Sec2-78p–GFP/GAL-YPT31 (NY2430), and Sec2-59p–GFP/ GAL-YPT32 (NY2428) show restored localization of Sec2 proteins when grown on YPGal. (B) Approximately 100 cells were counted for each experiment and the percentage of cells showing bud tip and mother–daughter neck localization of Sec2p–GFP, Sec2-78p–GFP, and Sec2-59p–GFP. 60% of wild-type cells show polarized localization of Sec2p–GFP. Sec2-78p and Sec2-59p–GFP show <1% of localized protein. When Ypt32p is overproduced (YPGal), restored localization of Sec2 proteins is observed in 46% of cells. (C) Overexpression of Sec4p from the GAL promoter does not affect the localization of Sec2-78p–GFP (NY2446). Western blot was probed with αSec4p antibody.
Quantification of these results showed that in wild-type yeast, grown in YPD or YPGal, 60% of the cells had a polarized concentration of Sec2–GFP (Fig. 2 B). The remaining wild-type cells were at stages of the cell cycle where a concentrated localization of Sec2 could not be detected. In the cases of Sec2-78–GFP and Sec2-59–GFP, <1% of the cells had correctly localized protein. However, when Ypt32p was overexpressed, 46% of sec2-78 and sec2-59 cells showed concentration at the bud tips and mother–daughter necks.
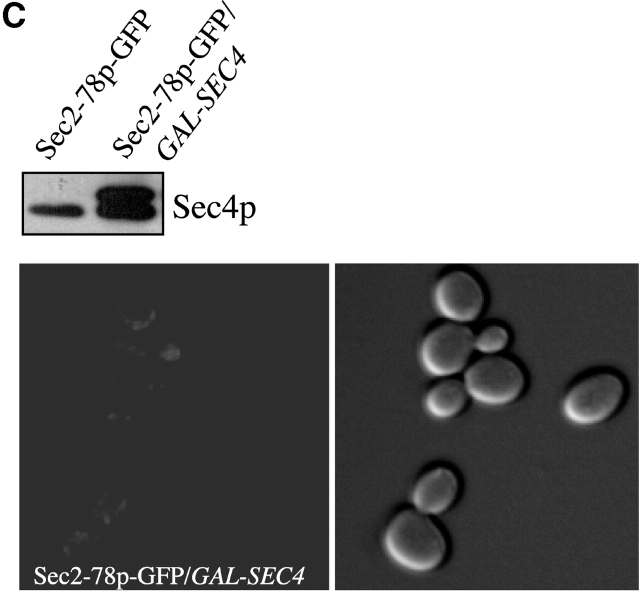
In contrast, overexpression of Sec4p from the GAL promoter did not cause any relocalization of Sec2-78p–GFP (Fig. 2 C), even though it was able to suppress the growth defect of sec2-78 at nonpermissive temperatures (Fig. 1 C). Overexpression of Ypt1p had no effect on the localization of Sec2-78p– and Sec2-59p–GFP, in agreement with its inability to suppress the growth defects of these mutants at the nonpermissive temperatures (unpublished data; Fig. 1 C).
Overexpression of Ypt32 restores the localization of Sec4p in sec2-78 mutant cells
In wild-type cells Sec4p, like Sec2p, localized to sites of polarized secretion. In sec2-78 cells, Sec4p was partially localized to bud tips and necks at the permissive temperature (25°C), but this localization was lost upon shifting to 37°C (Walch-Solimena et al., 1997; Fig. 3). Because overexpression of Ypt32p restores localization of Sec2-78p–GFP, we investigated whether it could also restore Sec4p localization in sec2-78 cells at 37°C. For these experiments we used a 2μ plasmid with the constitutive promoter GPD to overexpress Ypt32p. We were unable to use the GAL-YPT32 construct because our immunofluorescence protocol does not work well on cells that have been grown on galactose. Strains NY10 (WT), NY2179 (sec2-78), and NY2431 (sec2-78, 2μ GPD-YPT32) were used to observe Sec4p localization (Fig. 3 A).
Figure 3.
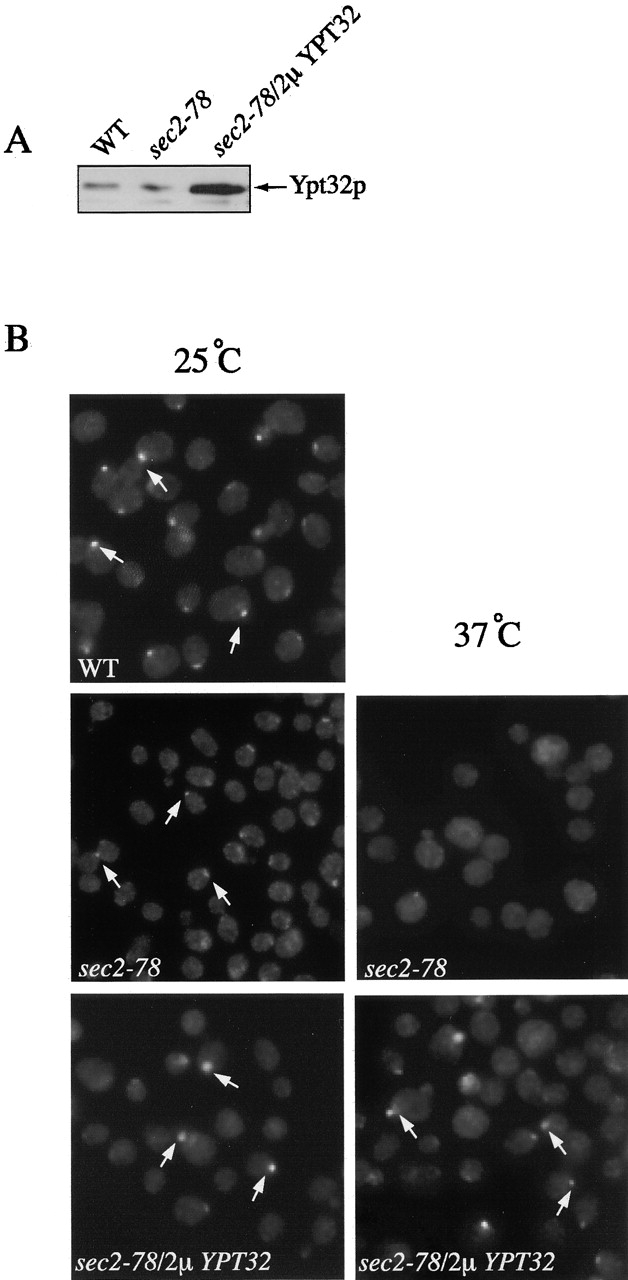
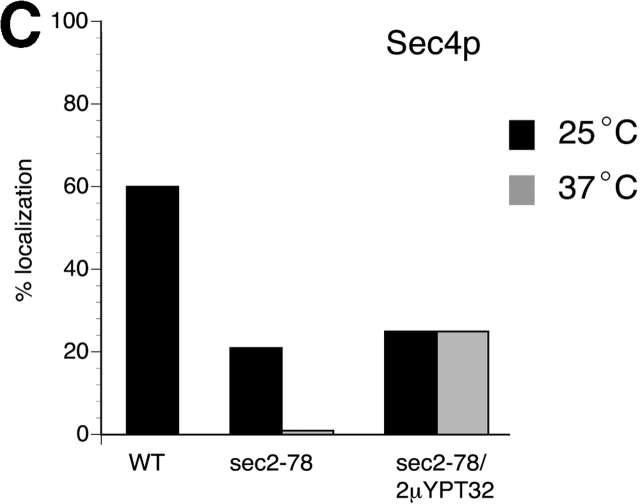
Ypt32p overexpression partially restores the localization of Sec4p. (A) For these experiments, sec2-78 cells (NY2179) were transformed with a 2μ URA3 plasmid overproducing YPT32 from the GPD promoter (NY2431). Overexpression was confirmed by Western blot probed with αYpt32p antibody. (B) For Sec4p immunofluorescence, cells were grown overnight in YPD at 25°C, shifted to 37°C for 2 h, fixed, and labeled with αSec4p antibody. At 25°C, wild-type (NY10), sec2-78 (NY2179), and sec2-78/2μYPT32 cells (NY 2431) show Sec4p staining preferentially at the bud tip and necks (arrows). In sec2-78 cells, diffuse staining is observed after a temperature shift to 37°C, but upon Ypt32p overexpression, localization of Sec4p is partially restored (arrows). (C) Approximately 100 cells were counted for each experiment and the percentage of cells showing bud tip and mother–daughter neck localization of Sec4p was calculated. Wild-type yeast have 60% localized Sec4p, whereas sec2-78 and sec2-78/2μYPT32 yeast exhibit 21% and 25% localization before the temperature shift. After shifting to 37°C, no localized Sec4p is observed in sec2-78 cells. However, sec2-78/2μYPT32 exhibit 25% localization of Sec4p.
Polarized localization of Sec4p was observed in 60% of wild-type yeast cells but only in 21% of sec2-78 cells at the permissive temperature (Fig. 3, B and C). When sec2-78 cells were shifted to 37°C, the concentration of Sec4p in bud tips and necks was completely lost. Overexpression of Ypt32p had little effect upon the localization of Sec4p at 25°C, but after the shift to 37°C, Sec4p remained partially concentrated (25%) at bud tips and necks.
Two-hybrid assay
The yeast two-hybrid system was used to explore possible interactions between Sec2p and Ypt32p (Chien et al., 1991). We made various point mutations in YPT32 based on homology with other small G proteins like Ras and Sec4p (Walworth et al., 1989). These alleles were fused with the GAL4 DNA binding domain in the pAS-CYH2 plasmid. SEC2 was fused to the GAL4 DNA activation domain in pACTII. The SEC2 construct was then screened against the YPT32 constructs. Expression of the fusion proteins was confirmed by Western blotting with anti-HA antibody (12CA5), which recognizes the HA epitope immediately adjacent to the GAL4 domain. All proteins were expressed at similar levels (unpublished data).
Ypt32S27N contains a substitution of serine for asparagine that is analogous to the dominant blocking allele RasN17. This mutant binds GDP preferentially but probably adopts a conformation intermediate between GDP- and GTP-bound states (Farnsworth et al., 1991). The Ypt32E49Q mutant exhibits cold sensitivity, and this position has been reported to be part of the effector domain (Matsuda et al., 2000). The Ypt32Q72L mutation lowers the intrinsic rate of GTP hydrolysis as demonstrated in other Rab proteins (Der et al., 1986). Ypt32N126I contains an asparagine to isoleucine mutation in the highly conserved NKXD box. This results in a protein that is unable to bind nucleotides (Walter et al., 1986). Ypt32ΔCys contains a deletion of COOH-terminal cysteines that are necessary for geranylgeranylation and membrane attachment of the protein.
The results of the two-hybrid assay are shown in Table I. Sec2p was found to interact with wild-type, Ypt32E49Q, Ypt32Q72L, and Ypt32ΔCys, but not with Ypt32S27N and Ypt32N126I. The interaction between Sec2p and the Ypt32p constructs was weaker than that observed for Ypt32p with GDI and Sec2p with Sec4S34N (preferentially binds GDP; unpublished data). This could indicate that the interaction between Ypt32p and Sec2p is a weak or transient interaction.
Table I. Two-hybrid interaction between Ypt32p and Sec2p.
| Ypt32 construct | Amino acid change | Class | Sec2pa |
|---|---|---|---|
| Wild type | + | ||
| S27 | Ser27→Asn | Binds GDP preferentially | − |
| E49 | Glu49→Gln | Effector domain? | + |
| Q72 | Gln72→Leu | GTPase deficient | + |
| N126 | Asn126→Ile | Nucleotide binding deficient | − |
| ΔC | Cys221,222→Δ | Wild-type protein lacking COOH-terminal prenylation | + |
β-Galactosidase activity was determined by filter assay. Plus (+) represents a positive and minus (−) a negative indication of β-galactosidase activity.
Sec2p binds Ypt32p preferentially in its GTP-bound form
To evaluate the interaction between Sec2p and Ypt32p in greater detail, we performed in vitro binding experiments with purified proteins. Because of difficulties in expressing full-length Sec2p in bacteria, glutathione-S-transferase (GST)–Sec2p was expressed under the control of the strong GAL1 promoter in yeast and purified using affinity chromatography. A band of apparent molecular weight of 120 kD was observed by Coomassie blue staining of the SDS-PAGE gel. This band corresponds to GST–Sec2p, as confirmed by Western Blot analysis using anti-Sec2 and anti-GST antibodies. We have previously shown that Sec2p with a COOH-teminal GFP tag is fully functional (Elkind et al., 2000). To ask whether the NH2-terminal GST fusion also results in active protein, several tests were performed. First, the GST–SEC2-pNB529 plasmid (pNB1153) suppressed the ts growth defect of a sec2-78 yeast strain. Second, GST–SEC2 can function as the sole copy of the essential gene SEC2 in yeast (unpublished data). Third, the purified GST–Sec2p is catalytically competent as it has exchange activity on Sec4p in vitro (see below).
In the binding experiment, GST–Sec2p attached to glutathione-Sepharose beads was incubated with purified Ypt32p. Binding of GST–Sec2p to three different conformations of Ypt32p, the nucleotide-free, GDP-bound, and GTP-γ-S–bound forms, was examined. GST alone bound to the beads was used as a control for nonspecific binding. As shown in Fig. 4, all three forms of Ypt32p were able to bind to Sec2p. The apparent affinity of Sec2p for the GTP-γ-S–loaded form of Ypt32p was somewhat higher than its affinity for the other nucleotide states examined in the assay. However, under these experimental conditions, only ∼40–50% of Ypt32p was being consistently loaded with the appropriate nucleotide. It is therefore possible that the difference between GTP-γ-S bound and other forms is larger than it appears in the data shown. As a control, we performed an identical binding experiment with Sec4p. In agreement with our previous results (Walch-Solimena et al., 1997), Sec2p interacted preferentially with the nucleotide-free conformation of Sec4p. Weak binding to Sec4p-GDP was also detected, but no binding of activated Sec4p-GTP-γ-S was seen. The efficiency of the Ypt32–Sec2p binding appeared to be slightly lower than the efficiency of the Sec4p–Sec2p interaction. Under the same experimental conditions, four times more Sec4p bound to Sec2p than Ypt32p. This may be due to the transient nature of this interaction. Similar, but very small, amounts of endogenous Ypt32p and Sec4p copurified with GST–Sec2p from yeast (unpublished data). Therefore, it is possible that when GST–Sec2p is immobilized on beads, its Ypt32p binding site might be less accessible than its Sec4p binding site.
Figure 4.
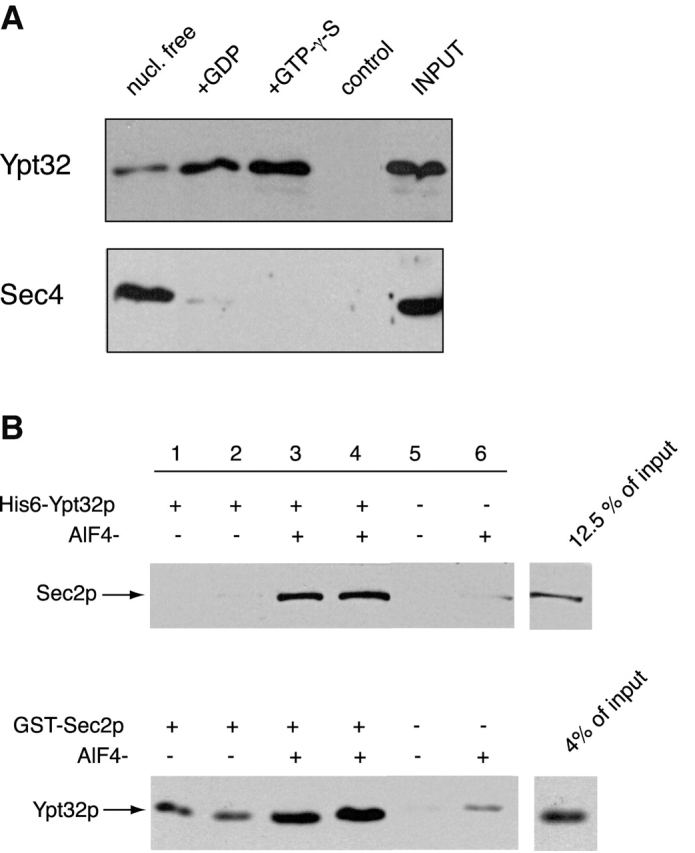
Sec2p binds to Ypt32p with higher apparent affinity for its activated form. (A) GST–Sec2p immobilized on glutathione-Sepharose beads was incubated with Ypt32p as described in the Materials and methods. Ypt32p was either stripped of nucleotide (nucleotide free, lane 1) or loaded with GDP (lane 2) or GTP-γ-S (lane 3). Control (lane 4) represents Ypt32p binding to the GST protein immobilized on glutathione-Sepharose beads. Input represents 5% (1.5 ng) of total Ypt32p used in binding mixtures. An identical experiment was performed for Sec4p. Input represents 20% (6 ng) of Sec4p protein used per binding tube. The beads were washed and the bound proteins were eluted with SDS-PAGE sample buffer. The eluates were then resolved on an SDS-PAGE gel, and Western blot analysis was performed using αYpt32p and αSec4p antibodies. (B) In the top panel, His6–Ypt32p (1 μM) was incubated with Ni-NTA beads and a portion of lysate, prepared from yeast overexpressing GST–Sec2p in a buffer containing PBS and 10 mM imidazole with or without 50 μM AlCl3 and 10 mM NaF. After incubation for 60 min at 4°C, the beads were washed and the bound proteins were analyzed by SDS-PAGE. GST–Sec2p was detected by αSec2p antibody. In the bottom panel, GST–Sec2p (10 nM) immobilized on glutathione-Sepharose beads was incubated with His6–Ypt32p (3 nM) in a buffer containing PBS and 1 mg/ml BSA with or without 50 μM AlCl3 and 10 mM NaF. Bound Ypt32p was detected by αYpt32p antibody.
To further characterize Sec2p–Ypt32p binding, we performed an additional binding experiment using AlF4− (Fig. 4 B). AlF4− is known to bind tightly to small G protein–GDP–Mg2+–GAP complexes (Mittal et al., 1996; Ahmadian et al., 1997). Significantly higher Sec2p–Ypt32p binding was observed when AlF4− was included in the binding mixtures (Fig. 4 B, lanes 3 and 4) than when it was omitted (lanes 1 and 2). The AlF4− stimulation of binding suggested that Sec2p may have GAP activity on Ypt32p. With stoichiometric amounts of Sec2p and Ypt32p, we observed a fivefold increase in GTP hydrolysis by Ypt32p (unpublished data). Although the GAP activity was detectable, it was not quantitatively comparable to the 105-fold stimulation typically seen with GAPs for other members of the Ras superfamily.
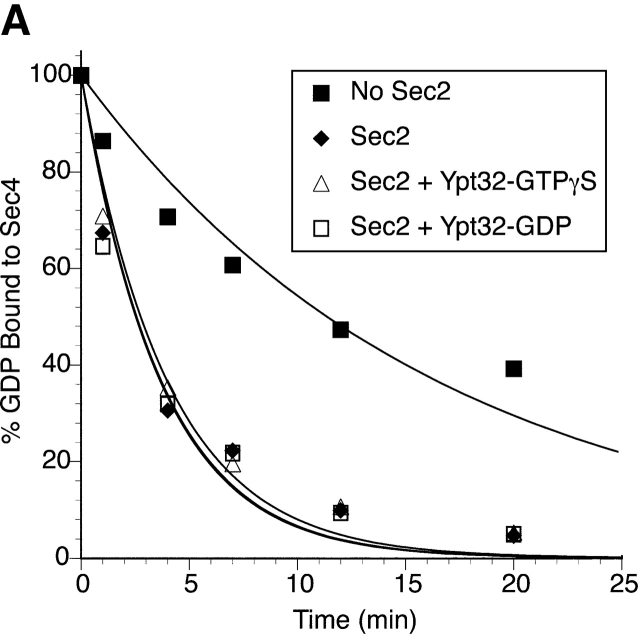
Sec2p does not have exchange activity on Ypt32p
To assess whether Sec2p functions as a GEF for Ypt32p, an in vitro activity assay was performed (Fig. 5). Purified Ypt32p was preloaded with [3H]GDP and the time course of nucleotide release was measured. GST–Sec2p, even at the concentration of 0.25 μM, did not stimulate the nucleotide release from 0.2 μM Ypt32p. On the other hand, as shown previously, substoichiometric amounts of Sec2p were sufficient to stimulate [3H]GDP release from Sec4p (Walch-Solimena et al., 1997). In our hands, with 0.2 μM Sec4p and 10 nM GST–Sec2p, threefold stimulation of the GDP release rate was observed (unpublished data). A tenfold higher amount of GST–Sec2p resulted in almost a 30-fold stimulation of nucleotide release from Sec4p.
Figure 5.
Sec2p is not a GEF for Ypt32p. Release of [3H]GDP from Ypt32p (0.2 μM) in the absence and presence of 100 nM purified GST–Sec2p was measured at 30°C. Increasing the GST–Sec2p concentration to 250 nM did not result in a higher activity (unpublished data). Release of [3H]GDP from Sec4p (0.2 μM) in the absence and presence of 100 nM GST–Sec2p was measured at 12°C. At the times indicated, 10 μl of reaction mixtures were withdrawn and Rab-bound radioactivity was determined by the filter-binding assay. Data represent one of three independent similar experiments.
Ypt32p has no effect on the exchange activity of Sec2p on Sec4
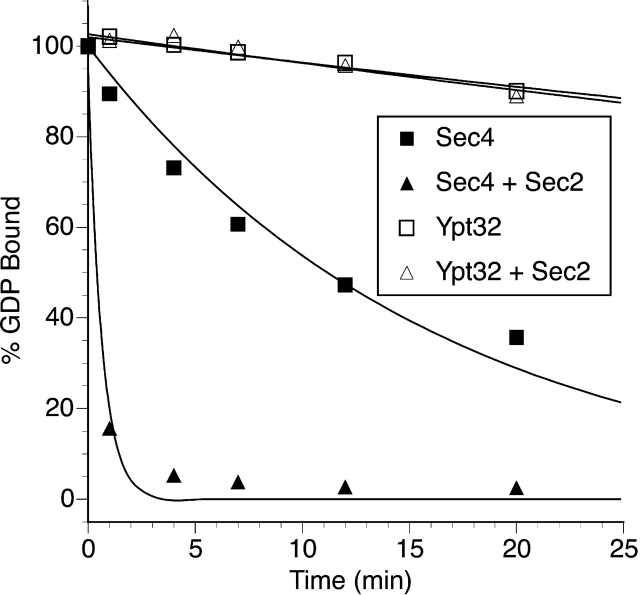
We have established that Sec2p binds to Ypt32p, but does not catalyze nucleotide exchange on it. To further explore the function of this complex, we investigated its ability to catalyze [3H]GDP release from Sec4p. In particular, we wanted to ask whether Ypt32p might modulate Sec2p activity on Sec4p (Fig. 6 A). GST–Sec2p, at a concentration of 20 nM, stimulated the nucleotide release from Sec4p by approximately fivefold. Neither activation nor inhibition of this activity was observed when 1 μM Ypt32p in either its GDP- or GTP-γ-S–bound conformations were included in the assay mixture. Under these buffer conditions, Sec2p bound to Ypt32p equally well as in the phosphate buffer used for our binding assays (unpublished data). Therefore, it is unlikely that Ypt32p functions as a regulator of the catalytic activity of Sec2p.
Figure 6.
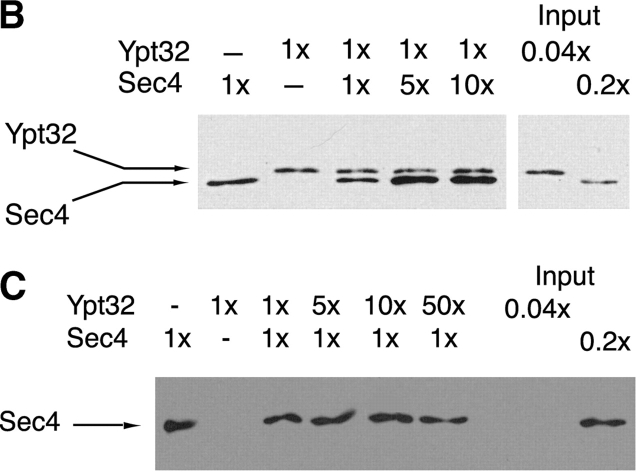
Ypt32p does not regulate the Sec2p exchange activity on Sec4p and Ypt32p and Sec4p binding sites on Sec2p are independent. (A) [3H]GDP release from Sec4p (0.2 μM) was measured in the presence of 20 nM GST–Sec2p or 20 nM GST–Sec2p and 1 μM Ypt32 preloaded with GDP or GTP-γ-S. Intrinsic nucleotide release from Sec4p is shown as well for comparison. To preload Ypt32p with nucleotides, 7.6 μM protein was incubated with 1 mM GDP or GTP-γ-S in preloading buffer (PBS, 1 mM EDTA, 1 mM MgCl2) as described in the Materials and methods. After the incubation, MgCl2 was adjusted to 5 mM and the excess nucleotide was removed by gel filtration using a Sephadex G-25 column (Amersham Pharmacia Biotech). Protein concentration was then determined using the Bio-Rad Laboratories protein assay with BSA as a standard. The final concentration of Ypt32p in the assay was 1 μM. Data represent one of three independent similar experiments. (B) Purified Sec4p (lane 1; 1× corresponds to 30 ng) and Ypt32-GTP-γ-S (lane 2; 1× corresponds to 30 ng) were mixed at the indicated ratios (lanes 3, 4, and 5) and incubated with GST–Sec2p immobilized on beads. The beads were washed and the bound proteins were eluted with SDS-PAGE sample buffer. The eluates were then resolved on 14% SDS-PAGE gel, and Western blot analysis was performed using αYpt32p. The cross-reactivity of the Ypt32p antibody with Sec4p allowed detection of both proteins. (C) Complementary experiment to A was performed. In this experiment, the amount of Sec4p was kept constant (1× corresponds to 30 ng) while varying the concentration of Ypt32. Sec4p was detected by Western analysis using monoclonal αSec4p antibody.
Ypt32p and Sec4p bind to distinct sites on Sec2p
The Sec4p binding site on Sec2p has been previously shown to be located within the NH2-terminal half of the molecule. To address whether Ypt32p binds to the same region on Sec2p, competition experiments were conducted (Fig. 6, B and C). A tenfold excess of Sec4p (30 nM) did not displace Ypt32p from its binding site on Sec2p (Fig. 6 B). Furthermore, Ypt32p and Sec4p appeared to bind to Sec2p independently, with no observable cooperativity in binding. In this experiment, the total amount of the Rab proteins was at least threefold higher than the estimated amount of GST–Sec2p in the assay (10 nM). This implies that Ypt32p and Sec4p do not share the same binding site on Sec2p. In a complementary experiment, Ypt32p at a fiftyfold excess did not compete off Sec4p from its binding to Sec2p (Fig. 6 C). These results are also consistent with the lack of effect of Ypt32p on the exchange activity of Sec2p. If both proteins were to compete for the same binding site on Sec2p, inhibition of Sec2p exchange activity on Sec4p due to the presence of Ypt32p would be expected.
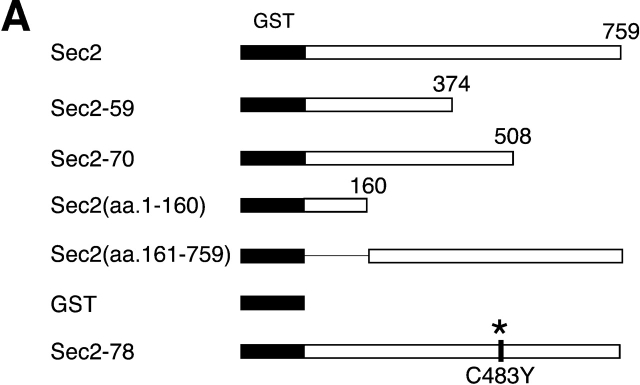
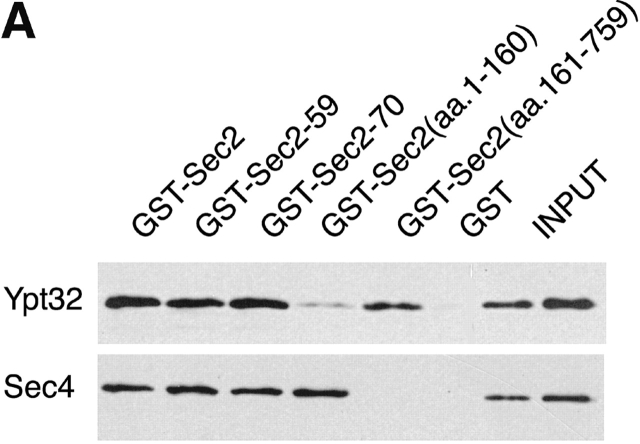
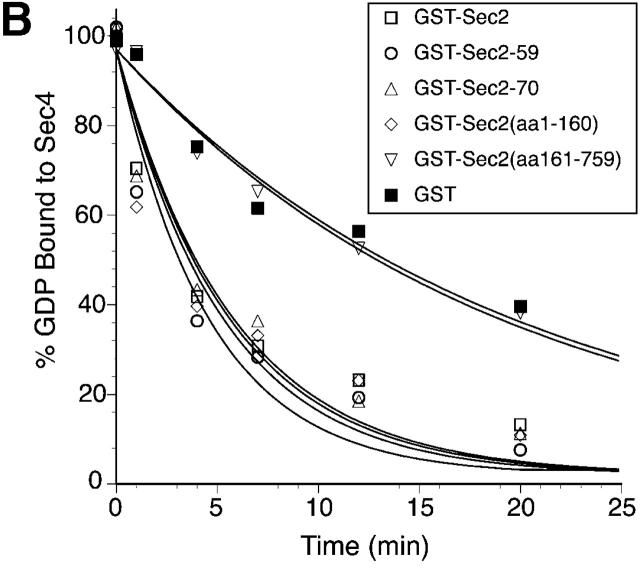
To further define the Ypt32p and Sec4p binding sites on Sec2p, expression vectors for several truncated Sec2p alleles have been constructed and the corresponding proteins were expressed as GST fusions in yeast. As shown in a schematic diagram (Fig. 7 A), the constructs included the truncated proteins encoded by the ts allele sec2-59 (aa 1–374) and the point mutant sec2-78 (Sec2C483Y) as well as additional truncations Sec2(aa 1–160), Sec2(aa 161–759), and allele sec2-70 (aa 1–508). All of the fusion proteins were purified from yeast lysates. Purification yielded bands of the appropriate molecular weights (Fig. 7 B). These constructs were immobilized on glutathione-Sepharose beads and tested for their ability to bind Sec4p and Ypt32p (Fig. 8 A). Binding of Ypt32p to all fusion proteins except GST–Sec2(aa 1–160)p was observed. Based on the differential binding of Ypt32p to GST–Sec2-59(aa 1–374)p and GST–Sec2(aa 1–160)p, we believe that the Ypt32p binding site on Sec2p is located within the NH2-terminal half of the Sec2p molecule, between amino acids 160 and 374. On the other hand, all of the Sec4p binding determinants appear to be located within the NH2-terminal coiled coil domain (aa 1–160) of Sec2p. The domain composed of the first 160 amino acids of Sec2p is also fully sufficient for its exchange activity (Fig. 8 B). No other part of the Sec2p molecule appears to contribute to the ability of Sec2p to catalyze the nucleotide exchange on Sec4p in vitro.
Figure7.
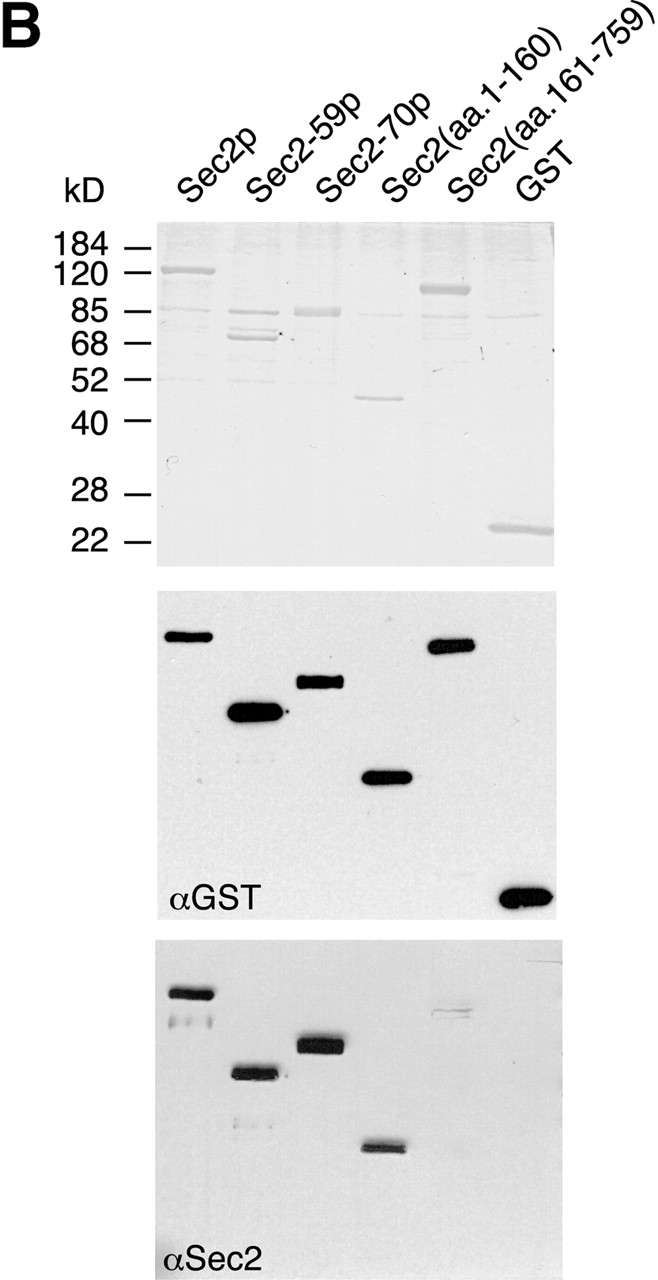
Expression and purification of GST–Sec2 protein constructs in yeast. (A) Schematic diagram of Sec2p constructs employed in this study. (B) Purified GST–Sec2 constructs were resolved on SDS-PAGE gel and the gel was stained with Coomassie blue. Proteins of appropriate molecular masses were observed. An additional protein band of apparent mol wt of 85 kD nonspecifically binds to the beads. Identity of purified GST–Sec2-derived proteins was confirmed by Western analysis using αSec2 and αGST antibodies. Sec2 antibody was raised against the NH2-terminal domain and does not effectively recognize Sec2p missing the first 160 amino acids.
Figure 8.
The Ypt32p binding site on Sec2p is located downstream from the Sec4p binding site. (A) In parallel experiments, Ypt32-GTP-γ-S or Sec4p (both 30 ng per tube; final concentration in the binding assay 3 nM) were incubated with full-length GST–Sec2p or various GST–Sec2 truncated proteins (Fig. 7) immobilized on beads. The beads were then washed and the bound proteins were resolved by SDS-PAGE and detected by Western blot analysis. Input represents 2 and 4% of total Ypt32p and 10 and 20% of total Sec4p protein used per binding mixture. (B) The NH2-terminal domain (aa 1–160) of Sec2p has full exchange activity on Sec4. [3H]GDP release from Sec4p (0.2 μM) in the total reaction volume of 60 μl was measured. 20 μl of beads containing attached GST–Sec2 constructs (for purification procedure see Materials and methods section) were included in the reaction mixtures. At the times indicated, 10 μl of reaction mixtures was withdrawn and Rab-bound radioactivity was determined by the filter-binding assay. Data represent one of two independent similar experiments.
Discussion
In this study, we identified Ypt31p and Ypt32p as suppressors of the ts sec2-78 mutation. Ypt31/32p are 83% identical and functionally redundant GTPases of the Rab family that have been implicated in transport through and/or out of the Golgi apparatus (Benli et al., 1996; Jedd et al., 1997). Accumulating evidence about Ypt31/32p involvement in multiple Golgi-trafficking events suggests complex functions for these GTPases, but a detailed mechanism of action has not been established. Sec2p is the essential nucleotide exchange factor for Sec4p and is required for the polarized delivery of Sec4p vesicles to exocytic sites (Walch-Solimena et al., 1997). Proper localization of Sec2p to sites of polarized growth is essential for its function. Earlier work has shown that COOH-terminal truncation of Sec2p produces a defective protein, causing a ts growth and secretion phenotype (Nair et al., 1990). Subsequent work identified a stretch of 58 amino acids within the COOH-terminal half of the molecule as necessary but not sufficient for the membrane localization of Sec2p (Elkind et al., 2000). Loss of this 58–amino acid region (aa 450–508) in previously characterized ts alleles (sec2-59 and sec2-41) or even just a point mutation within this domain (sec2-78) results in severe impairment of Sec2p localization and function. Sec2p associates with membranes largely by a Sec4p-independent mechanism (Elkind et al., 2000). Because analysis of its primary structure does not reveal any transmembrane regions, putative membrane interacting domains, or lipid modifications, we have proposed that there might be an interacting partner functioning perhaps as the receptor for Sec2p on the membranes of secretory vesicles (Elkind et al., 2000). In this report, we show that Ypt32p has the potential to fulfill this function, at least in part. We have shown that overexpression of Ypt31/32p can restore proper localization of Sec2-78p– and Sec2–59p–GFP. This appears to be a specific effect, as overexpression of Ypt1p or Sec4p does not restore Sec2-78p–GFP localization. Sec4p, like Ypt32p, suppresses the sec2-78 growth defect, but the mechanism is different from Ypt31/32p-driven suppression. These findings, together with the observation that Ypt32p and Sec2p directly interact in both two-hybrid and in vitro binding assays, suggest that Ypt31/32p GTPases might be involved in the recruitment of Sec2p to secretory vesicles. Although Ypt32p does not directly modulate the exchange activity of Sec2p, it may indirectly promote Sec2p function by recruiting Sec2p to its substrate, Sec4p. In budding yeast cells, Ypt31/32 proteins have been previously shown to localize to the sites of polarized growth, which is consistent with the novel function we are proposing (Jedd et al., 1997). This suggests that two Rab family proteins, Sec4 and Ypt32, might be functionally linked in a regulatory cascade through the exchange protein Sec2. Ypt32p participates in recruiting Sec2p to membranes of secretory vesicles; once on the vesicle, Sec2p catalyzes nucleotide exchange on its downstream GTPase Sec4p, which in turn leads to the polarized delivery of vesicles and their fusion with the plasma membrane. Sec2p may also help to inactivate Ypt32p, as suggested by the modest GAP activity observed. The secretory pathway consists of several distinct transport steps. It is likely that these individual transport steps are functionally linked to ensure proper transitions between them and thus coordination of the entire pathway. For many years, Rab GTPases have been known to be key regulators of membrane traffic. With multiple Rab proteins involved in multiple trafficking events, the concept of a cascade appears even more attractive. In a parallel study, a Ypt32p exchange activity present in yeast lysates has been found to bind to the GTP-bound form of the Rab GTPase Ypt1p, which functions at an earlier stage of the secretory pathway (W. Wang and S. Ferro-Novick, personal communication). Therefore, it is tempting to speculate that each Rab may act to recruit the exchange protein that activates its downstream Rab regulating the subsequent stage of transport.
Another example of coordinated GTPase function involving Ypt31/32p was recently proposed. YPT31/32 were shown to genetically interact with another family of guanine nucleotide exchangers, the Sec7 domain–containing Arf nucleotide exchangers (Jones et al., 1999). However, at present it is not clear how these genetic interactions translate into a biochemical mechanism. Perhaps, as in the case of Sec2p, Ypt31p and Ypt32p participate in recruitment of these exchangers to the proper sites of action. Genetic interactions of ypt31 with sec4 as well as with arf1 and ypt1 alleles were also reported (Yoo et al., 1999).
The Rab protein cascade mechanism we report is strikingly similar to the mechanism by which the functions of Bud1/Rsr1p and Cdc42p, two GTPases that regulate polarity establishment and bud formation in yeast, are coordinated. They are functionally linked through the action of Cdc24p, which serves as a nucleotide exchange factor for its downstream GTPase Cdc42p. Analogous to the Ypt32p–Sec2p interaction, BUD1/RSR1 was isolated as a multicopy suppressor of a cdc24-4 mutation (Bender and Pringle, 1989), and Bud1/Rsr1p was subsequently shown to bind directly to Cdc24p in its GTP-bound form (Park et al., 1997). Sequential action of two GTPases was also proposed to play a role in vacuole docking and fusion (Eitzen et al., 2000).
We were able to localize binding sites on Sec2p for Ypt32p and Sec4p. Despite the fact that these two GTPases are strongly related by sequence, they bind to different domains of the Sec2 protein. Sec4p binds to the exchange domain (aa 1–161), whereas the Ypt32p binding site is located further downstream (aa 161–374). This is consistent with the fact that only Sec4p is a substrate for nucleotide exchange. It is important to note that truncation of Sec2p at position 374, as in sec2-59, or mutation of position 483 from cysteine to tyrosine, as in sec2-78, causes mislocalization of Sec2p. Thus, localization can be lost despite the fact that the Ypt32p binding site is intact. Nonetheless, overproduction of Ypt32p restores the localization of Sec2-59–GFP or Sec2-78–GFP proteins. Therefore, it appears likely that there is yet another, unidentified component regulating Sec2p localization. We propose that this component, whose identity is presently unknown, requires the region of Sec2p downstream from position 374. When this interaction is lost, localization of Sec2p can be restored by a compensatory increase in the level of Ypt32p. This situation is somewhat analogous to that of the Rab5 effector EEA1 (Simonsen et al., 1998). In that case, both Rab5 and PI3P are important for recruiting the protein to endosomes. Overproduction of one ligand can compensate for the loss of the other.
In conclusion, we have uncovered new details regarding the regulation of Sec2p function. We identified Ypt32p as a new factor involved in recruiting Sec2p to sites of polarized growth. The current data are consistent with the existence of a Rab protein cascade regulating yeast exocytosis. In this cascade, the first Rab (Ypt32p) recruits the exchange factor for its downstream Rab (Sec4p). Our data also suggest the existence of an additional factor regulating Sec2p function.
Materials and methods
Genetic screen
The high copy suppressor screen was performed using a yeast genomic DNA library that was inserted into a BamHI site in the 2μ plasmid YEp24. This library was introduced into NY2179 (Matα, ura3-52, sec2-78) by transformation. 27,000 transformants were screened for growth at the restrictive temperature of 37°C. A total of 68 suppressors were isolated. These were tested on 5-fluorooratic acid plates for plasmid dependency and all isolated strains were dependent on the presence of the plasmid for suppression. We tested for the presence of SEC2 and SEC4 by PCR; 26 suppressors were positive for SEC4 but none of them were positive for SEC2. This implies that our screen was not performed to saturation or that the library isn't complete. The suppressors were digested with HindIII and grouped into categories. Representatives of each group were then sequenced and four genomic fragments were identified as sec2-78 suppressors.
Plasmid construction and strains
Construction of GAL-YPT1, GAL-YPT32, and GAL-SEC4 (pNB829–833) was described earlier (Grote and Novick, 1999). To create pNB1162, pNB1176, and pNB1177, YPT32, YPT1, and SEC4 (BamHI/HindIII fragments from pNB831, 829, 833) were cloned into pNB530. To create GAL-YPT31 (pNB1178), the gene was PCR amplified from genomic DNA. The product was cloned into pNB530 by digesting with BamHI/HindIII. Plasmids pNB829–833 were digested with ClaI to direct integration into the leu2-3,112 gene of NY2179 (Matα leu2-3,112 ura3-52 sec2-78 GAL +) to create strains NY2440, 2427, and 2441. Plasmid pNB1178 was digested with NcoI to direct integration into the ura3-52 gene of NY26 (Matα, ura3-52, sec2-59), NY2179, and NY2152 (Mata sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78–GFP] his3-Δ200 ura3-52 Gal + ) to create strains NY2444, 2445, and 2430. Plasmids pNB1162, 1176, and 1177, digested with NcoI, were integrated into NY26, creating strains NY2426, 2442, and 2443. pNB1162, digested with NcoI, was integrated into NY2147 (Mata sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-59–GFP] his3-Δ200 ura3-52 Gal + ) and NY2152 (Mata sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78–GFP] his3-Δ200 ura3-52 Gal +) to create strains NY2428 and 2429, whereas pNB1177, linearized with NcoI, was transformed into NY2152 to create NY2446 (Table II).
Table II. Strain list.
| Strain | Genotype |
|---|---|
| Y190 | MATa ura3-52 his3-Δ200 ade2-101 lys2-801 trp1-901 leu2-3,112 gal4-542 gal80-538 URA3::GAL-LacZ LYS2::GAL-HIS3 cyhr |
| NY10 | MATα ura3-52 |
| NY26 | MATα ura3-52 sec2-59 |
| NY603 | MATα leu2-3,112 ura3-52 pep4::URA3 Gal+ |
| NY2146 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP] his3-Δ200 ura3-52 Gal+ |
| NY2147 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-59-GFP] his3-Δ200 ura3-52 Gal+ |
| NY2152 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78-GFP] his3-Δ200 ura3-52 Gal+ |
| NY2179 | MATα leu2-3,112 ura3-52 sec2-78 Gal+ |
| NY2426 | MATα ura3-52::[URA3 GALp-YPT32; pNB1162] sec2-59 |
| NY2427 | MATα leu2-3,112::[LEU2 GALp-YPT32; pNB831] ura3-52 sec2-78 Gal+ |
| NY2428 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-59-GFP] his3-Δ200 ura3-52::[URA3 GALp-YPT32; pNB1162] Gal+ |
| NY2429 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78-GFP] his3-Δ200 ura3-52::[URA3 GALp-YPT32; pNB1162] Gal+ |
| NY2430 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78-GFP] his3-Δ200 ura3-52::[URA3 GALp-YPT31; pNB1178] Gal+ |
| NY2431 | MATα ura3-52 sec2-78 + [GPDp-YPT32 2μ; pNB1163] |
| NY2432 | MATα leu2-3,112::[LEU2 GALp-GST-SEC2; pNB1153] ura3-52 pep4::URA3 Gal+ |
| NY2433 | MATα leu2-3,112::[LEU2 GALp-GST; pNB1154] ura3-52 pep4::URA3 Gal+ |
| NY2434 | MATα leu2-3,112::[LEU2 GALp-GST-sec2(aa.1-160); pNB1157] ura3-52 pep4::URA3 Gal+ |
| NY2435 | MATα leu2-3,112::[LEU2 GALp-GST-sec2-59(aa.1-374); pNB1155] ura3-52 pep4::URA3 Gal+ |
| NY2436 | MATα leu2-3,112::[LEU2 GALp-GST-sec2-70(aa.1-508); pNB1156] ura3-52 pep4::URA3 Gal+ |
| NY2437 | MATα leu2-3,112::[LEU2 GALp-GST-sec2(aa.161-759); pNB1159] ura3-52 pep4::URA3 Gal+ |
| NY2438 | MATα leu2-3,112::[LEU2 GALp-GST-sec2-78; pNB1160] ura3-52 pep4::URA3 Gal+ |
| NY2439 | MATα leu2-3,112::[LEU2 GALp-GST-sec2(aa.1-450); pNB1161] ura3-52 pep4::URA3 Gal+ |
| NY2440 | MATα leu2-3,112::[LEU2 GALp-YPT1; pNB829] ura3-52 sec2-78 Gal+ |
| NY2441 | MATα leu2-3,112::[LEU2 GALp-SEC4; pNB833] ura3-52 sec2-78 Gal+ |
| NY2442 | MATα ura3-52::[URA3 GALp-YPT1; pNB1176] sec2-59 |
| NY2443 | MATα ura3-52::[URA3 GALp-SEC4; pNB1177] sec2-59 |
| NY2444 | MATα ura3-52::[URA3 GALp-YPT31; pNB1178] sec2-59 |
| NY2445 | MATα leu2-3,112 ura3-52::[URA3 GALp-YPT31; pNB1178] sec2-78 Gal+ |
| NY2446 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78-GFP] his3-Δ200 ura3-52::[URA3 GALp-SEC4; pNB1177] Gal+ |
pNB1163 was created by subcloning YPT32 (as a BamHI/HindIII fragment from pNB831) into pNB854, a 2μ plasmid containing the strong GPD promoter. The resulting plasmid was introduced into NY2179, creating strain NY2431. The YPT32 point mutations used in the two-hybrid assay were constructed by PCR-based mutagenesis, except for YPT32N126I, which was previously described (Grote and Novick, 1999). Fusion constructs with GAL4 DNA binding domain (plasmid pAS1-CYH) and with the GAL4 DNA activation domain (plasmid pACTII) were obtained by in-frame ligation of PCR products.
To create the GST–SEC2 construct, DNA encoding Sec2p was PCR amplified using pNB978 as a template. The product (SEC2; 2.3 kb) was digested with BamHI and XhoI and ligated to pGEX4T-1 predigested with the same enzymes, resulting in pNB1152. PNB1152 was then used as a template for another round of PCR amplification with primers 5′-ATATCTG CAGATGTCCCCTATACTAGGTTATTGG-3′ and 5′-ATATCTCGAGAAG CTTATTGCTGTTCCTGGGC-3′. The product (GST–SEC2; 3.0 kb) was digested with PstI and HindIII and ligated to pNB529 (integrating yeast vector with GAL1 promoter and ADH terminator) resulting in pNB1153. pNB1153 was linearized with ClaI and introduced into the protease-deficient pep4::HIS3 yeast strain NY603, resulting in NY2432. To induce protein expression, cells were grown in YPGal (2% galactose).
DNA regions coding for GST–Sec2-59 (aa 1–374), GST–Sec2-70 (aa 1–508), and GST–Sec2(aa 1–160) were amplified from pNB1152 as well. The products (GST–sec2-59, 1.8 kb; GST–sec2-70, 2.2 kb; GST–sec2(aa 1–160), 1.15 kb) were digested with PstI and HindIII and ligated to pNB529, resulting in pNB1155, pNB1156, and pNB1157.
To prepare GST–sec2(aa 161–759), part of the SEC2 gene was first amplified using pNB978 as a template. The product was digested with BamHI and XhoI and ligated to pGEX4T-1, resulting in pNB1158. GST–sec2(aa 161–759) was then amplified from pNB1158 using the same primers described above, resulting in pNB1159. The resulting plasmids were linearized and transformed into the protease-deficient pep4::HIS3 yeast strain NY603, resulting in NY2433–2437 (Table II).
GFP and immunofluorescence
Cells containing Sec2 proteins tagged with GFP, with and without overexpression of Ypt31p, Ypt32p, and Sec4p were grown overnight to midlog phase (A600 = 0.5–1.0) in YPGal at 25°C. Cells were fixed in ice cold methanol and acetone, washed three times with phosphate buffer (PBS), sonicated for 5 s, and observed under the microscope. Sec4p was visualized in strains NY10, 2179, and 2431 by indirect immunofluorescence. Strains were grown overnight in YPD at 25°C to a final cell density (A600) between 0.5 and 1.0 U. The cells were then either immediately fixed or shifted to the restrictive temperature (37°C) for 1 h. Preparation of samples was performed essentially as described (Walch-Solimena et al., 1997). GFP and Sec4p immunolabeling were visualized with a ZEISS Axiophot 2 microscope using a 100× objective. Images were acquired with a Photometrics Quantix CCD camera.
Two-hybrid assay
S. cerevisiae strain Y190 (Mata, ura3-52, his3-Δ200, ade2-101, lys2-801, trp1-901, leu2-3,112, gal4-542, gal80-538, URA3::GAL-LacZ, LYS2::GAL-HIS3, cyhr) was simultaneously transformed with DNA activation and DNA binding domain constructs. Transformants were monitored for expression of β-galactosidase activity using an X-Gal filter lift assay. For this, yeast colonies were transferred to filter paper (no. 1; Whatman Inc.), permeabilized by submerging twice in liquid nitrogen for 10 s, and then laid onto a second filter presoaked in Z buffer (100 mM sodium phosphate, 10 mM KCl, 1 mM MgSO4) containing 38 mM β-mercaptoethanol and 0.35 mg/ml X-Gal. The filters were incubated at 30°C for 1–2 d.
In vitro binding assay
For a typical binding experiment, 50–75 OD units of yeast overexpressing GST–Sec2p or GST–Sec2 truncations were resuspended in lysis buffer containing PBS, 0.5% Triton X-100, 5 mM DTT, and protease inhibitors. Cells were disrupted in a bead beater using 0.5-mm zirconia/silica beads (beads and instrument from Biospec Products). Lysates were then cleared by centrifugation at 16,000 g for 10 min at 4°C. Triton X-100 was adjusted to 1% and supernatants were then incubated with 400 μl of a 50% (vol/vol) slurry of glutathione-Sepharose 4B (Amersham Pharmacia Biotech) beads for 60 min while rotating the samples at 4°C. After the incubation, the beads were spun at 5,000 g and washed four times with 1 ml of ice cold PBS buffer. Hexa-histidine–tagged (His6)–Ypt32p and (His6)–Sec4p were purified from Escherichia coli as previously described (Du and Novick, 2001). GST–Sec2 fusion protein immobilized on glutathione-Sepharose beads (20 μl of beads; estimated amount of GST–Sec2p on beads was 0.5 μg) was incubated with 3 nM Ypt32p in PBS buffer containing 1 mg/ml BSA, 1 mM DTT, and 1 mM MgCl2. Sec4p binding experiments were conducted in the same manner in PBS buffer containing 1 mg/ml BSA, 1 mM DTT, and 5 mM MgCl2. The total volume of incubation mixtures was 400 μl. After incubating for 60 min at room temperature, the resin was washed with the incubation buffer without BSA and bound products were separated by SDS-PAGE. To preload Ypt32p with GDP or GTP-γ-S, 400 nM protein was incubated with 1 mM nucleotide in PBS buffer containing 1 mg/ml BSA, 1 mM EDTA, 1 mM MgCl2, and 1 mM DTT for 1 h at room temperature. Sec4p preloading buffer contained PBS, 1 mg/ml BSA, 5 mM MgCl2, and 1 mM DTT. To analyze the binding of the nucleotide-free forms of Ypt32p and Sec4p to GST–Sec2p, the Rab proteins were incubated with GST–Sec2p immobilized on beads in PBS buffer containing 5 mM EDTA, 1 mg/ml BSA, and 1 mM DTT. After the incubation, the beads were washed with PBS buffer containing 5 mM EDTA.
GDP displacement assay
GDP displacement activity was monitored as previously described (Walch-Solimena et al., 1997). Ypt32p (0.4 μM) was preloaded with [3H]GDP (0.8 μM; 27 Ci/mmol) by incubation in 50 mM Tris, pH 8, 100 mM KCl, 1 mM EDTA, 1 mM MgCl2, 1 mg/ml BSA, and 1 mM DTT for 30 min at 30°C. After the incubation, MgCl2 was adjusted to 6 mM. Sec4p (0.4 μM) was preloaded with [3H]GDP (0.8 μM; 27 Ci/mmol) by incubation in a buffer containing 50 mM Tris, pH 8, 100 mM KCl, 1 mM EDTA, 6 mM MgCl2, 1 mg/ml BSA, and 1 mM DTT. Reactions were initiated by the addition of 0.5 mM GDP and either purified GST–Sec2 protein or control buffer (total volume 30 μl) to 30 μl of [3H]GDP preloaded Ypt32p and Sec4p. To purify GST–Sec2p, 1 liter of yeast (1.2 OD/ml) was grown in YPGal (2% galactose). Cells were resuspended in 10 ml of lysis buffer and lysed as described above. The lysate was centrifuged at 90,000 g for 45 min at 4°C. The supernatant was incubated with 3 ml of 50% slurry of glutathione-Sepharose 4B at 4°C for 60 min. After the incubation, the beads were spun and washed 3 times with 15 ml of ice cold PBS buffer. The bound protein was eluted with three 1-ml portions of elution buffer (50 mM Tris, pH 8, 10 mM glutathione). The eluted fractions were pooled, buffer exchanged, and concentrated using Ultrafree-4 centrifugation units (Millipore) with a 10,000 mol wt cut off. The purified protein was stored at −20°C in a buffer containing 20 mM Tris, pH 8, 100 μM PMSF, and 40% glycerol. GDP displacement from Ypt32p was conducted at 30°C. To lower the high intrinsic GDP release rate of Sec4p, experiments with Sec4p were conducted at 12°C. 10-μl aliquots were withdrawn at the times indicated and placed into 1 ml of ice cold stop buffer solution (25 mM Tris, pH 8, 20 mM MgCl2). Rab-associated radioactivity was determined by filter binding followed by scintillation counting.
Acknowledgments
We thank S. Ferro-Novick for providing Ypt32p antibody, Wei Wang for help with the exchange assays, and Ellen France and Eric Grote (Yale University School of Medicine) for critical reading of the manuscript and helpful discussions.
This work was supported by a grant to P. Novick from the National Institutes of Health (CA46128).
D. Ortiz and M. Medkova contributed equally to this paper.
C. Walch-Solimena's present address is Max Planck Institute of Molecular Biology and Genetics, Pfotenhauerstrasse 108, 01307 Dresden, Germany.
Footnotes
Abreviations used in this paper: GAP, GTPase-activating proteins; GEF, guanine nucleotide exchange factor; GFP, green fluorescent protein; GST, glutathione-S-transferase; HA, hemagglutinin; ts, temperature sensitive; YPD, YP medium containing glucose; YPGal, YP medium containing galactose; YPGR, YP medium containing galactose and raffinose.
References
- Ahmadian, M.R., R. Mittal, A. Hall, and A. Wittinghofer. 1997. Aluminium fluoride associates with the small guanine nucleotide binding proteins. FEBS Lett. 408:315–318. [DOI] [PubMed] [Google Scholar]
- Bender, A., and J.R. Pringle. 1989. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. USA. 86:9976–9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli, M., F. Doring, D.G. Robinson, X. Yang, and D. Gallwitz. 1996. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Chien, C.T., P.L. Bartel, R. Sternglanz, and S. Fields. 1991. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl. Acad. Sci. USA. 88:9578–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der, C.J., T. Finkel, and G.M. Cooper. 1986. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 44:167–176. [DOI] [PubMed] [Google Scholar]
- Du, L.L., and P. Novick. 2001. Purification and properties of a GTPase-activating protein for yeast Rab GTPases. Methods Enzymol. 329:91–99. [DOI] [PubMed] [Google Scholar]
- Echard, A., F. Jollivet, O. Martinez, J.J. Lacapere, A. Rousselet, I. Janoueix-Lerosey, and B. Goud. 1998. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 279:580–585. [DOI] [PubMed] [Google Scholar]
- Eitzen, G., E. Will, D. Gallwitz, A. Haas, and W. Wickner. 2000. Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J. 19:6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind, N.B., C. Walch-Solimena, and P.J. Novick. 2000. The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. J. Cell Biol. 149:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth, C.L., M.S. Marshall, J.B. Gibbs, D.W. Stacey, and L.A. Feig. 1991. Preferential inhibition of the oncogenic form of RasH by mutations in the GAP binding/”effector” domain. Cell. 64:625–633. [DOI] [PubMed] [Google Scholar]
- Govindan, B., R. Bowser, and P. Novick. 1995. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 128:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, E., and P.J. Novick. 1999. Promiscuity in Rab-SNARE interactions. Mol. Biol. Cell. 10:4149–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., D. Roth, C. Walch-Solimena, and P. Novick. 1999. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland, P.G., M. Tecklenberg, and R.A. Sclafani. 1997. Overexpression of the protein kinase Pak1 suppresses yeast DNA polymerase mutations. Mol. Gen. Genet. 256:45–53. [DOI] [PubMed] [Google Scholar]
- Hume, A.N., L.M. Collinson, A. Rapak, A.Q. Gomes, C.R. Hopkins, and M.C. Seabra. 2001. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., J. Mulholland, and N. Segev. 1997. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 137:563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S., G. Jedd, R.A. Kahn, A. Franzusoff, F. Bartolini, and N. Segev. 1999. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics. 152:1543–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T.S., S.L. Reck-Peterson, N.B. Elkind, M.S. Mooseker, P.J. Novick, and J.A. Cooper. 2000. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell. 11:1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre, L.A., R. Kumar, C.M. Hales, J. Navarre, S.G. Bhartur, J.O. Burnette, D.W. Provance, Jr., J.A. Mercer, M. Bahler, and J.R. Goldenring. 2001. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell. 12:1843–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, N., T. Ueda, Y. Sasaki, and A. Nakano. 2000. Overexpression of PRA2, a Rab/Ypt-family small GTPase from pea Pisum sativum, aggravates the growth defect of yeast ypt mutants. Cell Struct. Funct. 25:11–20. [DOI] [PubMed] [Google Scholar]
- Mittal, R., M.R. Ahmadian, R.S. Goody, and A. Wittinghofer. 1996. Formation of a transition-state analog of the Ras GTPase reaction by Ras-center-Dot-Gdp, tetrafluoroaluminate, and GTPase-activating proteins. Science. 273:115–117. [DOI] [PubMed] [Google Scholar]
- Nair, J., H. Muller, M. Peterson, and P. Novick. 1990. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J. Cell Biol. 110:1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., and D. Botstein. 1985. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 40:405–416. [DOI] [PubMed] [Google Scholar]
- Park, H.O., E. Bi, J.R. Pringle, and I. Herskowitz. 1997. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc. Natl. Acad. Sci. USA. 94:4463–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S.R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487–491. [DOI] [PubMed] [Google Scholar]
- Pruyne, D.W., D.H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and P.J. Novick. 1987. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 49:527–538. [DOI] [PubMed] [Google Scholar]
- Schott, D., J. Ho, D. Pruyne, and A. Bretscher. 1999. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147:791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., R. Lippe, S. Christoforidis, J.M. Gaullier, A. Brech, J. Callaghan, B.H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 394:494–498. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena, C., R.N. Collins, and P.J. Novick. 1997. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, M., S.G. Clark, and A.D. Levinson. 1986. The oncogenic activation of human p21ras by a novel mechanism. Science. 233:649–652. [DOI] [PubMed] [Google Scholar]
- Walworth, N.C., B. Goud, A.K. Kabcenell, and P.J. Novick. 1989. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 8:1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl, T., H. Klier, H. Ammer, F. Lottspeich, and V. Magdolen. 1993. The HYP2 gene of Saccharomyces cerevisiae is essential for aerobic growth: characterization of different isoforms of the hypusine-containing protein Hyp2p and analysis of gene disruption mutants. Mol. Gen. Genet. 241:305–311. [DOI] [PubMed] [Google Scholar]
- Yoo, J.S., R. Grabowski, L. Xing, H.H. Trepte, H.D. Schmitt, and D. Gallwitz. 1999. Functional implications of genetic interactions between genes encoding small GTPases involved in vesicular transport in yeast. Mol. Gen. Genet. 261:80–91. [DOI] [PubMed] [Google Scholar]