Abstract
RanGAP1 was the first documented substrate for conjugation with the ubiquitin-like protein SUMO-1. However, the functional significance of this conjugation has not been fully clarified. We sought to examine RanGAP1 behavior during mitosis. We found that RanGAP1 associates with mitotic spindles and that it is particularly concentrated at foci near kinetochores. Association with kinetochores appeared soon after nuclear envelope breakdown and persisted until late anaphase, but it was lost coincident with nuclear envelope assembly in telophase. A mutant RanGAP1 protein lacking the capacity to be conjugated to SUMO-1 no longer associated with spindles, indicating that conjugation was essential for RanGAP1's mitotic localization. RanBP2, a nuclear pore protein that binds SUMO-1–conjugated RanGAP1 during interphase, colocalized with RanGAP1 on spindles, suggesting that a complex between these two proteins may be involved in mitotic targeting of RanGAP1. This report shows for the first time that SUMO-1 conjugation is required for mitotic localization of RanGAP1, and suggests that a major role of SUMO-1 conjugation to RanGAP1 may be the spatial regulation of the Ran pathway during mitosis.
Keywords: RanGAP1; SUMO-1; kinetochore; Ran; RanBP2
Introduction
Ran is a GTPase that is required for nuclear transport, cell cycle control, mitotic spindle formation, and postmitotic nuclear assembly (for review see Sazer and Dasso, 2000). Ran is regulated by a cytosolic GTPase–activating protein, RanGAP1, and by a chromatin-bound nucleotide exchange factor, RCC1 (Sazer and Dasso, 2000). The distribution of Ran-GTP provides important spatial information that directs cellular activities during different parts of the cell cycle (for review see Dasso, 2001). During interphase, the localization of RCC1 and RanGAP1 predicts that nuclear Ran is GTP-bound and cytosolic Ran is GDP-bound. This compartmentalization determines the direction of nuclear transport by promoting the loading and unloading of transport receptors in a manner that is appropriate to the nucleus or cytosol (Gorlich and Kutay, 1999).
In mitosis, microtubules (MTs)* are stabilized in the vicinity of chromatin by a factor(s) whose concentration is inversely proportional to distance from chromosomes (for review see Dasso, 2001). This stabilization contributes toward the assembly of bipolar mitotic spindles. Ran is implicated in spindle assembly through observations in M-phase Xenopus egg extracts, including the finding that elevated levels of Ran-GTP promote spontaneous MT polymerization in a manner that is independent of chromosomes (Dasso, 2001). Since a significant fraction of RCC1 remains chromatin-associated in mitosis (Carazo-Salas et al., 1999), it has been hypothesized that Ran-GTP could be a diffusionally distributed stabilization factor that contributes to the localized stabilization of MTs near condensed chromosomes.
Given the proposed roles of Ran-GTP as a spatial signal during both interphase and mitosis, knowledge of the distribution of Ran's regulators will be essential for understanding the control and function of this pathway. We have been particularly interested in understanding how RanGAP1 is distributed as cells progress through mitosis. In metazoans, RanGAP1 is conjugated with SUMO-1, a small ubiquitin-like protein (Matunis et al., 1996; Mahajan et al., 1997; Saitoh et al., 1997). SUMO-1 shares only ∼18% identity with ubiquitin at the amino acid level, and it is covalently linked through isopeptide bonds to lysine residues in other target proteins in a manner that is very similar to ubiquitin (for review see Melchior, 2000).
The activity of SUMO-1–conjugated RanGAP1 (RanGAP1–SUMO-1) as a Ran GTPase–activating protein is not substantially altered (Saitoh et al., 1997). However, SUMO-1 modification causes RanGAP1 to associate with RanBP2, a large nuclear pore protein, and Ubc9, the E2 enzyme for SUMO-1 conjugation (Mahajan et al., 1997; Saitoh et al., 1997; Matunis et al., 1998). The association of RanGAP1 with RanBP2 may facilitate nuclear transport. This idea is supported by the capacity of antibodies to block transport by inhibiting pore-associated RanGAP1 (Mahajan et al., 1997). However, yeast homologues of RanGAP1 are not subject to SUMO-1 modification, nor is the necessity for tight association of RanGAP1 with the pore obvious from our current knowledge of nuclear transport (Gorlich and Kutay, 1999). Alternatively, it is possible that SUMO-1 modification of RanGAP1 could shape Ran-GTP gradients in mitosis or promote the repolarization of Ran-GTP across the nuclear envelope as nuclei assemble after mitosis. Consistent with the former idea, RanGAP1 associates with the spindle during mitosis (Matunis et al., 1996). However, it has not been shown how this association is achieved or regulated.
We sought to examine the behavior of RanGAP1 during the passage of cells through mitosis. We found that RanGAP1 localized to mitotic spindles and kinetochores. Binding to spindles was dependent upon the SUMO-1 conjugation of RanGAP1, revealing a novel role for this modification in localizing RanGAP1 during mitosis. When compared with RanGAP1–SUMO-1, RanBP2 showed an overlapping but nonidentical distribution, indicating that the complex between these proteins may be maintained in mitosis and serve as part of the mechanism whereby RanGAP1 targets to the spindle. These observations have important implications for models of spindle assembly that rely upon the mitotic distribution of Ran-GTP.
Results and discussion
RanGAP1 associates with the mitotic spindle
A fraction of RanGAP1 is targeted to the nuclear pore during interphase (Matunis et al., 1996; Mahajan et al., 1997). This fraction can be visualized by immunofluorescence if cells are treated briefly with digitonin before fixation to release soluble cytoplasmic RanGAP1 protein. Using similar strategies, it was demonstrated that a portion of RanGAP1 is associated with the mitotic spindle (Matunis et al., 1996), although the mechanism of this targeting has not been reported.
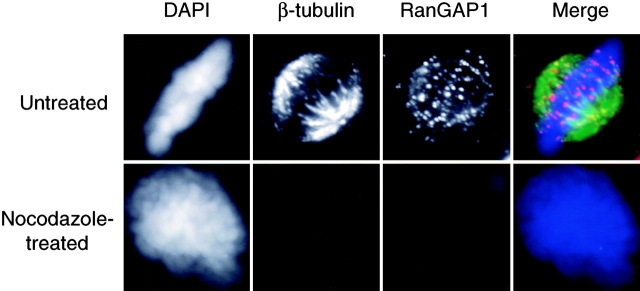
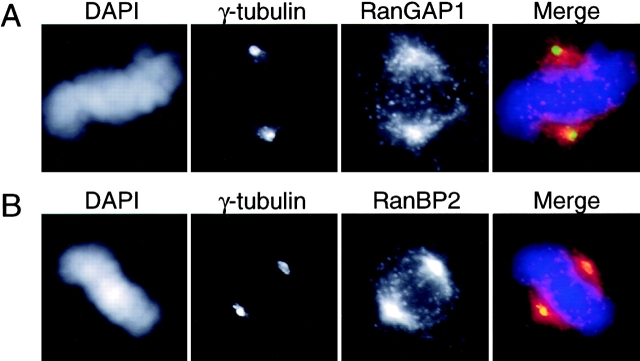
We wished to determine more precisely where RanGAP1 localized on the spindle. In initial experiments, we stained digitonin-permeabilized HeLa cells with antibodies against RanGAP1 (Matunis et al., 1996) and either anti–β-tubulin antibodies to visualize spindle MTs (Fig. 1) or anti–γ-tubulin antibodies to visualize centrosomes (Fig. 2 A). The cells were also stained with the DNA dye DAPI. Mitotic cells were identified through their chromosome morphology and photographed. We found that RanGAP1 associates with the body of the spindle, but that this association did not extend into the immediate vicinity of spindle poles: RanGAP1 extensively overlapped with β-tubulin, except at the poles (Fig. 1), but RanGAP1 did not colocalize extensively with γ-tubulin (Fig. 2 A). A second site of RanGAP1 staining was a series of prominent dots corresponding to the plus ends of the kinetochore MTs visualized by anti-β-tubulin staining (Fig. 1). Repetition of this experiment with two other independently generated antibodies against RanGAP1 (Mahajan et al., 1998; Saitoh et al., 1997) gave essentially identical results.
Figure 1.
RanGAP1 localizes to spindles in a microtubule-dependent manner. HeLa cells were untreated or treated with 2 μM nocodazole for 2 h and then permeabilized with digitonin and fixed in formaldehyde. The cells were stained for β-tubulin (green) and RanGAP1 (red) using specific antibodies and counterstained with DAPI (blue) for DNA.
Figure 2.
RanGAP1 does not localize at the immediate vicinity of spindle poles. HeLa cells were stained with either anti-RanGAP1 or anti-RanBP2 and anti–γ-tubulin antibodies after permeabilization and fixation to observe the localization of these proteins at spindle poles. (A) γ-Tubulin (green) and RanGAP1 (red), as recognized by corresponding antibodies. (B) γ-Tubulin (green), RanBP2 (red), and DNA were visualized by staining with DAPI (blue).
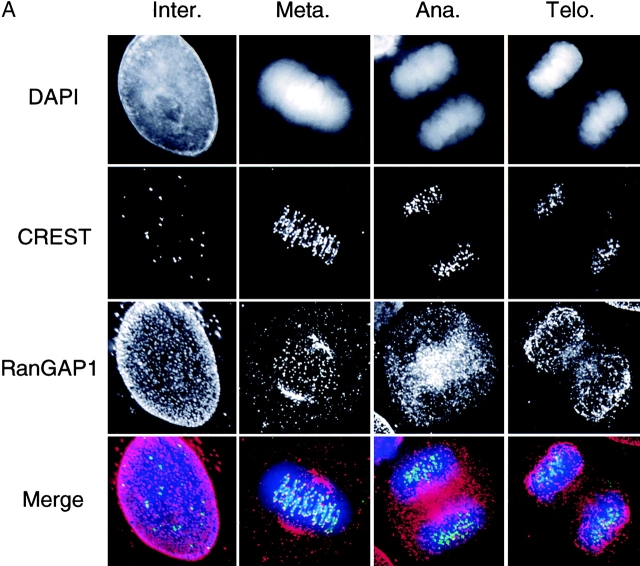
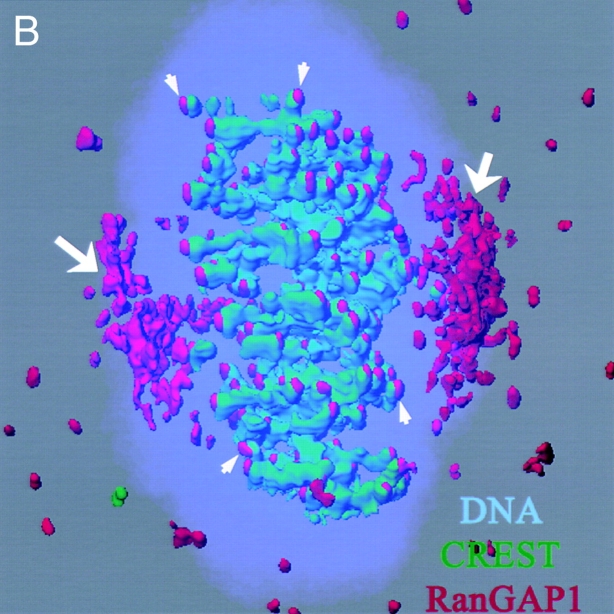
For a more precise examination of the second RanGAP1 population, we performed coimmunofluorescence using anti-RanGAP1 antibodies and CREST sera, which contain human autoantibodies against centromeric proteins (Fig. 3 A). We observed that RanGAP1 and CREST staining were distinct in interphase, when RanGAP1 associated with pores and CREST antigens resided within the nucleus. After nuclear envelope breakdown, these two patterns came into juxtaposition. Their relative distribution was particularly striking in metaphase: as chromosomes aligned on the metaphase plate, each dot of CREST staining was associated with a single dot of RanGAP1 staining, located immediately adjacent to and poleward from the centromere (Fig. 3 B). RanGAP1 and CREST staining continued to be closely associated through anaphase. This close association became much less apparent when cells entered telophase, as the nuclear envelope reformed and RanGAP1 again associated with pores. These observations suggest that RanGAP1 is localized on or very near kinetochores through much of mitosis.
Figure 3.
RanGAP1 localization through mitosis. Deconvolution images of HeLa cells at different stages of mitosis were taken and processed. The cells were stained with DAPI (blue), CREST sera (green), and anti-RanGAP1 antibodies (red). (A) Projection images constructed from the optical sections of cells at different stages of mitosis. (B) Model generated using the Imaris/Surpass software package showing the association of RanGAP1 with the spindle in a metaphase cell. RanGAP1 associated with spindle body is indicated with arrows. Arrowheads indicate kinetochore-associated RanGAP1.
To determine whether RanGAP1 depended upon MTs for its targeting to kinetochores, we examined cells that had been treated with 2 μM nocodazole for 2 h (at which time the MTs are almost completely disrupted) before digitonin permeabilization, fixation, and staining (Fig. 1). In this case, there was no digitonin-resistant RanGAP1 staining in mitotically arrested cells with condensed chromosomes. The lack of such staining suggests either that the kinetochore sites to which RanGAP1 is targeted contain MTs, or that the binding site for RanGAP1 is unstable when microtubules are disrupted.
It has been widely suggested that gradients of Ran-GTP could help to orient the mitotic spindle in metazoans by locally stabilizing MTs in the vicinity of chromosomes, based upon the localization of Ran's nucleotide exchange factor, RCC1 (for review see Dasso, 2001; Kahana and Cleveland, 2001). While this is an attractive model, it is likely that the true situation is more complicated and that the location of other Ran pathway components can play a role in regulating Ran-GTP distribution. In particular, our observations suggest that RanGAP1 localization on the spindle and kinetochores could locally alter the concentrations of Ran-GTP available to mediate MT stabilization. It was therefore of interest to determine how RanGAP1 was targeted to the spindle and how such an association might be regulated.
SUMO-1 conjugation targets RanGAP1 to the spindle during mitosis
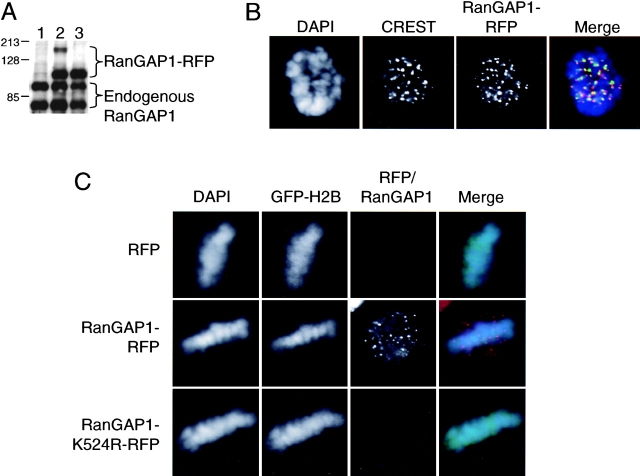
We wished to determine whether SUMO-1 plays a role in RanGAP1's targeting to the spindle during mitosis. To do this, we transfected a pDsRed1-N1 vector expressing a fusion of human RanGAP1 and the red fluorescent protein (RFP) (RanGAP1-RFP) into HeLa cells. In all cases, we simultaneously cotransfected the cells with a GFP–histone H2B marker to unambiguously identify the transfected cells. We performed several experiments to verify that RanGAP1-RFP serves as a reliable indicator of RanGAP1 behavior and localization: RanGAP1-RFP was subject to modification by SUMO-1 (Fig. 4 A, lane 2) and localized in a manner that was indistinguishable from endogenous RanGAP1 in interphase (unpublished data). It also localized in a pattern resembling the endogenous RanGAP1 during mitosis (Fig. 4 B), suggesting that the targeting of RanGAP1 to spindles was not compromised by fusion to the RFP moiety.
Figure 4.
SUMO-1 conjugation targets RanGAP1 to the spindle during mitosis. (A) Whole cell extracts from HeLa cells transfected with pDsRed1-N1 (lane 1), pDsRed1-RanGAP1-WT (lane 2), and pDsRed1-RanGAP1-K524R (lane 3) were analyzed by Western blot using polyclonal anti-RanGAP1 antibodies. (B) A mitotic cell showing the localization pattern of RanGAP1-RFP (red) and CREST staining (green). DNA is stained using DAPI (blue). Note that RanGAP1-RFP localization is similar to untagged endogenous RanGAP1 (Figs. 1 and 2). (C) HeLa cells were transfected with pDsRed1-N1 (RFP) or pDsRed1-RanGAP1-WT (RanGAP1-RFP) or pDsRed1-RanGAP1-K524R (RanGAP1-K524R-RFP). pBOS-H2BGFP (GFP-H2B) was used as a transfection marker (Kanda et al., 1998). Cells were permeabilized, fixed, and stained for DNA using DAPI (blue) and observed for GFP-H2B (green) and RanGAP1-RFP (red). Note that RFP and RanGAP1-K524R-RFP do not show any specific signal on spindle or kinetochores. (D) HeLa cells were transfected with pDsRed1-RanGAP1-WT (top) and pDsRed1-RanGAP1-K524R (bottom) and fixed without digitonin permeabilization. Immunofluorescence was performed with anti–β-tubulin antibody (green) and the cells were stained with DAPI (blue). Mutant and wild-type RanGAP1-RFP are shown in red. Note that cells expressing RanGAP1-RFP show distinct dots of RanGAP1 near the positive ends of microtubules, but these foci are absent in RanGAP1-RFP-K524R–transfected cells.
Notably, we observed that kinetochore-associated RanGAP1-RFP was brighter in comparison to the microtubule-associated RanGAP1-RFP signal than would have been anticipated from immunofluorescence experiments (compare Fig. 1 to Fig. 4 C). While there are several possible explanations for this difference, we suspect that kinetochore-associated RanGAP1 was under represented by immunofluorescence due to restricted accessibility to antibodies. Although we have not quantitated the amounts of RanGAP1 on kinetochores and other parts of spindles, it is our feeling that kinetochores are likely to be the most concentrated site of RanGAP1 localization during metaphase.
Mutation of a single acceptor lysine in mouse RanGAP1 (RanGAP1-K526R) can abolish interphase RanGAP1 conjugation to SUMO-1 and simultaneously disrupt its association with nuclear pores (Matunis et al., 1998). We made a homologous mutation in the human RanGAP1 fusion protein (RanGAP1-K524R-RFP), and observed that it abolished SUMO-1 conjugation (Fig. 4 A, lane 3). We identified transfected mitotic cells by the presence of the GFP–histone H2B marker and examined the localization of RanGAP1-K524R-RFP. In all nonpermeabilized cells in which the GFP–histone H2B marker was visible, we also saw clear expression of RanGAP1-K524R-RFP (unpublished data). As predicted, we found that RanGAP1-K524R-RFP did not associate with pores in digitonin-permeabilized interphase cells (unpublished data).
Notably, RanGAP1-K524R-RFP was also strikingly absent from spindles and kinetochores in digitonin-permeabilized mitotic cells containing GFP-histone H2B (n = 50) (Fig. 4 C). It is unlikely that this absence reflects poor expression or instability of RanGAP1-K524R-RFP, since 100% of the cells with the GFP-histone H2B cotransfection marker showed strong RanGAP1-K524R-RFP fluorescence when examined without digitonin permeabilization (unpublished data). Furthermore, the overall expression of mutant and wild-type RanGAP1-RFP appeared similar when fixation was performed without prior permeabilization (Fig. 4 D). Staining of mitotic cells expressing either RanGAP1-RFP or RanGAP1-K524R-RFP with antitubulin antibodies showed that these proteins did not disrupt spindle formation (Fig. 4 D). Thus, the failure of the mutant RanGAP1 to bind to spindles and kinetochores is most likely due to its inability to undergo SUMO-1 conjugation rather than some indirect effect on spindle structure. Remarkably, we observed that we could detect some elevation in the RanGAP1-RFP levels at kinetochores even in cells that had not been digitonin permeabilized (Fig. 4 D), although the distribution of RanGAP1-RFP on the remainder of the spindle was difficult to discern because of the high background from soluble RanGAP1-RFP. This enrichment was entirely absent in the cells expressing RanGAP1-K524R-RFP. Together, these observations strongly suggest that SUMO-1 conjugation is not only essential for targeting RanGAP1 to nuclear pores, but also is required for RanGAP's association with mitotic spindles and kinetochores.
Our findings suggest that a major role of SUMO-1 conjugation to RanGAP1 may be the spatial regulation of the Ran pathway during open mitosis. Although Ran plays a role in spindle assembly in fission yeast (Fleig et al., 2000), there is no general dissipation of the Ran-GTP gradient at mitosis in budding or fission yeast because they undergo a closed mitosis during which the nuclear envelope does not break down (for review see Sazer and Dasso, 2000). Moreover, Ran does not relocalize to the cytoplasm at any point in the yeast cell cycle, nor does a substantial fraction of RanGAP1 enter the nucleus. It is thus difficult to imagine how a gradient of Ran-GTP might be established in yeast nuclei where spindle formation occurs. It appears likely that there will be fundamental differences between yeast and metazoans in the way Ran functions during mitosis (Sazer and Dasso, 2000). The restriction of SUMO-1 conjugation of RanGAP1 to metazoans may therefore reflect its particular role in the localization of RanGAP1 during spindle assembly in the absence of an intact nuclear envelope.
RanBP2 colocalizes with RanGAP1 throughout the cell cycle
In Xenopus egg extracts, RanGAP1–SUMO-1 remains associated with RanBP2 in mitosis (unpublished data), raising the question of whether RanBP2 might also be targeted to the spindle. To evaluate this possibility, we performed immunofluorescent staining of digitonin-permeabilized cells with antibodies against RanGAP1 and RanBP2 (Fig. 5). As above, the cells were also stained with the DNA dye DAPI. We found that RanBP2 associated with mitotic spindles in a pattern that extensively overlapped with the staining of RanGAP1. This colocalization was particularly evident at the kinetochores. Notably, however, RanBP2 staining extended beyond RanGAP1 staining to regions near spindle poles that contained γ-tubulin (Fig. 2 B). As with RanGAP1, RanBP2 staining was abolished by prior treatment of the mitotic cells with nocodazole (unpublished data). Performance of this experiment with two independently generated antibodies against RanBP2 (Yokoyama et al., 1995; Saitoh et al., 1997) gave essentially identical results. Our observations indicate that RanBP2 is targeted to the same sites on mitotic spindles as RanGAP1, as well as to additional distinct sites.
Figure 5.
RanBP2 colocalizes with RanGAP1 in mitosis. HeLa cells were stained with DAPI (blue), anti-RanBP2 (green), and anti-RanGAP1 (red). Note the strong staining of spindle poles by anti-RanBP2 antibodies but not by anti-RanGAP1.
Given that RanGAP1 and RanBP2 form a tight complex throughout the cell cycle in Xenopus egg extracts (Saitoh et al., 1997; unpublished data), the simplest interpretation of these results would be that these proteins associate with MTs and kinetochores as a complex. Consistent with this notion, we observed that a short COOH-terminal region of human RanGAP1 (amino acids 401–587), which corresponds to the region in mouse RanGAP1 that is sufficient for SUMO-1 conjugation and RanBP2 association in interphase (Matunis et al., 1998), could associate with spindle and kinetochores in mitosis (unpublished data). The RanGAP1–RanBP2 complex may be less stable near spindle poles, such that RanGAP1 is released from the MTs in this region while RanBP2 remains associated. This model for RanGAP1 association with the spindle makes two predictions: first, the dissociation of the RanGAP1 from RanBP2 occurs in response to some factor(s) localized near spindle poles. One attractive possibility would be that this complex becomes destabilized through loss of SUMO-1 conjugation to RanGAP1. Second, RanBP2 can bind to sites on the spindle in a manner that is independent of RanGAP1. It is possible that RanBP2 could associate with MTs directly or that it is targeted to MTs through binding to other spindle-associated proteins.
Several pore-associated proteins have recently been reported to associate with mitotic spindles, including hNup133, hNup107 (Belgareh et al., 2001), PBC68 (Theodoropoulos et al., 1999), and Rae1 (Wang et al., 2001). Conversely, the hMad1 and hMad2 proteins, which play essential roles in the spindle assembly checkpoint localize to the nuclear pore during interphase (Campbell et al., 2001). Together with our findings, these observations suggest a relationship between the nuclear pore and the spindle. Although the nature of this relationship remains to be demonstrated, it is attractive to speculate that an essential requirement for the same set of proteins in both interphase pores and mitotic spindles could enforce a reciprocal relationship between these two structures (i.e., the assembly of one structure would be mutually exclusive with the persistence of the other), and thereby structurally differentiate the interphase state from mitosis.
In summary, we have shown that RanGAP1 is targeted to kinetochores and mitotic spindles. This targeting is achieved through SUMO-1 conjugation, and may involve interactions with RanBP2. Our observations suggest that Ran-GTP gradients may be regulated in a complex fashion during mitosis, and that SUMO-1 modification of RanGAP1 may be used in metazoans extensively for this purpose.
Materials and methods
Reagents
Antibodies against human RanBP2 were a gift from T. Nishimoto (Kyushu University, Fukuoka, Japan). Monoclonal mouse anti-RanGAP1 antibodies were obtained from Zymed Laboratories. Polyclonal goat anti-RanGAP1 antibody was a gift from F. Melchior (Max Planck Institute for Biochemistry, Martinsried, Germany). Polyclonal guinea pig antibodies against Xenopus RanBP2 and polyclonal rabbit antibodies against Xenopus RanGAP1 were as described previously (Saitoh et al., 1997). CREST serum was a gift from I. Ouspeski (NCI, Bethesda, MD). Monoclonal anti–γ-tubulin antibodies were obtained from Sigma-Aldrich, and polyclonal anti–β-tubulin antibodies were from Santa Cruz Biotechnology, Inc. Unless otherwise specified, other reagents were obtained from Sigma-Aldrich.
Constructs
Human RanGAP1 cDNA cloned into pET 23a was a gift from V. Gerke (University of Munster, Munster, Germany). For transient expression in mammalian cells, the human RanGAP1 coding region was PCR-amplified and sub-cloned into the SmaI site of pDsRed1-N1 (CLONTECH Laboratories, Inc.). The K524R mutation was introduced by a PCR-based procedure using the mutant primer and Platinum Taq HiFi (GIBCO BRL/Life Technologies) and confirmed by DNA sequencing. pBOS-H2BGFP vector was from BD PharMingen.
Cell culture and immunofluorescence microscopy
HeLa cells were grown in DME supplemented with 10% FBS (Gemini Bio-Products) in the presence of antibiotics in a humidified incubator at 37 C. HeLa cells (∼40% confluent) were transfected with pDsRed1-N1 (RFP), pDsRed1-RanGAP1-WT (RanGAP1-RFP), or pDsRed1-RanGAP1-K534R (RanGAP1-KR-RFP) by electroporation. pBOS-H2BGFP (GFP-H2B) was cotransfected at 20 times less concentration to identify the transfected cells. The cells were processed for immunofluorescence microscopy after 2–3 d as described below. GFP- or RFP-tagged proteins were visualized using appropriate filters.
In all figures except Fig. 4 D, HeLa cells were permeabilized for immunofluorescence with 0.005% digitonin in transport buffer (110 mM KOAc, 20 mM HEPES, pH 7.3, 2 mM Mg(OAc)2, 0.5 mM EGTA, 2 mM DTT, 1 μg/ml each of leupeptin, pepstatin, and aprotinin) for 4 min and fixed with 3.7% formaldehyde in PBS for 15 min. In Fig. 4 D, transfected HeLa cells were fixed with 3.7% formaldehyde in PBS and permeabilized using 0.2% Triton X-100 in PBS. In all cases, the cells were incubated with monoclonal anti-RanGAP1 antibodies (1:200 dilution), polyclonal anti-RanBP2 antibodies (1:500), polyclonal anti–β-tubulin antibodies (1:100), monoclonal anti–γ-tubulin antibodies (1:100), or CREST (1:10,000) sera as indicated, followed by appropriate fluorescent secondary antibodies. Samples were counterstained with DAPI and mounted in Vectashield antifade mounting medium (Vector Laboratories). Slides were examined with a ZEISS Axioskop fluorescence microscope and images were collected and analyzed with Openlab software.
Deconvolution microscopy
Cells were imaged on a DeltaVision microscope system (Applied Precision). This system consists of an inverted microscope IX70 (Olympus) with a 1.35 NA 100× objective and FITC, Rhodamine, and Cy5 filter sets, a Photometrics CH350 12-bit camera (Photometrics) with a KAF1400 chip, and a UNIX-based Silicon Graphics O2 workstation with SoftWoRx software installed. Camera wells were not binned, yielding a pixel size of 0.07 μm in x and y. z steps were set to 0.07 μm, yielding cubic voxels. Image sizes in x, y, and z were 384 × 384 × 140–200. Images were deconvolved with the SoftWoRx software package (Applied Precision) using Decon3d with the default settings and experimental PSF. Three-dimensional models of the nuclei were built with the Imaris/Surpass software package (Bitplane AG, Zurich, Switzerland). An intensity threshold was set in each color to define objects that were then surface rendered. Images for display (Fig. 3 A) were constructed by projecting the sum of the optical sections using Openlab software.
Acknowledgments
We would like to thank Y. Azuma and A. Arnaoutouv, B. Quimby, and M. Cavenagh for helpful discussions and critical reading of the manuscript.
J. Joseph was supported by HFSP Research Grant RG0229/1999-M.
Footnotes
Abbreviations used in this paper: MT, microtubule; RFP, red fluorescent protein.
References
- Belgareh, N., G. Rabut, S.W. Bai, M. van Overbeek, J. Beaudouin, N. Daigle, O.V. Zatsepina, F. Pasteau, V. Labas, M. Fromont-Racine, et al. 2001. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J. Cell Biol. 154:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, M.S., G.K. Chan, and T.J. Yen. 2001. Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J. Cell Sci. 114:953–963. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., G. Guarguaglini, O.J. Gruss, A. Segref, E. Karsenti, and I.W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 400:178–181. [DOI] [PubMed] [Google Scholar]
- Dasso, M. 2001. Running on Ran: nuclear transport and the mitotic spindle. Cell. 104:321–324. [DOI] [PubMed] [Google Scholar]
- Fleig, U., S.S. Salus, I. Karig, and S. Sazer. 2000. The fission yeast ran GTPase is required for microtubule integrity. J. Cell Biol. 151:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607–660. [DOI] [PubMed] [Google Scholar]
- Kahana, J.A., and D.W. Cleveland. 2001. Cell cycle. Some importin news about spindle assembly. Science. 291:1718–1719. [DOI] [PubMed] [Google Scholar]
- Kanda, T., K.F. Sullivan, and G.M. Wahl. 1998. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8:377–385. [DOI] [PubMed] [Google Scholar]
- Mahajan, R., C. Delphin, T. Guan, L. Gerace, and F. Melchior. 1997. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 88:97–107. [DOI] [PubMed] [Google Scholar]
- Mahajan, R., L. Gerace, and F. Melchior. 1998. Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J. Cell Biol. 140:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis, M.J., E. Coutavas, and G. Blobel. 1996. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135:1457–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis, M.J., J. Wu, and G. Blobel. 1998. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J. Cell Biol. 140:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior, F. 2000. SUMO—nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16:591–626. [DOI] [PubMed] [Google Scholar]
- Saitoh, H., R. Pu, M. Cavenagh, and M. Dasso. 1997. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc. Natl. Acad. Sci. USA. 94:3736–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer, S., and M. Dasso. 2000. The ran decathlon: multiple roles of Ran. J. Cell Sci. 113:1111–1118. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos, P.A., H. Polioudaki, M. Koulentaki, E. Kouroumalis, and S.D. Georgatos. 1999. PBC68: a nuclear pore complex protein that associates reversibly with the mitotic spindle. J. Cell Sci. 18:3049–3059. [DOI] [PubMed] [Google Scholar]
- Wang, X., J.R. Babu, J.M. Harden, S.A. Jablonski, M.H. Gazi, W.L. Lingle, P.C. de Groen, T.J. Yen, and J.M. van Deursen. 2001. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. J. Biol. Chem. 276:26559–26567. [DOI] [PubMed] [Google Scholar]
- Yokoyama, N., N. Hayashi, T. Seki, N. Pante, T. Ohba, K. Nishii, K. Kuma, T. Hayashida, T. Miyata, U. Aebi, et al. 1995. A giant nucleopore protein that binds Ran/TC4. Nature. 376:184–188. [DOI] [PubMed] [Google Scholar]