Abstract
β-Amyloid peptides (Aβ) are the major component of plaques in brains of Alzheimer's patients, and are they derived from the proteolytic processing of the β-amyloid precursor protein (APP). The movement of APP between organelles is highly regulated, and it is tightly connected to its processing by secretases. We proposed previously that transport of APP within the cell is mediated in part through its sorting into Mint/X11-containing carriers. To test our hypothesis, we purified APP-containing vesicles from human neuroblastoma SH-SY5Y cells, and we showed that Mint2/3 are specifically enriched and that Mint3 and APP are present in the same vesicles. Increasing cellular APP levels increased the amounts of both APP and Mint3 in purified vesicles. Additional evidence supporting an obligate role for Mint3 in traffic of APP from the trans-Golgi network to the plasma membrane include the observations that depletion of Mint3 by small interference RNA (siRNA) or mutation of the Mint binding domain of APP changes the export route of APP from the basolateral to the endosomal/lysosomal sorting route. Finally, we show that increased expression of Mint3 decreased and siRNA-mediated knockdowns increased the secretion of the neurotoxic β-amyloid peptide, Aβ1-40. Together, our data implicate Mint3 activity as a critical determinant of post-Golgi APP traffic.
INTRODUCTION
The hallmark of Alzheimer's disease is the presence in brain of plaques that contain Aβ peptides, formed by the result of proteolytic processing of the β-amyloid precursor protein (APP). APP is a ubiquitous, single-pass transmembrane protein of unknown function that is highly expressed in brain. Processing involves the sequential actions of α- or β- plus γ-secretases, each of which are found in multiple cellular locations (Kuentzel et al., 1993; Selkoe, 1998; Yan et al., 2001). Although processing may occur at any step, the trans-Golgi network (TGN) is the earliest site at which APP processing is thought to commence, and APP is internalized from the cell surface to endosomal compartments where γ-secretase also acts (Selkoe et al., 1996; Greenfield et al., 1999; Lah and Levey, 2000; Bagshaw et al., 2003).
The C-terminal domain of APP contains the tyrosine-based sorting motif, YENPTY, which is conserved across species and a determinant of APP traffic (Perez et al., 1999; Bonifacino and Traub, 2003; King et al., 2004). Such sorting motifs function by binding specific adaptors to facilitate their selective import into budding carriers and ensure specificity in cargo selection and coat recruitment. The highly conserved phosphotyrosine binding (PTB) domains in all three Mint proteins have been shown previously to bind directly to the YENPXY motif of APP (Borg et al., 1996, 1998; Zhang et al., 1997; Sastre et al., 1998; Tanahashi and Tabira, 1999; Tomita et al., 1999; Ho et al., 2002; Araki et al., 2003). Other PTB domain containing proteins have similarly been shown to bind APP, including the Fe65 (Sabo et al., 1999) family, Dab2 (Howell et al., 1999), JIP1b (King et al., 2004), and ARH (Noviello et al., 2003). These proteins can each impact APP traffic and processing in a variety of ways, although where they act is unknown (Selkoe, 1998; Sabo et al., 1999; Lau et al., 2000; King et al., 2003).
Mint1/X11α/APBA1, Mint2/X11β/APBA2/X11-like, and Mint3/X11γ/APBA3/X11-like 2 comprise a family of proteins that share highly conserved PTB and dual PDZ domains, with divergent N termini. We have shown previously that all three Mints bind to the activated form (GTP-bound) of ADP-ribosylation factor (Arf) via both the PTB and second postsynaptic density 95/disc-large/zona occludens (PDZ) domains (Hill et al., 2003) and that Mint3 localizes to late Golgi/TGN membranes in several cell lines (Biederer and Sudhof, 2000; Hill et al., 2003). Because Mint1 and Mint2 are fairly specifically expressed in neurons, they have been the focus of studies of Mints in brain. However, like APP, Mint3 is ubiquitously expressed, including in the brain, and the Mint3 message has been specifically localized to neurons in mice (Okamoto et al., 2001). Mint1 is found in a heterotrimeric complex with Cask and Velas, and it is involved in synaptic transmission at the cell surface (Butz et al., 1998). Mint2 shares with Mint1 the Munc18 interacting domain, which is lacking in Mint3. Mints lack a transmembrane domain or any defined lipid binding domains so their actions to alter traffic of transmembrane proteins is thought to result from specific protein interactions at the cytosolic face of membranes. We hypothesized that traffic of APP requires the actions of Mint3, acting as an Arf-dependent adaptor, to aid in the formation of specific transport carriers (Hill et al., 2003). The current studies support this hypothesis and also implicate Mint2 in post-Golgi traffic of APP in neurons.
MATERIALS AND METHODS
Cell Culture and Lentiviral Infections
The human neuroblastoma SH-SY5Y, HeLa, and human embryonic kidney (HEK)293 cell lines were grown in DMEM medium supplemented with 10% fetal bovine serum and 100 μg/ml penicillin and streptomycin. Cells that overexpress human Mint3, human APP695-Swe or APP695-Swe, and Mint3 were generated by infecting SH-SY5Y cells (5 × 106/well) with the appropriate lentivirus at 1–4 × 109 IU/ml. Infected cell lines were grown under the same conditions as controls, and they did not show any changes in cellular morphology, in comparison with noninfected cells.
Antibodies, Plasmids, and Lentiviruses
The antibody used for immunoblotting of APP in this study was a rabbit polyclonal, directed against the C-terminal 15 residues, and it was purchased from Synaptic Systems (Goettingen, Germany). We determined that APP770 1 and APP695 were each expressed in SH-SY5Y cells and migrated in 7.5% polyacrylamide SDS gels to positions corresponding to ∼148 and ∼120 kDa, respectively, based upon previously published data (Buxbaum et al., 1990, 1998; Cordy et al., 2003). Antibody APP369 (a gift from Sam Gandy, Thomas Jefferson University, Philadelphia, PA) is a rabbit polyclonal raised against the C terminus of APP, and it was used for immunofluorescent staining of APP. Other antibodies used in this study included the mouse monoclonals to Mint3, GM130, TGN38, early endosome antigen 1 (EEA1), syntaxin 6, γ-adaptin (a subunit of the adaptor protein [AP]-1 adaptin complex), and α-adaptin (a subunit of the AP-2 adaptin complex), each purchased from BD Biosciences Transduction Laboratories, Lexington, KY). Other commercially available antibodies included monoclonals directed against the cation-independent mannose 6-phosphate receptor (CI-M6PR; Affinity BioReagents, Golden, CO), clathrin heavy chain (Affinity BioReagents), KDEL-receptor (Stressgen, Ann Arbor, MI), Na+/K+ ATPase (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), δ-adaptin (a subunit of the AP-3 adaptin complex; Developmental Studies Hybridoma bank), transferrin receptor (TfR; Zymed Laboratories, South San Francisco, CA), Fe65 (Upstate Cell Signaling Solutions, Charlottesville, VA), and polyclonal antibodies to cathepsin D (Dako North America, Carpinteria, CA), Mint1 (Sigma-Aldrich, St. Louis, MO), and Mint3 (Santa Cruz Biotechnology, Santa Cruz, CA). Both mouse monoclonal (9E10) and rabbit polyclonal antibodies (Upstate Cell Signaling Solutions) to the c-myc epitope were used as controls. Rabbit polyclonal antisera directed against Arf3 (Cavenagh et al., 1994), GGA1 (Boman et al., 2000), and GGA2 and GGA3 (McKay and Kahn, 2004) were generated and characterized previously in our laboratory. A polyclonal antibody to Mint2 (UT-69) and Rab 6 were the generous gifts from Toshiharu Suzuki (University of Tokyo, Japan) and Joachim Kremerskothen (University of Münster, Münster, Germany), respectively.
The open reading frame of human Mint3 or APP was cloned in place of the green fluorescent protein (GFP) sequence in the pFUGW transfer vector (Lois et al., 2002). A single hemagglutinin epitope was introduced at the N terminus of Mint3 by polymerase chain reaction (PCR), and the sequence of the PCR product was confirmed by DNA sequencing. To produce viruses, HEK293T cells at 60–70% confluence were cotransfected with pFUGW-derivatives (e.g., containing the human Mint3 open reading frame) or pCMO2 (APP695-Swe), the Δ8.9 HIV-1 packaging vector, and pVSVG envelope glycoprotein in a ratio of 4:3:2. After overnight incubation, 20 ml of fresh medium was added to each dish and conditioned for 48–72 h. Medium was collected, and debris was removed by centrifugation at 800 × g, before supernatants were filtered through 0.45-μm membranes. Supernatants were then spun for 2 h at 16°C in a Beckman SW28 rotor (Beckman Coulter, Fullerton, CA) at 20,000 rpm. The supernatant was discarded, and the viral pellet resuspended in sterile phosphate-buffered saline (PBS). Virus particles were collected by centrifugation in a Beckman SW41 rotor at 28,000 rpm for 2 h at 16°C. The final viral pellet was resuspended in 150 μl of PBS and stored in 10-μl aliquots at −80°C. Based on GFP expression in HEK293 cells infected with serial dilutions of lentivirus, this protocol consistently yielded a viral titer of 1–2 × 109 infectious units (IU)/ml.
Subcellular Fractionation and Vesicle Purification
Cells were grown in 20- × 15-cm dishes to ∼95% confluence (∼3–5 × 108 cells), before harvesting in PBS/EDTA and collection by sedimentation at 800 × g for 5 min. All subsequent steps were performed at 4°C. Cells were washed once and lysed in lysis buffer (38 mM potassium aspartate, 38 mM potassium glutamate, 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS)-KOH, pH 7.2, 5 mM reduced glutathione, 5 mM sodium carbonate, 2.5 mM magnesium sulfate, and 2 mM EGTA) supplemented with anti-protease mixture (Sigma-Aldrich) by using a cell cracker with a 25.4-μm ball bearing (Clift-O'Grady et al., 1998). The homogenate was sedimented for 5 min at 1000 × g to obtain pellet P1 and the postnuclear supernatant, which was further fractionated by centrifugation at 24,000 × g for 15 min to generate pellet P2 and the high-speed supernatant (S2). S2 was then loaded on top of 15–45% sucrose gradients prepared in 20 mM MOPS-KOH, pH 7.2, and spun at 100,000 × g for 1 h in a SW41Ti rotor. When different cell lines were directly compared, S2 supernatants were normalized for protein content before loading onto sucrose gradients. Sixteen fractions (700 μl) were collected from the top, and they were then analyzed by immunoblot. Pooled fractions were brought to 30% Optiprep, and fresh anti-protease mixture was added before being loaded at the bottom of a SW55Ti tube and then overlaid consecutively with 25% (2 ml) and 10% (1 ml) Optiprep, all prepared in 20 mM MOPS-KOH, pH 7.2. Vesicles were allowed to “float up” by spinning these gradients for 3 h at 250,000 × g in the SW55Ti rotor before fractions (300 μl) were collected from the top. Equal volumes from each fraction were analyzed by immunoblot.
Immunoisolation of Vesicles and Immunogold Labeling
Magnetic beads (Dynal Biotech, Oslo, Norway), coated with either anti-mouse (M480) or anti-rabbit (M280) immunoglobulin (IgG, were incubated with either mouse monoclonal antibodies to Mint3 or rabbit polyclonal antibody to APP, respectively in PBS/5% bovine serum albumin (BSA) for 2 h at room temperature, according to the Salazar et al. (2005). Controls included substitution of either mouse monoclonal or rabbit polyclonal c-myc antibodies. Antibody-coated magnetic beads were washed three times for 5 min each with PBS/5% BSA before incubating with light vesicle fractions from Optiprep gradients at 4°C for 4 h in the presence of the complete protease inhibitor mixture. Vesicles captured on magnetic beads were fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate, pH 7.2, and processed for transmission electron microscopy at the Emory Electron Microscopy Core Facility (Atlanta, GA). For immunogold labeling, vesicles isolated using mouse monoclonal Mint3 antibody were incubated sequentially with rabbit anti-APP antibody for 2 hours followed by goat anti-rabbit IgG, conjugated to 10-nm gold (British Bio Cell International, Cardiff, South Glamorgan, United Kingdom) for 1 h at 4°C before fixing in 2.5% glutaraldehyde in phosphate buffer at 4°C overnight.
Small Interference RNA (siRNA)
Knockdown of the expression of Mint3 was carried out in HeLa or HEK293 cells, as described previously (Volpicelli-Daley et al., 2005), by using pSUPER-based vectors (Brummelkamp et al., 2002). Four sequence-independent constructs were generated toward each target RNA and the two constructs that demonstrated the greatest effectiveness in decreasing cellular levels of the target protein were used. Only those phenotypes present for both of the constructs for any one target were considered specific, as a guard against off-target effects. Target sequences were determined with the help of on-line search algorithms at the Dharmacon RNA Technologies (Boulder, CO) and BD Biosciences (San Diego, CA) websites and BLAST searches of the National Center for Biotechnology Information databases found no other human cDNAs identical to the target sequences. Time course and plasmid concentration dependence studies were performed to determine the optimal conditions for protein depletion.
For immunofluorescence studies using Mint3 knockdowns, HeLa cells were transfected with 5 μg of pSUPER-based plasmid using Lipofectamine 2000, according to the manufacturer's recommendations. The next day, cells were replated at lower cell density.
Immunofluorescence
Cells grown on polylysine-coated coverslips were fixed with 2% paraformaldehyde, rinsed, and permeabilized with blocking buffer (0.05% saponin, 5% normal goat serum, and 1% bovine serum albumin in phosphate-buffered saline). Cells were incubated in the following primary antibodies diluted in blocking buffer overnight at 4°C: Mint3 (1:200; BD Biosciences Transduction Laboratories), APP369 (1:1000), EEA (1:1000; BD Biosciences Transduction Laboratories), syntaxin 6 (1:200; BD Biosciences Transduction Laboratories). Rinses were performed using 0.05% saponin/phosphate-buffered saline. Goat anti-rabbit or mouse secondary antibodies (Invitrogen, Carlsbad, CA) conjugated to Alexa-488 or Alexa-594 (1:500) were diluted in blocking buffer. Cells were mounted with Prolong antifade mounting media (Invitrogen). Confocal microscopy was performed on an LSM 510 (Carl Zeiss, Thornwood, NY) as described previously (Volpicelli-Daley et al., 2005).
Immunohistochemistry
Blocks of hippocampus and cerebellum from control human brain were fixed for 24–48 h in 4% paraformaldehyde, cryoprotected in 30% sucrose, and frozen. Frozen blocks were cut into 50-μm sections. Sections were treated with hydrogen peroxide, washed in Tris buffer, blocked with normal serum, and incubated with anti-Mint3 polyclonal antibodies (1:100; Santa Cruz Biotechnology) overnight at 4°C. On day 2, sections were incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), followed by avidin-biotin-peroxidase complex (Elite ABC kit; Vector Laboratories). Color development was performed with 3,3′-diaminobenzidine. Control sections incubated without primary antibody or with primary antibody that was previously incubated with purified recombinant Mint3 (100 μg) showed negligible staining.
Quantitation of Cells Exhibiting Enlarged Puncta and Colocalization of Proteins in Fixed Cells
Cells were cultured, fixed, and developed as described above. MetaMorph imaging system software (Molecular Devices, Sunnyvale, CA) was used to quantify the extent of colocalization on unprocessed images as described previously (Volpicelli et al., 2001). Scoring of cells was performed on an LSM510 confocal microscope (Carl Zeiss). In those cells in which enlarged (> 0.5-μm) punctate staining was evident, the number of large puncta per cell was scored. Cells were scored as having zero, one to four, or more than four large puncta.
Assaying the Post-Golgi Traffic of APP
Cells were transfected with pSUPER-based plasmids and plated onto coverslips. Two days later, they were transfected with the plasmids directing expression of human FLAG-tagged APP (pcDNA3-FLAG-APP695) or the APPΔ681-690 mutant (pcDNA3-FLAG-APP695Δ681-690), deleted for residues 681-690 (681GYENPTYKFF690) near the C terminus of the human protein (gift from T. Suzuki, Hokkaido University, Japan) (Taru and Suzuki, 2004). This mutant has previously been shown to lose binding to Mint2 in yeast two-hybrid (Tomita et al., 1999) and coimmunoprecipitation (Taru and Suzuki, 2004) assays, and the effect of Mint2 on APP phosphorylation in cells (Taru and Suzuki, 2004). The cells were rinsed with fresh media, and then incubated at 37°C for 16 h. Cells were then incubated at 19.5°C for 4 h with addition of 100 μg/ml cycloheximide for the last 2 h, before release by incubation at 37°C for up to 90 min before fixation and staining. For vesicle fractionation, SH-SY5Y-APP695-Swe cells were incubated at 19.5°C for 4 h before switching to 37°C for 15 min. Cells were processed by homogenization and fractionation immediately, as described above.
Determination of Secreted Aβ Levels
Overexpression of human Mints was achieved in HEK293 cells by transient transfection using the pBK vector, which drives expression off the cytomegalovirus promoter, and Aβ1-40 levels were determined in conditioned media as described previously (Offe et al., 2006). Empty vector was used as a control. Aβ1-40 levels were determined in conditioned medium after 48 h, beginning 24 h after transfection. For determination of Aβ1-40 production in Mint3-depleted cells, HEK293 cells were transfected with 5 μg of plasmid by using Lipofectamine 2000, as described above, and replated at lower densities the following day. On day 3, cells were retransfected to obtain the maximal knock down of Mint3 in these cells. In these studies, conditioned medium was collected for 48 h, beginning 24 h after the second transfection. Aβ1-40 levels were measured using the human β-amyloid1-40 enzyme-linked immunosorbent assay (ELISA) kit (BioSource, Camarillo, CA) according to the manufacturer's instructions and normalized to total cellular protein. Plates were read at 450 nm on a Spectra Max Plus plate reader (Molecular Devices). Experiments were performed in triplicate. Cell extracts were blotted for Mint1, Mint2, and Mint3 antibodies described above, to confirm expression of each protein.
RESULTS
Mint3 Is Expressed in Human Brain
The production of neurotoxic Aβ peptides is thought to contribute to the development of plaques and neuronal loss in brains of Alzheimer's patients. Because APP and the proteases involved in its processing are each transmembrane proteins that traverse the secretory and endocytic pathways, it has proven difficult to determine the site of Aβ production in cells. This is further complicated by the fact that only a minor fraction of the protein is cleaved to yield Aβ peptides. One approach to unravel these important questions and identify potential sites of intervention is to identify the regulators of APP traffic and develop models of the critical sorting steps. Among the adaptors known to bind APP and influence the rate of Aβ production are the Mint family of proteins. We previously identified all three Mints as Arf-dependent adaptors and hypothesized that one or more acts to regulate the traffic of APP (Hill et al., 2003). Because Mint1 and Mint2 are preferentially expressed in the brain they have become the focus of studies investigating their potential to regulate APP traffic and processing. In contrast, Mint3 has been largely ignored in such studies, at least in part due to the mistaken belief that it is not expressed in brain.
To assess the potential importance of Mint3 in APP traffic, we first asked whether it is expressed in human brain. Human brain tissue sections from neurologically normal control cases were prepared for free-floating immunohistochemistry by using a rabbit polyclonal antibody to human Mint3, as described under Materials and Methods. Granule cells of the dentate gyrus were rarely stained. In the cerebellar cortex, many Purkinje cells displayed strong immunoreactivity (Figure 1A). Strong neuronal immunostaining for Mint3 also was observed in some pyramidal neurons of the hippocampal field CA1 and in many neurons in the subiculum (Figure 1C). The immunostaining within hippocampal and cerebellar neurons often appeared as fine cytosolic puncta. Diffuse immunoreaction product was present in neuropil in the molecular layers of the hippocampus and cerebellar cortex, with comparatively little cellular staining of granule cell layers and white matter. Competition of the primary antibody with purified human Mint3 (Figure 1, B and D) or omission of primary antibody (data not shown) abolished the staining of Mint3 antibody in the Purkinje cells and pyramidal neurons, confirming the specificity of staining in these areas. This pattern of Mint3 expression in human brain is very similar to that described previously for Mint2, and it differs from that of Mint1 in mice (Nakajima et al., 2001). Thus, we show that Mint3 is expressed in human neurons and its pattern of expression is consistent with an adaptor capable of influencing APP traffic with potential relevance to Alzheimer's disease. To better develop models of APP–Mint interactions in cells we used human SH-SY5Y neuroblastoma cells, and we initially focused on Mint3 but also found that Mint2 behaves very similarly.
Figure 1.
Mint3 is expressed in neurons of normal human brains. Immunohistochemical staining for Mint3 in normal human brain. (A) In the cerebellar cortex, many Purkinje cells were strongly immunopositive, whereas brain sections in the same region of the cerebellar cortex incubated primary antibody that had been previously incubated with antigen (B) showed negligible staining. (C) In the subiculum, immunoreactive puncta fill the perikarya of pyramidal neurons and extend into apical dendrites. Prior competition with antigen (D) again eliminates the specific staining of Mint3. Bar, 50 μm.
Enrichment of APP Vesicles from SH-SY5Y Cell Homogenates
To determine the nature of protein adaptors used to selectively move APP within neuronally derived cells, we set out to isolate APP vesicles and examine the adaptor protein content. Whereas the majority of APP is predicted to be resident in the larger organelles (endoplasmic reticulum [ER], Golgi, TGN, endosomes, and plasma membrane), our goal was the enrichment of the small subpopulation of vesicles in transit between organelles. We chose SH-SY5Y cells because of their prior use in related studies and because they express APP and previously identified coat proteins at readily detectible levels. Because of the transient nature of adaptor association with membranes and carriers, we monitored the fractionation of the cargo, focusing on the small subset of APP that is predicted to reside in carriers in transit at any one time. The two longer variants of mature, glycosylated APP migrate together in 7.5% SDS polyacrylamide gels at ∼150 kDa, whereas APP695 migrates to a position corresponding to ∼115 kDa. Optimal conditions for enrichment of APP-containing vesicles included highly controlled cell lysis, differential centrifugation, and consecutive velocity and equilibrium density gradient fractionations, as described under Materials and Methods.
Cell lysis was achieved by shearing, via passage of cells through a cell cracker under conditions that largely retain the integrity of large organelles and allow their separation from the smaller and lighter vesicles by differential centrifugation. The cell homogenate was sequentially fractionated to obtain the postnuclear, 1000 × g pellet (P1), the 24,000 × g pellet (P2), and supernatant (S2). Equal amounts of protein from these fractions were then analyzed by immunoblotting to determine where the APP and organelles of the secretory and endocytic membrane traffic fractionated. S2 has been shown previously to contain small vesicles, including synaptic vesicles and coated vesicles, in addition to soluble proteins (Lichtenstein et al., 1998; Salazar et al., 2005), and so was expected to contain a subpopulation of cargos and adaptors that can be used to identify specific vesicles.
We found substantial amounts of APP and previously established binding partners, Mints (1–3) and Fe65, in S2 (Figure 2A). The S2 contained ∼45–50% of total cell protein under these conditions; yet, it is largely devoid of markers of secretory organelles. Most of the organelles were found in P1 or P2, as monitored by immunoblotting for markers of each organelle; including the Golgi (GM130), trans-Golgi network (TGN38), endoplasmic reticulum (KDEL receptor), plasma membrane (Na+-K+ ATPase), early endosomes (EEA1), recycling endosomes (TfR), and lysosomes (cathepsin D) (Figure 2B). The presence of smaller amounts of both recycling endosomes (TfR) and early endosomes (EEA1) in S2 is consistent with this fraction containing light vesicles, whereas the larger/denser organelles are effectively removed by differential centrifugation. Because APP is glycosylated in its transit through the Golgi stacks, and in this study we followed only the mature, fully glycosylated forms of APP, we are enriching for a post-Golgi form of APP.
Figure 2.
Fractionation of traffic components by differential centrifugation of SH-SY5Y homogenates. SH-SY5Y cells were lysed and homogenates prepared before fractionation by differential centrifugation, to generate P1 and P2 pellets and supernatant, S2, as described under Materials and Methods. Equal amounts of protein (15 μg) were resolved by SDS-PAGE and analyzed by immunoblotting with the antibodies indicated on the left of each panel. (A) The distribution of endogenous APP splice variants (APP770 and APP695), Mint 1-3, Fe65, and mannose 6-phosphate receptors (CI-M6PR) is shown. (B) The fractionation of organelle markers for medial-Golgi (GM130), trans-Golgi network (TGN38), endoplasmic reticulum (KDELR), plasma membrane (Na+/K+-ATPase), recycling endosomes (TfR), early endosomes (EEA1), and lysosomes (cathepsin D) in these same fractions is shown. (C) The distribution of vesicle coat proteins AP-1, AP-2, and AP-3 (as determined by immunoblotting with antisera specific to γ-adaptin, α-adaptin, and δ-adaptin, respectively), GGA1-3, β-COP (a component of COPI), clathrin, and the regulatory GTPases Arf3 and Rab6 are shown.
We also screened these fractions for the presence of proteins required for the formation of, and selectively marking, specific types of vesicles; including clathrin, protein adaptors/coat proteins or complexes, and regulatory GTPases. These proteins and complexes exist in a soluble pool from which they can be selectively recruited into budding vesicles, at which they are involved in cargo selection or vesicle coating, and may become incorporated into the mature vesicle. Indeed, it is the lumenal or transmembrane protein cargoes and cytosolic coats that are used to define different carriers. We found virtually every such protein to be present in the S2 fraction (Figure 2C). Thus, the S2 fraction is largely devoid of organelles of secretory and endocytic traffic, and it is enriched in proteins and lighter vesicles that are involved in vesicular traffic. Note that S2 also contains all soluble/cytosolic proteins and that both clathrin and adaptor proteins/complexes can rapidly dissociate from membranes under a variety of conditions, so that we expect both vesicular and soluble forms of such proteins in S2.
To further resolve the vesicular carriers of APP from the larger and more abundant organelles, specifically recycling endosomes that were seen to contaminate S2, the S2 was loaded on top of linear sucrose gradients (15–45%) before centrifugation at 100,000 × g for 1 h. Recycling endosomes (TfR; Supplemental Figure 1) were found to migrate into the gradient and away from the APP. The adaptors and regulatory GTPases assayed all remained at the top of the gradient (fractions 1–2; Supplemental Figure 1). Whereas soluble proteins would be expected to remain at the top of the gradient, the presence of transmembrane proteins, including APP, in these fractions is evidence that light vesicles or membranes are also present. Thus, velocity sedimentation allowed only slight further enrichment for the lighter vesicle components of SH-SY5Y cells, but importantly, it resolved the bulk of recycling, and perhaps other, endosomes.
Isolation of APP- and Mint3-containing Vesicles
The most abundant contaminants in the pool from the sucrose gradients are expected to be soluble proteins, including both those present originally in cytosol and the adaptors and associated proteins that had dissociated from membranes or vesicles during purification. Thus, a method was sought that would effectively resolve soluble and vesicular proteins. We chose to “float up” the light vesicles by using equilibrium sedimentation under conditions in which soluble proteins would not migrate up into the gradient. Fractions 1–2 of the sucrose gradients were pooled and brought to 30% Optiprep, and then 25% and 10% Optiprep layers were overlaid, before centrifugation for 3 h at 250,000 × g. We typically collected 16 fractions from these gradients, and fractions 11–16 correspond to the 30% Optiprep layer at the bottom of gradients. Soluble proteins (Arf3, Rab6, etc.) remained at the bottom (fractions 10–16) in this gradient (data not shown). In addition, a large percentage of membrane proteins (including APP) also remained near the bottom (data not shown), likely the result of aggregation or their presence in denser membranes or carriers. However, a subpopulation of APP was consistently found to float up into the less dense portion of the gradient, peaking in fractions 5–6, migrating near the 10/25% Optiprep interface. This peak contains both the APP695 and APP751/770 splice variants, and it represents only ∼0.08% of the total protein from cell homogenates (Figure 3). The adaptors and regulatory GTPases analyzed were overwhelmingly seen to stay near the bottom of the gradients. Arf-dependent adaptors, including AP-1 (γ-adaptin), AP-3 (δ-adaptin), GGA1, and COPI (β-COP) remained at the bottom of the gradient, and they were not seen in fractions 3–7 (Figure 3). To push the sensitivity of our analyses, these immunoblots were also developed using higher sensitivity enhanced chemiluminescence reagents, which increases the sensitivity >5-fold in our assays; yet, still we saw no evidence for the presence of any of these adaptors, with the exception of Mint2 and Mint3 (see below), which cofractionated with APP. Importantly, neither Fe65 nor Arfs were found in these fractions, effectively excluding the possibility of binding of adaptors from cytosol to APP during vesicle enrichment.
Figure 3.
Flotation of light vesicles enriched in APP and Mint3 by Optiprep density sedimentation. The pooled fractions from the sucrose velocity centrifugation were brought to 30% Optiprep before under loading in a discontinuous Optiprep gradient, as described under Materials and Methods. Equal volumes (45 μl) from each fraction were analyzed by immunoblot, by using our most sensitive detection method. S2 (20 μg of protein) was used as positive control. Exposure times varied and were the maximum time allowable without background interference, particularly for those antigens showing no reactivity in fractions 3–7 (shown) out of the 16 fractions collected.
In contrast to other coat proteins or adaptors, Mint3 was found to comigrate with APP into the lighter vesicle fractions. Like APP, the majority of Mint3 was near the bottom of the gradient, but a subpopulation of Mint3 was always found to comigrate with APP into the lighter fractions, peaking at fractions 5–6 (Figure 3). In addition, the fairly constant ratio of immunostaining between APP and Mint3 in these fractions and in different preparations suggested a close relationship between the two. Commercially available mouse monoclonal antibodies to Mint1 (BD Biosciences and Sigma-Aldrich) or Mint2 (BD Biosciences) failed to reveal either protein in the lighter, vesicular APP fractions. However, when we probed these fractions with more sensitive, polyclonal antibodies specific to Mint2, obtained from Dr. Toshiharu Suzuki (Tomita et al., 1999), we found that a subset of Mint2 also copurified with APP and Mint3 (Figure 3). Failure to detect immunoreactivity cannot be taken as evidence that a protein is not present in a preparation, due to differences in sensitivities between antibodies. However, it is clear in Figure 3 that each of the antibodies used readily detected their antigens in S2, but it was only Mint3 and Mint2 that were detected in the light vesicle fractions. Thus, Mints are relatively highly enriched over each of the other adaptor proteins examined.
Overexpression of APP Increases the Amount of APP and Mint2 and Mint3 in the Light Vesicle Fractions
As a further test of our hypothesis that Mint2 and Mint3 are specifically found on APP-containing vesicles, we asked whether an increase in the expression of APP is accompanied by a corresponding increase in Mints in the vesicle preparation. For comparison, we also increased the expression of Mint3, either alone or in concert with the elevated levels of APP, to test whether this would increase the amount of APP in the light vesicle fraction. We used the human APP695 variant that carries two mutations (K670N, M671L) that were found in a Swedish family with early-onset familial Alzheimer's disease and is referred to as APP695-Swe (Citron et al., 1992). The increased expression of APP695-Swe, Mint3, or both (Figure 4A) was achieved by infecting SH-SY5Y cells with the corresponding engineered lentiviruses. Note that the increase in Mint3 expression is greater than that of APP, as a percentage of endogenous proteins. Also, changing the level of either Mint3 or APP did not produce any change in the level of the other protein (Figure 4A). This proved an efficient way to increase expression of these proteins without other evident changes in cell or organelle morphologies (e.g., no changes in the staining of markers for Golgi, early or late endosomes, or recycling endosomes were evident).
Figure 4.
Increased expression of APP695-Swe resulted in increased APP, Mint3, and Mint2 in the light vesicle fractions. (A) SH-SY5Y cells were infected with lentiviruses that resulted in increased expression of APP695-Swe, Mint3, or both proteins. Equal amounts (15 μg) of total cell homogenates were analyzed for APP and Mint3 expression by immunoblot. (B) Cells were homogenized and equal amounts of protein in S2 were sequentially fractionated by sucrose velocity and Optiprep equilibrium density centrifugation. Equal volumes from fractions 4–10 of the Optiprep gradient were analyzed by immunoblot, with the antibodies shown on the left, with constant exposure times to allow relative quantitation. (C) Fractions from Optiprep gradients of SH-SY5Y or APP695-Swe expressing cells were analyzed for Mint2, as described in B. (D) Enrichment of APP, Mint3, and Mint2 in light vesicles was visualized by comparing equal amounts of protein (1.1 μg) from cell homogenate (H), P1, P2, or fraction 6 from an Optiprep gradient (V6). (E) SH-SY5Y cells expressing APP695-Swe were incubated at 19.5°C for 4 h to block protein exit from the TGN (top; 0 min) or cells were returned to 37°C for 15 min (bottom) before homogenization and light vesicle preparation. Equal volumes from fractions 3–8 of the Optiprep gradients were analyzed by immunoblot, with the antibodies shown on the right and exposure times were constant between cell treatments to allow relative quantitation.
SH-SY5Y cells infected with the APP695-Swe or Mint3 lentivirus, or both, were harvested for vesicle isolation, by using the same procedures described above for uninfected cells. Protein assays were performed at each stage to ensure that the same amount of protein was carried forward at each step. Relative quantitation was achieved in immunoblotting by loading the same amount of protein and keeping constant exposure times for all blots by using the same antibody, rather than reprobing the same filter, which can result in loss of immunoreactivity. This resulted in very consistent profiles and recoveries of APP-containing vesicles. This was evident when we compared the amounts of APP and Mint3 in light vesicles isolated from SH-SY5Y cells or those overexpressing Mint3 alone (Figure 4B; compare top two sets of panels, SH-SY5Y vs. SH-SY5Y/Mint3), because no changes were observed in the protein profiles at any step in the preparation of vesicles when only Mint3 was overexpressed. In contrast, when vesicles were prepared from cells overexpressing APP695-Swe, we consistently observed a substantial increase in the amount of APP in the light vesicle fractions (Figure 4B; compare the top and third set of panels; SH-SY5Y to SH-SY5Y/APP695-Swe). Even more striking than the increase in APP695 in our light vesicle population was the increase in the amount of Mint3 that accompanied the APP695 in fractions 5–6. This is again in contrast to other adaptors that are recruited to membranes by activated Arf (e.g., GGA2 and γ-adaptin), which were not seen in fractions 5–6 even when APP expression and APP vesicles were increased (data not shown). The amount of Mint3 in fractions 5–6 did not increase when the cellular level of Mint3 (Figure 4B) was increased, indicating that the amount of Mint3 in cytosol is not a limiting factor in the formation of APP-containing vesicles. We also found that increasing the expression of APP695-Swe led to an increase in the Mint2 in fraction 5 and 6 (Figure 4C), whereas there was still no detectable Mint1 in these fractions under these conditions. Increasing the expression of Mint2 had no effect on APP levels in fractions 5 and 6 (data not shown). Thus, an excess of these Arf-dependent adaptors cannot by themselves drive the formation of carriers. Rather, it seems that the cargo, APP, is a limiting factor in vesicle biogenesis and when more APP is synthesized in cells, more is found in transit carriers, which in turn recruit more Mint2 and Mint3.
In efforts to quantify the fold enrichment of APP and Mint3 in our vesicle preparation, we compared the amounts of each protein by immunoblotting and comparison of cell homogenates to P1, P2, and fraction 6 (V6) from an Optiprep gradient (Figure 4D). Equal amounts of total protein were loaded, and the blots were underexposed to prevent an excess of signal in the V6 lane. Under these conditions APP and Mint3 are barely detectible in cell homogenates. We estimate from such data that APP and Mint3 are increased in specific activity >10-fold over cell homogenate. Although this number may seem low in comparison with a standard protein purification, it is consistent with published procedures for the purification of synaptic (15–18-fold) (Huttner et al., 1983; Floor et al., 1988) or AP-3 (∼16-fold; Craige et al., 2005; Salazar et al., 2005) coated vesicles. The fold purification of vesicles was estimated from that of Mint3, which in turn was determined by comparison of immunoreactivity in V6 to serial dilutions of a bacterially expressed recombinant Mint3 standard. This estimate reveals that Mint3 represents 0.1–1.0% of total protein in Optiprep fraction 6. Because Mint3 is expected to be dissociating from vesicles during purification, APP is expected to be present in even greater amounts. Mint2 was also found to be enriched in Optiprep fraction 6 in a similar manner as Mint3.
In our previous study (Hill et al., 2003), we showed that Mint3 and APP colocalize at the TGN. For this reason, we speculated that the enriched carriers described above were derived from the Golgi. As an initial test of this hypothesis, we performed a cold temperature (19.5°C) block of anterograde traffic from the Golgi, which leads to the accumulation of cargo at the late Golgi/TGN that moves out synchronously into post-Golgi carriers upon rewarming back to 37°C, as described previously (Kreis and Lodish, 1986; Lotti et al., 1992; Polishchuk et al., 2004). We asked whether the amount of APP and Mints in our enriched carrier preparation was increased shortly after release of the block. SH-SY5Y-APP695-Swe–expressing cells were incubated at 19.5°C for 4 h before processing for light vesicle fractionation. Subsequent incubation of the cells at 37°C for 15 min after removal of the temperature block resulted in substantial increases in APP, Mint3 (Figure 4E), and Mint2 (data not shown) in fractions 5 and 6. These data are consistent with the hypothesis that APP and Mint3/Mint2 accumulate at the Golgi/TGN during the temperature block and make a synchronous exit from the TGN upon release of the block.
APP and Mint3 Are Immunopurified on the Same Carriers
To further test the hypothesis that APP traffics on Mint3-coated carriers, vesicles were purified from the light vesicle fraction from the Optiprep gradients by using immunomagnetic isolation with antibodies to Mint3 or APP in the absence of detergents, to retain the vesicles and associated proteins. Immunomagnetic isolated vesicles were analyzed by transmission electron microscopy after fixation and processing as described under Materials and Methods. Vesicles were immunoisolated using antibodies to Mint3 (Figure 5B) or APP (Figure 5C). These preparations were repeated at least three times each with the same results. The average diameter of the immunomagnetic-isolated vesicles was determined by measuring the diameters of vesicles attached to magnetic beads in electron micrographs. Vesicles isolated with APP antibodies had an average diameter of 91.4 ± 20.3 nm (n = 23), and those obtained with Mint3 antibodies were 99.7 ± 14.6 nm (n = 12) (Supplemental Figure 2); consistent with them being present in the same population of vesicles as there is no statistical difference in diameter. Controls for the immunomagnetic isolation included the use of nonimmune IgG or a monoclonal antibody directed against an unrelated antigen (c-myc; Figure 5A) and secondary antibodies or magnetic beads alone. Vesicles were only very rarely ever found on beads in any of these cases.
Figure 5.
APP and Mint3 are present in the same vesicles. Light vesicles (fractions 4–6) from an Optiprep gradient were purified on magnetic beads by using antibodies specific to either the myc epitope (A; as negative control), Mint3 (B), or the C terminus of APP (C) before being analyzed by transmission electron microscopy, as described under Materials and Methods. Representative images are shown of vesicles bound to the surface of the magnetic beads. (D) Vesicles were immunoisolated using Mint3 monoclonal antibodies and were then sequentially incubated with the rabbit polyclonal APP C-terminal antibody and goat anti-rabbit IgG conjugated to 10-nm gold particles before fixation and analysis by electron microscopy, as described under Materials and Methods. (E) Gold-decorated vesicles bound to magnetic beads (from D) are shown enlarged. Figures are representative of three independent experiments. Bar, 100 nm.
We also asked whether the APP and Mint3 were found in the same vesicles by labeling immunomagnetic isolated Mint3 vesicles with APP antibodies conjugated to 10-nm gold particles before fixation in glutaraldehyde and processing for electron microscopy. When visualized by electron microscopy the Mint3 vesicles were found to be highly decorated with APP-gold (Figure 5, D and E). In contrast, when an unrelated polyclonal antibody (c-myc) was conjugated to gold and incubated with Mint3 vesicles, we found almost no gold particles decorating the vesicles. We conclude that we have purified a population of vesicles of a fairly uniform diameter (∼100 nm) that contain specifically APP and Mint3, but lack other known protein adaptors.
Knockdown of Mint3 Alters APP Traffic
Another prediction from our hypothesis that Mints are Arf-dependent adaptors involved directly in traffic of APP from the Golgi is that in the absence of Mints or in the absence of APP binding to Mints, the post-Golgi traffic of APP would be impaired or altered. To test this prediction, we performed siRNA experiments in HeLa cells, because they express only Mint3 and not Mint1 or Mint2. We used siRNAs expressed from pSUPER-based plasmids to specifically decrease the expression of Mint3. Four sequence-independent 19-nt sequences from the open reading frame of human Mint3 were generated to target the message, and the two that yielded the highest knockdown were used, to protect against off-target effects by expression of individual siRNAs. Optimization of transfected DNA and timing was performed by immunoblotting for Mint3 and revealed that expression of Mint3 was maximally reduced by day 2 and remained low on day 3. The effectiveness of knockdown of Mint3 was clearly evident by immunoblotting (Figure 6A).
Figure 6.
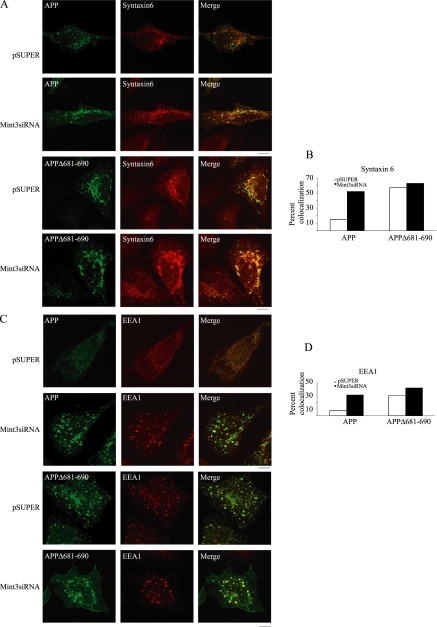
Decreased expression of Mint3 or mutation of the Mint3 binding domain causes missorting of APP. (A) HeLa cells were transfected with empty vector or either of two different Mint3 siRNA plasmids. The level of expression of Mint3 is shown on day 3 after transfection, as assessed by immunoblotting using antibodies to Mint3 or α-tubulin, which served as a loading control. (B–E) HeLa cells were transfected with empty vector or Mint3 siRNA plasmid and again 2 days later with plasmids driving expression of APP or APPΔ681-690. Analyses were performed 18 h later. (B) Cell lysates were prepared, and equal amounts of total cell protein (25 μg) were resolved in SDS gels, and immunoblotting was performed using antibodies to APP or Mint3. (C) The temperature block was imposed for 4 h before switching to 37°C for 0 or 15 min, as described under Materials and Methods. Cells were then fixed and labeled with antibodies specific to APP for confocal imaging. All images were captured at the same gain and exposure times. Bar, 10 μm. (D) The percentages of cells displaying APP staining in only small puncta (<0.5 μm; empty bars), only a few enlarged puncta (1–4 puncta >0.5 μm; gray bars), or many enlarged puncta (>4 puncta >0.5 μm; black bars) were quantified (APP: pSUPER, n = 142; and Mint3-siRNA, n = 127; APPΔ681-690: pSUPER, n = 138; and Mint3-siRNA, n = 154). Results shown are the average of two independent experiments. (E) Cells were either fixed before temperature block (steady state) or after the block (0 min) or with varying times shown after release from the block, before fixing and staining for APP. Note that steady-state cells do not contain enlarged puncta in either condition and that cells have returned to steady-state staining by 90 min after removal of the block.
We then asked whether Mint3 depletion affects the post-Golgi traffic of APP. Although HeLa cells express APP751, the endogenous levels were below the limits of detection so the overexpressed protein was monitored in these experiments. In contrast to some other basolaterally directed post-Golgi cargo (e.g., vesicular stomatitis virus G [VSVG] protein), APP is rapidly internalized from the cell surface by endocytosis so is not seen to accumulate at the plasma membrane. We again used the low temperature block to accumulate APP and Mint3 at the late Golgi/TGN. Depletion of Mint3 had no effect on the levels of APP expression or on traffic of APP from the ER to the Golgi either in steady-state (Figure 6E) or temperature-blocked cells (Figure 6C, top, or 6, left). Very soon after release of control (empty pSUPER-transfected) cells from the temperature block, APP was found to exit the Golgi region and appeared as small puncta, with diameters rarely exceeding 0.3 μm, and a fairly uniform cellular distribution (see 15-min time point in Figure 6E). In contrast, in cells depleted for Mint3 the APP staining was very different, with a large amount present in enlarged puncta, typically with diameters of 1–3 μm or even larger (Figure 6, C and E). The presence of APP in enlarged puncta was transient, because it was only very rarely observed in cells that had not undergone temperature block or in cells 90 min after release from the block (Figure 6E). Quantification revealed that in control cells ∼70% of cells displayed small punctate staining of APP with no large puncta, whereas in Mint3 siRNA cells ∼87% of cells contained one or more enlarged puncta staining positively for APP. Note that normal Golgi staining can appear as large puncta in HeLa cells so the differences between control and Mint3 siRNA cells is likely even larger than that indicated in Figure 6D. Importantly, the Golgi itself does not seem to be changing under these conditions, because GM130 staining was not altered by the temperature block or release (data not shown). Because these enlarged puncta are dramatically increased in numbers and sizes in cells recently released from temperature block, we think they result from the synchronous exit of a bolus of APP from the TGN. This conclusion is further supported by the observation that they are not observed in cells in which APP is not overexpressed (data not shown).
As an additional test of the importance of APP binding to Mints to its exit from the TGN, we obtained a mutant form of human APP695 that is deleted for the Mint binding motif, termed APP695Δ681-690, from Dr. Toshiharu Suzuki (Hokkaido University, Japan) (Taru and Suzuki, 2004). APP binds Mints via the YENPXY motif in its cytosolic tail (Borg et al., 1996; McLoughlin and Miller, 1996; Zhang et al., 1997; Okamoto and Sudhof, 1998) and in the absence of this motif cannot bind Mints (Tomita et al., 1999; Taru and Suzuki, 2004). We compared the post-Golgi traffic of wild-type APP695 to that of APP695Δ681-690 in HeLa cells, using the temperature block and release protocol for accumulating cargo at the TGN and following its exit, respectively. No difference in the levels of APP-expressed (Figure 6B) or steady-state distribution of APP was observed when HeLa cells overexpressing APP695 were compared with APP695Δ681-690 (Figure 6C). However, 15 min after release from temperature block cells expressing APPΔ681-690 were found to accumulate enlarged puncta that stained with APP antibodies (Figure 6C, bottom). This staining was very similar to that seen 15 min after release of cells expressing wild-type APP but depleted for Mint3 (Figure 6C, top). When cells expressing APPΔ681-690 were also depleted of Mint3, the enlarged punctate staining of APP was exaggerated in appearance (Figure 6C), although it was no different when quantified using our simple scoring system (Figure 6D). Note the similarities in appearance of APP staining in cells depleted for Mint3 and in those containing Mint3 but expressing APP that cannot bind Mint3 (Figure 6D). In addition, we found no differences in post-Golgi traffic of APPΔ681-690 in the absence or presence of Mint3. Together with the published reports from our group and others of direct binding of APP to Mints, the simplest interpretation of these data are that binding of APP to Mint3 is important to its post-Golgi traffic.
To allow us to identify the importance of the Mint3–APP interaction on the routing of post-Golgi traffic of APP, we again accumulated each protein at the late Golgi/TGN by using the temperature block protocol and we followed colocalization with markers of the secretory and endocytic pathways. It has been previously established that APP exits the TGN in polarized and nonpolarized cells by using the basolateral sorting machinery (Haass et al., 1994; Simons et al., 1995; Tienari et al., 1996a,b; Kaether et al., 2000), so it can be found on post-Golgi carriers costaining with markers of this pathway, including VSVG protein. Results in our laboratory using HeLa, Chinese hamster ovary, or primary cultured mouse hippocampal neuronal cells have confirmed these findings (data not shown). Quantification of confocal images using MetaMorph software was used to confirm any apparent changes in the extent of colocalization of APP with markers of the secretory and endocytic pathways. No changes were evident in the costaining of APP with markers of lysosomes (LAMP1), multivesicular bodies (CD63), recycling endosomes (TfRs), or Golgi (giantin; Hill et al., 2003) in control versus Mint3 siRNA cells after temperature block or 15 min after release (data not shown).
Only minimal colocalization of APP with syntaxin 6 was evident in control cells by eye (Figure 7A, top) or by quantitation with MetaMorph (15%; Figure 7B), as might be expected for this basolaterally sorted cargo and marker of the endosomal/lysosomal route. Similarly, colocalization of the staining of the early endosome marker EEA1 and that of APP was only 8% (Figure 7C, top, and D) in control cells. Importantly, upon depletion of Mint3 the overlap with APP staining increased from 15% to 52% with syntaxin 6 (Figure 7A, second row panels, and B) and from 8% to 31% with EEA1 (Figure 7C, second row panels, and D). The high degree of costaining of APP with two different markers of the endosomal/lysosomal sorting route 15 min after release from temperature block was also seen in cells expressing the Mint3-binding defective mutant APPΔ681-690 (Figure 7, B and D). However, in this case the colocalization with syntaxin 6 and EEA1 was already high in control cells and not dependent upon depletion of Mint3, although the combination of APPΔ681-690 and Mint3 depletion yielded the greatest extent of colocalization of APP with syntaxin 6 or EEA1. The fact that APPΔ681-690 was found in enlarged puncta that colocalize with the markers of endosomal/lysosomal system in control cells, similar to what was seen with wild-type APP in Mint3-depleted cells, suggests that Mint3 binding to APP is required for its normal routing from the Golgi and in the absence of Mint3, missorting of APP occurs from the basolateral toward the endosomal/lysosomal route. Note that neither the temperature block nor depletion of Mint3 alone caused a change in the size or distribution of syntaxin6 or EEA1 staining puncta (Supplemental Figure 3). Because endosomes have been identified as a source of Aβ in cells, we also asked whether the missorting that occurs when APP cannot bind Mint3 at the TGN results in any changes in Aβ generation.
Figure 7.
Decreased expression of Mint3 caused increased APP localization to enlarged endosomal structures. (A–D) Cells were transfected with siRNA and APP or APPΔ681-690 expression plasmids, before imposition of the temperature block and 15-min release, as described in the legend to Figure 6, before fixing and staining for APP and syntaxin 6 (A) or EEA1 (C). Colocalization of APP (green, top two rows of panels) or of APPΔ681–690 (green, bottom two rows) with syntaxin 6 (A; red) or EEA1 (C; red) is dramatically increased (facing page). in cells depleted of Mint3 or with APP mutated in the Mint binding domain, as seen by overlapping (yellow) pixels in the merged image. Quantification of APP and syntaxin 6 (B) or APP and EEA1 (D) colocalization was performed using MetaMorph software, as described under Materials and Methods. Note that colocalization of APPΔ681-690 with both syntaxin 6 or EEA1 is higher than in controls and is largely independent of Mint3 expression levels. Empty bars in B and D are for control (empty pSUPER plasmid) and filled bars are for Mint3siRNA-transfected cells. This experiment was repeated twice with similar results and the quantitation shown is from a single experiment. Bar, 10 μm.
Altered Traffic of APP Resulting from Mint3 siRNA Also Alters Its Processing
Because the processing of APP by secretases is tightly correlated with its traffic in cells it was reasonable to speculate that a change in traffic could alter processing; particularly because endosomes have been identified as a likely source of Aβ production (Selkoe et al., 1996). However, because APP is entering the endosomal system through a route that may not be normal for this cargo it is not clear what changes in processing, if any, may ensue. It has been shown previously that overexpression of Mint1 or Mint2 decreased the secretion of Aβ peptides and increased the levels of APP (Borg et al., 1998; Sastre et al., 1998; Tomita et al., 1999), although we could find no reports of the effects of changes in Mint3 expression on Aβ secretion. HEK293 cells have been used extensively to monitor effects of gene expression on the processing of endogenous APP; thus, they were used here. Cells were transiently transfected with Mint3 or control DNA, and the accumulation of Aβ1-40 in the media was determined by ELISA. Although Mint3 overexpression did not change the levels of full-length APP (data not shown), a substantial decrease in Aβ1-40 secretion (70.7 ± 8.2%, mean ± 1 SD; n = 3; p < 0.001) was seen in cells expressing elevated levels of Mint3, compared with empty vector-transfected controls (Figure 8A). This effect was comparable with the decreased secretion of Aβ that resulted from increased expression of Mint1 (74.4 ± 4.7%, mean ± 1 SD; n = 3; p < 0.001) or Mint2 (69.0 ± 4.4%, mean ± 1 SD; n = 3, p < 0.001) (Figure 8A). In contrast, decreased expression of Mint3, after siRNA treatment, was found to produce a modest increase (130.0 ± 8.0%, mean ± 1 SD; n = 3, p < 0.01) in the amount of Aβ1-40 secreted into the medium of HEK293 cells (Figure 8B). The extent of knockdown of Mint3 achieved in HEK293 cells was comparable with that in HeLa cells (compare Figure 6A with 8C). Thus, Mint3 shares with Mint1 and Mint2 the ability to alter the processing of APP and the levels of secreted Aβ1-40, although neither the site of Aβ generation nor the site of Mint effects on Aβ secretion are known.
Figure 8.
Mint3 expression levels impact the rate of secretion of Aβ1-40. HEK293 cells were transiently transfected with either empty vector (control) or vectors directing expression of human Mint1, Mint2, or Mint3. The medium was changed the next day and collected for analysis 48 h later. (A) Conditioned media from cells expressing the different Mints were assayed for Aβ1-40, as described under Materials and Methods. Values represent Aβ1-40 levels relative to controls (mean ± 1 SD; n = 3; **p < 0.001). (B) Cells were transfected with equal amounts of the control or Mint3-siRNA plasmids on day 0 and again on day 3 to maximize knockdown of the protein. Media were replaced on day 4 and conditioned media collected on day 6 for determination of Aβ1-40 levels (n = 3; *p < 0.01). (C) Cells were treated as described in B, and the level of expression of Mint3 was determined in cell lysates (20 μg/lane) by immunoblotting, with α-tubulin used as a loading control. Triplicates are shown of control and Mint3-depleted cell lysates.
DISCUSSION
The current study focused on testing the hypothesis that APP uses one or more Mints as adaptors in its traffic from the TGN. Given the previously published evidence that 1) APP binds directly to each of the Mints, 2) APP and Mint3 colocalize at the late Golgi/TGN, 3) changes in the levels of APP or Mint3 alter the other's localization at the TGN, and our observations reported here that 4) APP and Mint3/Mint2 copurify in vesicle preparations from SH-SY5Y cells, 5) increased expression of APP results in increased amounts of both APP and Mint3/Mint2 in the same vesicle population, 6) APP and Mint3 were coimmunoprecipitated from the purified vesicles, 7) APP antibodies specifically label the surface of Mint3-containing vesicles, and 8) siRNA of Mint3 results in alterations in the post-Golgi traffic of APP, we conclude that Mint3, and likely Mint2, are protein adaptors that are required for the proper sorting and basolaterally directed exit of APP from the Golgi and as such that they have the potential to alter the intracellular processing of APP into toxic Aβ peptides.
The first report of Mint3 message showed it to be expressed in the brain (Okamoto and Sudhof, 1998) and in situ hybridization revealed that the message was seen in “large neurons in layers I–VI of cerebral cortex … and in the pyramidal neurons and granular cells in the hippocampus” (Okamoto et al., 2001) in mouse brain. Yet, Mint3 has persistently been mischaracterized as not being expressed in neurons. We showed that the Mint3 protein is prominently expressed in human pyramidal neurons in the hippocampus, and other sites. Staining in these cells is punctate in appearance with some concentrated perinuclear stain that is consistent with carriers of membrane proteins and Golgi, respectively. This expression pattern is very similar to that reported previously for Mint2 but distinct from that of Mint1 (Nakajima et al., 2001). Thus, Mint3 is expressed in human neurons including and specifically those regions and cell populations vulnerable to degeneration in Alzheimer's disease.
When the normal TGN exit of APP (equivalent to basolateral sorting in polarized cells) was blocked, by depletion of Mint3 by siRNA or when a mutant form of APP that was unable to bind Mint3 was expressed, we discovered that it was missorted to the endosomal/lysosomal route, as evidenced by large increases in the colocalization of APP with syntaxin 6 and EEA1. The observation of an alternative pathway for APP exit from the TGN also raises the possibility that some fraction of APP is always routed via the endosomal/lysosomal route (also see Ang et al., 2004 and related studies) and that it is the abundance and relative affinities of different binding partners that determines the percentage of APP that traffics between the different alternative paths, which in turn could have an important impact on processing and Aβ generation. For example, LR11/SorLa is a lipoprotein receptor with links to Alzheimer's disease (Andersen et al., 2005; Offe et al., 2006; Rogaeva et al., 2007) that binds directly to APP in their lumenal domains (Andersen et al., 2006). The cytosolic tail of LR11 binds to GGAs (Jacobsen et al., 2002) and is thus predicted to exit the TGN via endosomal/lysosomal routing and could provide the means for recruitment of APP into GGA carriers and direct traffic into the endosomal system.
The current data lend further support to our hypothesis (Hill et al., 2003) that Mints are Arf-dependent adaptors that act are important to APP traffic. Given the number of Arf-dependent adaptors that operate at almost every intracellular membrane and the obligate roles for Arfs in recruitment of these adaptors to the sites of nascent carrier budding, it is difficult today to name a carrier whose origins are Arf independent. The presence of Mint2 and Mint3 on our purified vesicles and absence of other known adaptors (GGA1-3, AP-1, AP-3, COPI, and Mint1) is evidence supporting a specific functional relationship between Mints and APP. Given the specific coenrichment of Mint2 and Mint3 with APP vesicles (Figure 4D) and our ability to detect each of the other adaptors in the S2 fraction (Figure 2C) but not on purified vesicles, we conclude that Mint2/Mint3 serve as the Arf-dependent adaptors for APP in this vesicle preparation. This reasoning also leads to the conclusion that Mint1 does not play a role in APP traffic of this subset of vesicular intermediates or that the binding of Mint1 to carriers is more labile and lost during purification. All three Mints share highly conserved PTB and dual PDZ domains in their central and C-terminal regions, respectively, whereas only Mint1 and Mint2 share the N-terminal Munc18 interacting domain. Only Mint1 contains a Cask binding domain, which allows it to assemble into stable, soluble, heterotrimeric complexes of Mint1/Cask/Velas (Butz et al., 1998). Thus, in contrast to results with the three GGA adaptors (Ghosh et al., 2003), it is likely that Mint1 and Mint3 exhibit distinctive locations and functions, whereas Mint2 may overlap with each of the other two.
We propose that the vesicles purified and partially characterized in this study represent Golgi-derived carriers of APP and Mint3. This is based upon the colocalization of APP and Mint3 at the TGN and coenrichment in our vesicle preparation of APP and Mint3 shortly after release from cold temperature block. But given the low abundance of these vesicles and small percentage of total cellular APP or Mint3, we cannot rule out other origins of this fairly uniform preparation of vesicles. For example, the purified vesicles could be endosomal in origin, although we feel this is less likely because at least recycling endosomes were efficiently removed by velocity sedimentation in sucrose gradients (Supplemental Figure 1).
The absence of Arf proteins in the purified vesicles may be surprising in light of earlier data showing suprastoichiometric levels of Arfs over COPI subunits when vesicles were produced in vitro in the presence of guanosine 5′-O-(3-thio)triphosphate (GTPγS) (Serafini et al., 1991; Rothman, 1994; Sohn et al., 1996). However, it later became evident that Arf GTPase activation proteins play essential roles in vesicle biogenesis and specificity of cargo selection (Goldberg, 1998; Lee et al., 2005), making GTP hydrolysis and dissociation of Arfs from the nascent bud or vesicle likely. The absence of stoichiometric levels of Arf on two different vesicle populations, containing different Arf-dependent coats, AP-3 (Salazar et al., 2005) and now Mint3, is consistent with models in which Arfs dissociate from membranes before scission of transport carriers or shortly thereafter.
Despite our limited understanding of the role of APP in normal cell physiology, the regulation of APP traffic is an important issue not only to cell biologists but also potentially clinically, because it directly impacts the rate and extent of processing of APP into the neurotoxic Aβ peptides. We found that increasing the expression of APP led to an increase in the number of vesicles in transit, whereas increasing the expression of Mint3 had no effect. This is similar to the recent report of Hirst et al. (2007) in which cargo is shown to be a key determinant in GGA recruitment to membranes, in contrast with previous data showing that increasing the expression of transmembrane protein cargos did not cause an increase in their traffic at the cell surface (Santini and Keen, 1996; Santini et al., 1998), and loss of mannose 6-phosphate receptor expression did not produce a change in the distribution or recruitment of its protein adaptor, AP-1, onto TGN membranes (Zhu et al., 1999). This speaks to a close physical and functional linkage between APP and Mint3/Mint2 in the regulation of APP exit from the Golgi that may clearly have an impact on the destination and processing of APP in neurons.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. T. Suzuki for the kind gift of Mint2 antibodies, Sam Gandy for APP antibody (APP369), and Joachim Kremerskothen for antibodies to Rab6. Our thanks also to Roman Polishchuk and other members of the Cell Biology and Oncology Department of the Consorzio Mario Negri Sud (Santa Maria Imbaro, Italy) for numerous helpful discussions including those on the use and value of temperature block and release experiments. The electron microscopy and immunogold labeling was provided by the expertise of Hong Yi and the staff of Emory's Electron Microscopy Core Facility. This work was supported by National Institutes of Health grants GM-67226 (to R.A.K. and P.S.-R.), AG-025688 (to A.I.L.), NS-42599 and GM-077569 (to V.F.), and the Alzheimer's Association 3-5205 (to R.A.K. and P.S.-R.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0465) on October 24, 2007.
APP is alternatively spliced and can be expressed in three different lengths of 695, 751, and 770 residues. Although the shortest form migrates well below the other two, the 751 and 770 proteins are most often found to run together. We do not distinguish between the 751 and 770 forms here.
REFERENCES
- Andersen O. M., et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. M., et al. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006;45:2618–2628. doi: 10.1021/bi052120v. [DOI] [PubMed] [Google Scholar]
- Ang A. L., Taguchi T., Francis S., Folsch H., Murrells L. J., Pypaert M., Warren G., Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Tomita S., Yamaguchi H., Miyagi N., Sumioka A., Kirino Y., Suzuki T. Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid beta-protein precursor metabolism. J. Biol. Chem. 2003;278:49448–49458. doi: 10.1074/jbc.M306024200. [DOI] [PubMed] [Google Scholar]
- Bagshaw R. D., Pasternak S. H., Mahuran D. J., Callahan J. W. Nicastrin is a resident lysosomal membrane protein. Biochem. Biophys. Res. Commun. 2003;300:615–618. doi: 10.1016/s0006-291x(02)02865-6. [DOI] [PubMed] [Google Scholar]
- Biederer T., Sudhof T. C. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J. Biol. Chem. 2000;275:39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Boman A. L., Zhang C., Zhu X., Kahn R. A. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Borg J. P., Ooi J., Levy E., Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. P., Yang Y., De Taddeo-Borg M., Margolis B., Turner R. S. The X11alpha protein slows cellular amyloid precursor protein processing and reduces Abeta40 and Abeta42 secretion. J. Biol. Chem. 1998;273:14761–14766. doi: 10.1074/jbc.273.24.14761. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T. R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Butz S., Okamoto M., Sudhof T. C. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso R. P., Ramabhadran T. V., Unterbeck A. J., Greengard P. Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc. Natl. Acad. Sci. USA. 1990;87:6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Thinakaran G., Koliatsos V., O'Callahan J., Slunt H. H., Price D. L., Sisodia S. S. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J. Neurosci. 1998;18:9629–9637. doi: 10.1523/JNEUROSCI.18-23-09629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenagh M. M., Breiner M., Schurmann A., Rosenwald A. G., Terui T., Zhang C., Randazzo P. A., Adams M., Joost H. G., Kahn R. A. ADP-ribosylation factor (ARF)-like 3, a new member of the ARF family of GTP-binding proteins cloned from human and rat tissues. J. Biol. Chem. 1994;269:18937–18942. [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L., Desnos C., Lichtenstein Y., Faundez V., Horng J. T., Kelly R. B. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. doi: 10.1006/meth.1998.0662. [DOI] [PubMed] [Google Scholar]
- Cordy J. M., Hussain I., Dingwall C., Hooper N. M., Turner A. J. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B., Salazar G., Faundez V. New York: Wiley Interscience; 2005. Isolation of synaptic vesicles. [DOI] [PubMed] [Google Scholar]
- Floor E., Schaeffer S. F., Feist B. E., Leeman S. E. Synaptic vesicles from mammalian brain: large-scale purification and physical and immunochemical characterization. J. Neurochem. 1988;50:1588–1596. doi: 10.1111/j.1471-4159.1988.tb03048.x. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Griffith J., Geuze H. J., Kornfeld S. Mammalian GGAs act together to sort mannose 6-phosphate receptors. J. Cell Biol. 2003;163:755–766. doi: 10.1083/jcb.200308038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- Greenfield J. P., Tsai J., Gouras G. K., Hai B., Thinakaran G., Checler F., Sisodia S. S., Greengard P., Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc. Natl. Acad. Sci. USA. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Teplow D. B., Selkoe D. J. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc. Natl. Acad. Sci. USA. 1994;91:1564–1568. doi: 10.1073/pnas.91.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K., Li Y., Bennett M., McKay M., Zhu X., Shern J., Torre E., Lah J. J., Levey A. I., Kahn R. A. Munc18 interacting proteins: ADP-ribosylation factor-dependent coat proteins that regulate the traffic of beta-Alzheimer's precursor protein. J. Biol. Chem. 2003;278:36032–36040. doi: 10.1074/jbc.M301632200. [DOI] [PubMed] [Google Scholar]
- Hirst J., Seaman M. N., Buschow S. I., Robinson M. S. The role of cargo proteins in GGA recruitment. Traffic. 2007;8:594–604. doi: 10.1111/j.1600-0854.2007.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. S., Marinescu V., Steinhilb M. L., Gaut J. R., Turner R. S., Stuenkel E. L. Synergistic effects of Munc18a and X11 proteins on amyloid precursor protein metabolism. J. Biol. Chem. 2002;277:27021–27028. doi: 10.1074/jbc.M201823200. [DOI] [PubMed] [Google Scholar]
- Howell B. W., Lanier L. M., Frank R., Gertler F. B., Cooper J. A. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell Biol. 1999;19:5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L., Madsen P., Nielsen M. S., Geraerts W. P., Gliemann J., Smit A. B., Petersen C. M. The sorLA cytoplasmic domain interacts with GGA1 and -2 and defines minimum requirements for GGA binding. FEBS Lett. 2002;511:155–158. doi: 10.1016/s0014-5793(01)03299-9. [DOI] [PubMed] [Google Scholar]
- Kaether C., Skehel P., Dotti C. G. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. D., Cherian K., Turner R. S. X11alpha impairs gamma- but not beta-cleavage of amyloid precursor protein. J. Neurochem. 2004;88:971–982. doi: 10.1046/j.1471-4159.2003.02234.x. [DOI] [PubMed] [Google Scholar]
- King G. D., Perez R. G., Steinhilb M. L., Gaut J. R., Turner R. S. X11alpha modulates secretory and endocytic trafficking and metabolism of amyloid precursor protein: mutational analysis of the YENPTY sequence. Neuroscience. 2003;120:143–154. doi: 10.1016/s0306-4522(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986;46:929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuentzel S. L., Ali S. M., Altman R. A., Greenberg B. D., Raub T. J. The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. Biochem. J. 1993;295:367–378. doi: 10.1042/bj2950367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lah J. J., Levey A. I. Endogenous presenilin-1 targets to endocytic rather than biosynthetic compartments. Mol. Cell Neurosci. 2000;16:111–126. doi: 10.1006/mcne.2000.0861. [DOI] [PubMed] [Google Scholar]
- Lau K. F., McLoughlin D. M., Standen C. L., Irving N. G., Miller C. C. Fe65 and X11beta co-localize with and compete for binding to the amyloid precursor protein. Neuroreport. 2000;11:3607–3610. doi: 10.1097/00001756-200011090-00041. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Yang J. S., Hong W., Premont R. T., Hsu V. W. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell Biol. 2005;168:281–290. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein Y., Desnos C., Faundez V., Kelly R. B., Clift-O'Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc. Natl. Acad. Sci. USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C., Hong E. J., Pease S., Brown E. J., Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Lotti L. V., Torrisi M. R., Pascale M. C., Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J. Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay M. M., Kahn R. A. Multiple phosphorylation events regulate the subcellular localization of GGA1. Traffic. 2004;5:102–116. doi: 10.1111/j.1600-0854.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- McLoughlin D. M., Miller C. C. The intracellular cytoplasmic domain of the Alzheimer's disease amyloid precursor protein interacts with phosphotyrosine-binding domain proteins in the yeast two-hybrid system. FEBS Lett. 1996;397:197–200. doi: 10.1016/s0014-5793(96)01128-3. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Okamoto M., Nishimura H., Obata K., Kitano H., Sugita M., Matsuyama T. Neuronal expression of mint1 and mint2, novel multimodular proteins, in adult murine brain. Brain Res. Mol. Brain Res. 2001;92:27–42. doi: 10.1016/s0169-328x(01)00126-7. [DOI] [PubMed] [Google Scholar]
- Noviello C., Vito P., Lopez P., Abdallah M., D'Adamio L. Autosomal recessive hypercholesterolemia protein interacts with and regulates the cell surface level of Alzheimer's amyloid beta precursor protein. J. Biol. Chem. 2003;278:31843–31847. doi: 10.1074/jbc.M304133200. [DOI] [PubMed] [Google Scholar]
- Offe K., Dodson S. E., Shoemaker J. T., Fritz J. J., Gearing M., Levey A. I., Lah J. J. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J. Neurosci. 2006;26:1596–1603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Nakajima Y., Matsuyama T., Sugita M. Amyloid precursor protein associates independently and collaboratively with PTB and PDZ domains of mint on vesicles and at cell membrane. Neuroscience. 2001;104:653–665. doi: 10.1016/s0306-4522(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Sudhof T. C. Mint 3, a ubiquitous mint isoform that does not bind to munc18–1 or -2. Eur. J. Cell Biol. 1998;77:161–165. doi: 10.1016/S0171-9335(98)80103-9. [DOI] [PubMed] [Google Scholar]
- Perez R. G., Soriano S., Hayes J. D., Ostaszewski B., Xia W., Selkoe D. J., Chen X., Stokin G. B., Koo E. H. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J. Biol. Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Polishchuk R., Di Pentima A., Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- Rogaeva E., et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sabo S. L., Lanier L. M., Ikin A. F., Khorkova O., Sahasrabudhe S., Greengard P., Buxbaum J. D. Regulation of beta-amyloid secretion by FE65, an amyloid protein precursor-binding protein. J. Biol. Chem. 1999;274:7952–7957. doi: 10.1074/jbc.274.12.7952. [DOI] [PubMed] [Google Scholar]
- Salazar G., Craige B., Wainer B. H., Guo J., De Camilli P., Faundez V. Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles. Mol. Biol. Cell. 2005;16:3692–3704. doi: 10.1091/mbc.E05-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Keen J. H. Endocytosis of activated receptors and clathrin-coated pit formation: deciphering the chicken or egg relationship. J. Cell Biol. 1996;132:1025–1036. doi: 10.1083/jcb.132.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Marks M. S., Keen J. H. Endocytic clathrin-coated pit formation is independent of receptor internalization signal levels. Mol. Biol. Cell. 1998;9:1177–1194. doi: 10.1091/mbc.9.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M., Turner R. S., Levy E. X11 interaction with beta-amyloid precursor protein modulates its cellular stabilization and reduces amyloid beta-protein secretion. J. Biol. Chem. 1998;273:22351–22357. doi: 10.1074/jbc.273.35.22351. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Yamazaki T., Citron M., Podlisny M. B., Koo E. H., Teplow D. B., Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann. N Y Acad. Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- Serafini T., Orci L., Amherdt M., Brunner M., Kahn R. A., Rothman J. E. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Simons M., Ikonen E., Tienari P. J., Cid-Arregui A., Monning U., Beyreuther K., Dotti C. G. Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites. J. Neurosci. Res. 1995;41:121–128. doi: 10.1002/jnr.490410114. [DOI] [PubMed] [Google Scholar]
- Sohn K., Orci L., Ravazzola M., Amherdt M., Bremser M., Lottspeich F., Fiedler K., Helms J. B., Wieland F. T. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J. Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi H., Tabira T. X11L2, a new member of the X11 protein family, interacts with Alzheimer's beta-amyloid precursor protein. Biochem. Biophys. Res. Commun. 1999;255:663–667. doi: 10.1006/bbrc.1999.0265. [DOI] [PubMed] [Google Scholar]
- Taru H., Suzuki T. Facilitation of stress-induced phosphorylation of beta-amyloid precursor protein family members by X11-like/Mint2 protein. J. Biol. Chem. 2004;279:21628–21636. doi: 10.1074/jbc.M312007200. [DOI] [PubMed] [Google Scholar]
- Tienari P. J., De Strooper B., Ikonen E., Ida N., Simons M., Masters C. L., Dotti C. G., Beyreuther K. Neuronal sorting and processing of amyloid precursor protein: implications for Alzheimer's disease. Cold Spring Harb. Symp. Quant. Biol. 1996a;61:575–585. [PubMed] [Google Scholar]
- Tienari P. J., De Strooper B., Ikonen E., Simons M., Weidemann A., Czech C., Hartmann T., Ida N., Multhaup G., Masters C. L., Van Leuven F., Beyreuther K., Dotti C. G. The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO J. 1996b;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- Tomita S., Ozaki T., Taru H., Oguchi S., Takeda S., Yagi Y., Sakiyama S., Kirino Y., Suzuki T. Interaction of a neuron-specific protein containing PDZ domains with Alzheimer's amyloid precursor protein. J. Biol. Chem. 1999;274:2243–2254. doi: 10.1074/jbc.274.4.2243. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L. A., Li Y., Zhang C. J., Kahn R. A. Isoform-selective effects of the depletion of ADP-ribosylation factors 1–5 on membrane traffic. Mol. Biol. Cell. 2005;16:4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli L. A., Lah J. J., Levey A. I. Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies. J. Biol. Chem. 2001;276:47590–47598. doi: 10.1074/jbc.M106535200. [DOI] [PubMed] [Google Scholar]
- Yan R., Han P., Miao H., Greengard P., Xu H. The transmembrane domain of the Alzheimer's beta-secretase (BACE1) determines its late Golgi localization and access to beta-amyloid precursor protein (APP) substrate. J. Biol. Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lee C. H., Mandiyan V., Borg J. P., Margolis B., Schlessinger J., Kuriyan J. Sequence-specific recognition of the internalization motif of the Alzheimer's amyloid precursor protein by the X11 PTB domain. EMBO J. 1997;16:6141–6150. doi: 10.1093/emboj/16.20.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Traub L. M., Kornfeld S. High-affinity binding of the AP-1 adaptor complex to trans-Golgi network membranes devoid of mannose 6-phosphate receptors. Mol. Biol. Cell. 1999;10:537–549. doi: 10.1091/mbc.10.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.