Abstract
In vertebrate embryos, signaling via the β-catenin protein is known to play an essential role in the induction of the dorsal axis. In its signaling capacity, β-catenin acts directly to affect target gene transcription, in concert with transcription factors of the TCF/LEF family. We have developed a cell-free in vitro assay for β-catenin signaling activity that utilizes transcriptionally active nuclei and cytoplasm from cleavage-blocked Xenopus laevis embryos. Under these assay conditions, we demonstrate that either addition of β-catenin protein or upstream activation of the β-catenin signaling pathway can induce the expression of developmentally relevant target genes. Addition of exogenous β-catenin protein induced expression of Siamois, XTwin, Xnr3, and Cerberus mRNAs in a protein synthesis independent manner, whereas a panel of other Spemann organizer-specific genes did not respond to β-catenin. Lithium induction of the β-catenin signaling pathway, which is thought to cause β-catenin accumulation by inhibiting its proteasome-dependent degradation, caused increased expression of Siamois in a protein synthesis independent fashion. This result suggests that β-catenin derived from a preexisting pool can be activated to signal, and that accumulation of this activated form does not require ongoing synthesis. Furthermore, activation of the signaling pathway with lithium did not detectably alter cytoplasmic β-catenin levels and was insensitive to inhibition of the proteasome- dependent degradation pathway. Taken together, these results suggest that activation of β-catenin signaling by lithium in this system may occur through a distinct activation mechanism that does not require modulation of levels through regulation of proteasomal degradation.
Keywords: β-catenin, Xenopus laevis, Siamois, signaling, assay
As a key intermediate in the Wnt signaling pathway, β-catenin and its homologues are known to play important roles in numerous developmental processes, including segmentation in Drosophila melanogaster (Peifer et al. 1991), cell fate determinations in Caenorhabditis elegans (Thorpe et al. 1997; Giarre et al. 1998), and dorsal axis formation in vertebrates (McCrea et al. 1993; Heasman et al. 1994; Funayama et al. 1995). In addition to its role in developmental events, the loss of control of β-catenin signaling has been shown to be an essential step in the progression of cancer in certain differentiated tissues (for review, see Gumbiner 1997; Bullions and Levine 1998).
As a central component of the cadherin adhesion complex, β-catenin is expressed widely in the cells of vertebrates. However, this cadherin-bound pool of β-catenin is not thought to directly participate in signaling. Rather, a pool of free β-catenin (not bound to cadherins) enters the nucleus (Fagotto et al. 1998) and, through direct interaction with transcription factors of the TCF/LEF family, affects transcriptional activation at target gene promoters (Behrens et al. 1996; Huber et al. 1996; Molenaar et al. 1996). Therefore, modulation of the levels or activity of these noncadherin bound pool(s) of β-catenin is likely to be crucial for effective control of the signaling pathway.
Maintenance of low levels of cytoplasmic β-catenin is brought about by ubiquitin-dependent degradation (Aberle et al. 1997; Orford et al. 1997; Jiang and Struhl 1998; Marikawa and Elinson 1998; for review, see Maniatis 1999). In cell culture systems, ubiquitin-dependent degradation of β-catenin is facilitated by the interaction of β-catenin with a number of proteins, including axin/conductin (Zeng et al. 1997; Behrens et al. 1998; Hart et al. 1998; Ikeda et al. 1998; Itoh et al. 1998; Kishida et al. 1998; Nakamura et al. 1998; Yamamoto et al. 1998), the adenomatous polyposis coli (APC)1 protein (Rubinfeld et al. 1993; Munemitsu et al. 1995), and Slimb/β-TRCP (Marikawa and Elinson 1998; Winston et al. 1999). In these systems, phosphorylation of NH2-terminal serine/threonine residues of β-catenin, directly or indirectly, by glycogen synthase kinase 3 (GSK-3; Yost et al. 1996) is thought to result in β-TRCP binding and subsequent delivery of targeted β-catenin to the proteasomal degradation pathway. Upstream activation of the Wnt signaling pathway is thought to result in the inactivation of GSK-3, and gives rise to a concomitant increase in the levels and/or signaling activity of β-catenin. While increased levels of β-catenin have been correlated with activation of the signaling pathway, other modes of regulation may exist (Young et al. 1998). For example, it is possible that regulation could occur at the level of nuclear import (Fagotto et al. 1998) or transcriptional activity at target gene promoters.
A number of target genes of β-catenin signaling have been identified, both in developmental and pathological cellular contexts. In Xenopus laevis, β-catenin/TCF responsive elements have been described in the promoters Siamois (Brannon et al. 1997), XTwin (Laurent et al. 1997), and Xnr3 (McKendry et al. 1997). In Drosophila, Engrailed and Ultrabithorax have been suggested to be transcriptionally responsive to β-catenin/TCF (Riese et al. 1997; van de Wetering et al. 1997). In colon cancer cells, c-myc and cyclin D1 have been identified as targets of the β-catenin signaling pathway (He et al. 1998; Shtutman et al. 1999; Tetsu and McCormick 1999). It is likely that β-catenin may target distinct sets of response genes, depending on the availability of regulatory cofactors or, alternatively, tissue-specific chromatin-based control of gene expression.
During early development in Xenopus, cortical rotation leads to the activation of β-catenin signaling in the dorsal region of blastula stage embryos and, subsequently, to the induction of the dorsal axis (for review, see Moon and Kimelman 1998). A number of studies have shown that β-catenin signaling is required for activities ascribed to the early dorsalizing inductive region known as the Nieuwkoop center (Heasman et al. 1994; Guger and Gumbiner 1995; Wylie et al. 1996). While secreted signals from the Nieuwkoop center are known to be sufficient for the induction of the Spemann organizer in overlying mesoderm (Nieuwkoop 1977; Wylie et al. 1996), recent data suggest that β-catenin signaling may also be able to induce Spemann organizer-specific genes in a cell autonomous manner (Darras et al. 1997; Laurent et al. 1997). In these studies, it is suggested that differential interpretation of β-catenin signaling along the animal/vegetal axis directs the formation of the Spemann organizer, even in the absence of Nieuwkoop-derived secreted signals (for review, see Kodjabachian and Lemaire 1998).
We have developed a cell-free assay system that utilizes cytoplasm and nuclei from cleavage-blocked embryos. This assay allows for detection of changes in gene expression upon addition of purified β-catenin protein or by addition of lithium, a GSK-3 inhibitor, and for the exclusion of indirect inductions that occur through cell–cell interactions or through intervening synthesis steps. We show that signaling induced by exogenous β-catenin in our cell-free assay system recapitulates direct inductive events that occur in whole embryos. This assay system has many advantages over whole embryo studies with regard to analysis of direct inductive events in the early embryo, and is shown to be useful for analysis of the of β-catenin signaling and regulation.
Materials and Methods
Preparation of Baculovirus-expressed β-Catenin Protein
β-Catenin protein containing an NH2-terminal 6-His tag was expressed in Sf9 cells using a baculoviral expression system, as described previously (Fagotto et al. 1998). Expressed protein was purified on NTA affinity resin (Qiagen).
Preparation of Cleavage-blocked (Coenocytic) Embryos
Eggs were obtained from HCG-treated Xenopus females (Nasco), and were fertilized by standard procedures. Cleavage blockade was achieved by a low-speed centrifugation procedure described by Newport and Kirschner 1982, and is described briefly below. 45 min after fertilization, embryos (in 0.1× MMR) were layered onto a 50% Ficoll cushion (in 0.1× MMR) and centrifuged at 500 g for 10 min at 15°C. Noncentrifuged sibling embryos were maintained as a reference for developmental stage. After centrifugation, cleavage blocked (coenocytic), multinucleate embryos and nonblocked sibling embryos were incubated at 18°C. Several hours after centrifugation, coenocytic embryos that showed clear evidence of attempted cleavage (evidenced by furrows in the lipid cap) were selected for use in the β-catenin signaling assay.
Cell-free Assay for β-Catenin Signaling
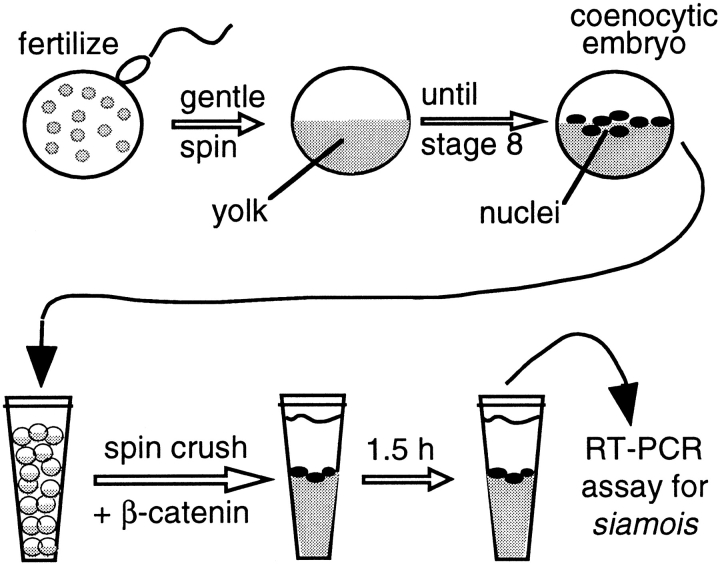
A schematic diagram of the cell-free assay used in these studies is shown in Fig. 1. When sibling control embryos reached stage 8 (unless otherwise noted), 20 coenocytic embryos were placed in 1.8-ml centrifuge tubes (one tube per experimental condition). To minimize dilution of cytoplasm upon disruption, 0.5 ml Versilube (Andpak-EMA) was layered over the embryos, followed by a brief spin at 300 g, which effectively separates embryos from aqueous buffer. The overlying separated buffer droplet and Versilube oil was aspirated gently and, as completely as possible, from the embryos. Before crushing embryos, a number of components were added over the intact coenocytic embryos. Proteinase inhibitors (PMSF + protease inhibitor cocktail II) were added to give the following cytoplasmic concentrations: 1 mM PMSF, 2 μg/ml leupeptin, 4 μg/ml aprotinin, 10 μg/ml antipain, 50 μg/ml benzamidine, 10 μg/ml STI, 100 μg/ml iodoacetamide. To provide for regeneration of cytoplasmic ATP stores, an energy mix was added to give cytoplasmic concentrations of 7.5 mM creatine phosphate, 1.0 mM ATP, and 50 μM magnesium chloride. To block protein synthesis, cycloheximide was added to give a cytoplasmic concentration of 5 μg/ml. Indicated amounts of β-catenin protein or lithium were then added to the embryos, followed by a 7-min spin crush centrifugation at 16,000 g (maximum centrifugal force in Eppendorf microcentrifuge) at 4–8°C. After spin crush, tubes were incubated at 22°C for 1.5 h, which is sufficient for maximal induction and expression of target genes, as indicated by semiquantitative reverse transcriptase (RT)-PCR. Increasing incubation time beyond this point was found to have detrimental effects on reproducibility.
Figure 1.
A cell-free assay system for β-catenin signaling. 40 min after fertilization, cleavage blockade of Xenopus embryos is brought about by a gentle spin over a Ficoll cushion (500 g), and then are incubated until nonblocked sibling embryos reach stage 8. β-Catenin, lithium, or other components are then introduced into embryonic cytoplasm by a 16,000 g spin crush, followed by an incubation to allow for induction of target genes in underlying nuclei.
Proteasome inhibitors ALLN and MG132 (both purchased from Sigma Chemical Co.; stocks were dissolved in ethanol at 25 mM) were included under certain experimental conditions in the cell-free assay. In these cases, the proteasome inhibitors were diluted 1:1,000 to give a cytoplasmic concentration of ∼25 μM.
Preparation of RNA and RT-PCR
Total RNA was isolated from crushed embryos using Ultraspec RNA isolation reagent (Biotecx). RNA from 20 embryos was resuspended in 400 μl of DEPC ddH2O (to give RNA concentrations of ∼300 μg/ml).
Detection of gene expression was carried out using standard RT-PCR procedures (Wilson and Melton 1994) using 32P-labeled dCTP and autoradiographic detection. Sequences of primer pairs used to detect the expression of various early Xenopus genes were as follows: EF-1α upstream, 5′ CAGATTGGTGCTGGATATGC 3′; EF-1α downstream, 5′ ACTGCCTTGATGACTCCTAG 3′; Siamois upstream, 5′ CGCGGATCCATGGCCTATGAGGCTGAAATGGAG 3′; Siamois downstream, 5′ GCTCTAGAGAAGTCAGTTTGGGTAGGGCT 3′; XTwin upstream, 5′ CATGACTTGTTGACTCTGA 3′; XTwin downstream, 5′ TGGCGTAGATCCCAGTAGA 3′; Xnr3 upstream, 5′ ATGGCATTTCTGAACCTG 3′; Xnr3 downstream, 5′ TCTACTGTCACACTGTGA 3′; Cerberus upstream, 5′ ATGTTACTAAATGTACTCAG 3′; Cerberus downstream, 5′ CTTGGCACCAGGCTTTTC 3′; Noggin upstream, 5′ ATGGATCATTCCCAGTGC 3′; Noggin downstream, 5′ TCTGTGCTTTTTGCTCTG 3′; Forkhead upstream, 5′ATGCTAAATAGAGTC-AAG3′; Forkhead downstream, 5′ GTAAGAGTATGGGGGCTT 3′; Xlim upstream, 5′ ATGGTTCACTGTGCTGGA 3′; Xlim downstream, 5′ GGGGTCACTGCCTGTTAC 3′; Follistatin upstream, 5′ ATGTTAAATGAAAGGATCCAG 3′; Follistatin downstream, 5′ TCTTCCCAGGGCCACAGTC 3′; Xotx2 upstream, 5′ ATGATGTCTTATCTCAAGC 3′; Xotx2 downstream, 5′ TGGCCTCCATTCTGCTGC 3′.
Protein Extractions, Western Blotting, and Metabolic Labeling of Proteins
Following standard electrophoretic and transfer procedures, Xenopus β-catenin was detected in Western blots using a polyclonal rabbit antibody directed against the NH2-terminal region of the protein. Proteins from crushed embryos were extracted with 1% NP-40 extraction buffer. For ConA precipitations of cadherin-bound β-catenin, 1/10 vol of a 1:1 slurry of ConA-Sepharose beads were added to protein extracts, and allowed to incubate 4 h overnight at 4°C. The efficacy of the ConA precipitations was indicated by the loss of detectable C-cadherin in Western blots. To test the effectiveness of cycloheximide inhibition of protein synthesis, 7 μCi of 35 S methionine (ProMix cell labeling mix; Nycomed Amersham, Inc., Pharmacia Biotech) was added to stage 8 coenocytic embryos before the spin crush step in the cell-free assay procedure. After 1.5 h, these samples were extracted in 1% NP-40, soluble material was separated electrophoretically, and visualized using fluorography. Immunoprecipitation of β-catenin was carried out using a rabbit polyclonal antibody directed against the NH2 terminus of the protein, followed by incubation with protein A–Sepharose beads.
Results
Cleavage Blockade Inhibits Normal Induction of β-Catenin Target Genes
In these studies, our goal was the development of a cell-free assay for analysis of the β-catenin signaling pathway. Such a system would require both transcriptionally active nuclei and cytoplasm that is competent to transmit a β-catenin dependent signal. Such a system would also require that, in the absence of added β-catenin (or alternative activators of the pathway), potential target genes of β-catenin signaling be either off or expressed at a low, basal level.
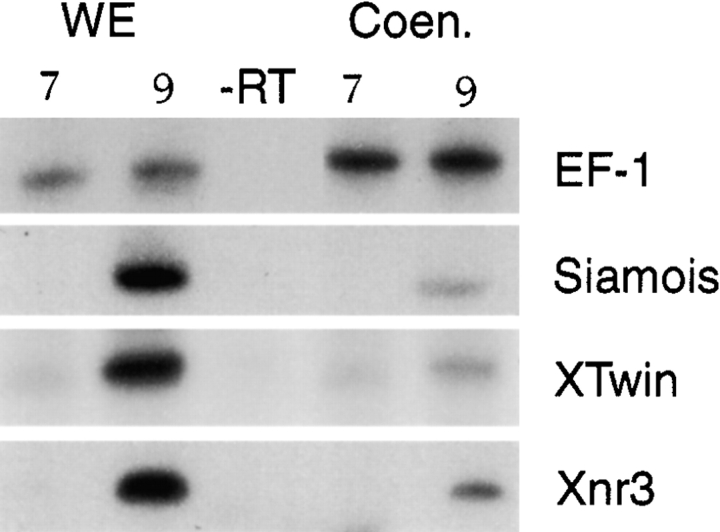
Cleavage blockade of embryos would be expected to preclude any intercellular communications that are required for specific activation of dorsal genes, and would result in a single, freely diffusing cytoplasm. The induction of a number of genes known to be downstream targets in the β-catenin signaling pathway during early development is significantly reduced in coenocytic embryos, compared with normal embryos (Fig. 2), although zygotic transcription occurs normally in these embryos (Newport and Kirschner 1982; Nelson and Gumbiner 1998). Therefore, the β-catenin mediated signaling events that induce target gene expression in normal embryos appear to be significantly reduced or disrupted in cleavage blocked embryos.
Figure 2.
Cleavage blockade reduces the normal expression of dorsal marker genes. Activation of β-catenin target genes and EF-1α (loading control) was assessed by RT-PCR in whole developing embryos (WE) and in coenocytic embryos (Coen.) at stage 7 (pre-MBT) and stage 9 (post-MBT).
A Cell-free Signaling Assay Recapitulates β-Catenin–dependent Inductive Events that Occur in the Embryo
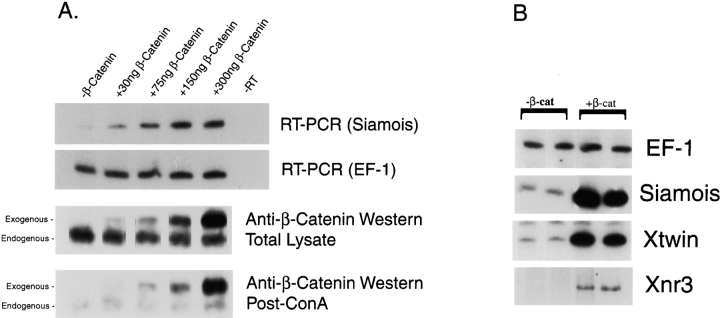
We developed a novel cell-free assay (see Materials and Methods and Fig. 1) that used endogenous nuclei and cytoplasm from cleavage-blocked embryos. Induction of the expression of Siamois, a known target gene of β-catenin signaling (Brannon et al. 1997; Nelson and Gumbiner 1998), was responsive to added β-catenin protein in a dose-dependent manner (Fig. 3 A). The levels of exogenous β-catenin required for these inductions was relatively low, compared with total endogenous β-catenin, but roughly similar to the levels of the nonglycoprotein-associated (presumably cytosolic) pools of β-catenin (Fig. 3 A).
Figure 3.
Target gene induction and β-catenin dose responsiveness in the cell-free assay system. A, Increasing amounts of β-catenin protein (as indicated) were added to 20 crushed embryos under standard cell-free assay conditions. RT-PCR (upper panel) analysis of Siamois expression revealed a dose-dependent induction. Western blot analysis of total lysate and ConA-Sepharose– treated (glycoprotein depleted) lysate was carried out using antibodies to the NH2-terminal region of β-catenin (lower two panels). Endogenous and free β-catenin show different electrophoretic mobility, due to the presence of a 6× His tag on the exogenous protein. Incubation of extracts with ConA-Sepharose effectively depleted C-cadherin below detectable levels (data not shown). B, In addition to Siamois, addition of β-catenin (1.5 μg/20 embryo condition) induces expression of XTwin and Xnr3 mRNA, as demonstrated by RT-PCR.
The addition of β-catenin into the crushed embryo system induced the expression of a number of genes previously described as direct targets of β-catenin signaling. β-catenin induced expression of Siamois, Xtwn, and Xnr3 (Fig. 3 B). These inductions demonstrate that all required steps for β-catenin signaling, including nuclear localization, interaction with TCF, and activation of transcription at target sites can all occur in this disrupted assay system.
β-Catenin Specifically Induces Siamois, Xnr3, XTwin, and Cerberus in a Protein Synthesis Independent Manner
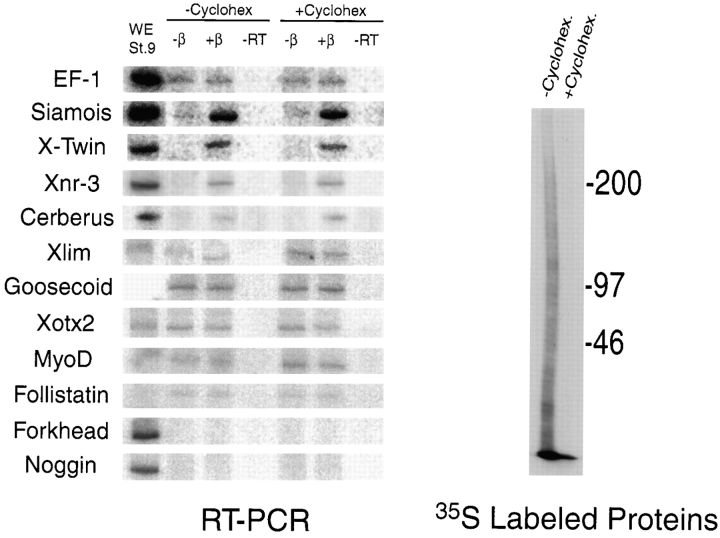
To further characterize the β-catenin induced signaling events that occur in the cell-free assay system, we carried out a comprehensive analysis of various early marker genes, focusing on those expressed in the Nieuwkoop center or Spemann organizer. In addition to inducing Siamois, Xnr3, and XTwin, β-catenin resulted in a weak, but reproducible, induction in the expression of Cerberus, a novel secreted factor expressed in the anterior endoderm that gives rise to head structures (Bouwmeester et al. 1996; Fig. 4). All of the genes induced by β-catenin were induced in a protein synthesis independent manner, suggesting these inductive events are direct. The ability of β-catenin to directly target Cerberus is a novel observation, as previous studies have suggested a requirement for intervening synthesis of Xtwn or Siamois protein. Goosecoid, which has been shown to respond to β-catenin signaling through intervening expression of XTwin and Siamois, is not induced by β-catenin protein under conditions where Siamois, Xnr3, and XTwin are induced. A number of other dorsal marker genes were evaluated (Fig. 4), none of which were induced by β-catenin, either in the presence or absence of protein synthesis.
Figure 4.
β-catenin induces a subset of dorsal marker genes in a protein synthesis independent manner. The standard cell-free assay (± β-catenin protein) was carried out with and without 5 μg/ml (cytoplasmic concentration) cycloheximide, as indicated. This dose of cycloheximide effectively inhibits protein translation in crushed embryos, as is evidenced by 35S incorporation (right). RT-PCR analysis (left) revealed that β-catenin induced Siamois, XTwin, Xnr3, and Cerberus in the presence or absence of protein synthesis. Other genes that were analyzed were not induced by β-catenin in any condition. The lane marked WE St. 9 represents an RT-PCR reaction conducted on cDNA derived from normal embryos at stage 9 (at the termination of the assay).
Lithium Induces Downstream Target Genes in the Cell-free Assay without New Synthesis of β-Catenin
It is known that lithium has dorsalizing effects on Xenopus embryos (Aoshima et al. 1986; Kao and Elinson 1988). While lithium is thought to inhibit the phosphoinositol cycle in Xenopus (Busa and Gimlich 1989), it is likely that the dorsalizing activity of lithium occurs through activation of the Wnt pathway by direct inhibition of GSK-3 (Stambolic et al. 1996; Hedgepeth et al. 1997). In the prevailing turnover model for Wnt signaling, inactivation of GSK-3 by lithium results in blockage of the ongoing ubiquitin-dependent degradation of cytosolic β-catenin as a result of its dephosphorylation. This stabilization of β-catenin is thought to lead to its accumulation in the cytoplasm, which in turn allows for activation of downstream dorsal target genes. In the turnover model, continuing synthesis of β-catenin would provide the source of protein that would allow for enhanced cytosolic levels.
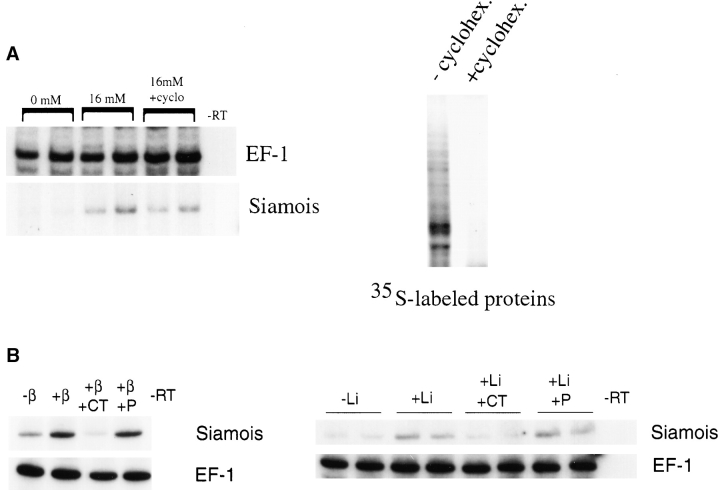
In the cell-free assay, Siamois was induced by the addition of lithium (Fig. 5 A). The inductive effect of lithium was dependent on β-catenin, as sequestration of β-catenin by addition of purified C-cadherin intracellular domain protein was inhibitory (Fig. 5 B). In contrast, an equivalent amount of the juxtamembrane portion of the intracellular domain of C-cadherin, which does not bind β-catenin, was not inhibitory (Fig. 5 B). Therefore, as in normal embryos, lithium can activate an endogenous signaling pool of β-catenin in the cell-free assay system. Induction of Siamois by lithium occurred in the absence of protein synthesis (Fig. 5 A), suggesting that the β-catenin responsible for this signaling event is derived from preexisting pools, not from newly translated protein. This lack of a requirement for ongoing protein synthesis was surprising in the context of the current turnover model for β-catenin–dependent signaling, in which increased levels of cytosolic β-catenin are responsible for activation of the signaling pathway.
Figure 5.
Lithium induction of Siamois mRNA expression. A, Lithium was introduced at a final cytoplasmic concentration of 16 mM in crushed embryo cytoplasm under standard assay conditions. RT-PCR analysis of gene expression reveals that this concentration of lithium induces Siamois in the presence or absence of protein synthesis. EF-1α serves as a loading control. Protein synthesis was blocked by inclusion of cycloheximide in the crushed embryo cytoplasm (final cytoplasmic concentration, 5 μg/ml), as is demonstrated by the loss of metabolically labeled protein bands in the right panel. B, RT-PCR analysis of Siamois expression reveals that addition of 2 μl of 1 mg/ml recombinant C-cadherin intracellular domain protein (CT) effectively blocks induction by exogenous β-catenin (left) or lithium (right), whereas equivalent amounts of the membrane-proximal portion (P) of the intracellular domain of C-cadherin (lacking catenin binding sites) has no effect (right and left).
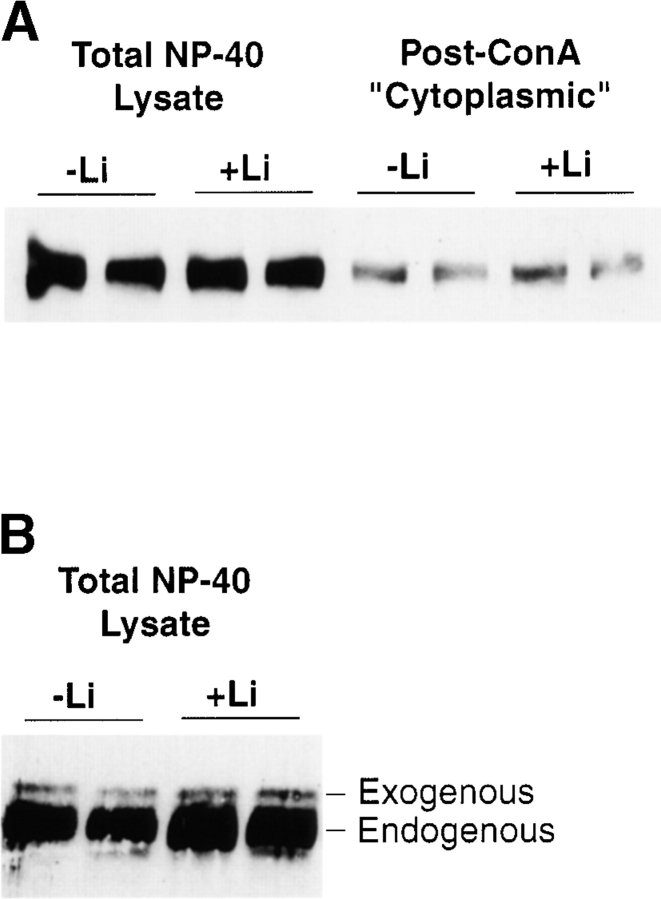
It is thought that proteasome-dependent degradation of β-catenin in the absence of upstream signal serves to block accumulation of cytosolic pools. In other systems, the signaling activity of lithium (Stambolic et al. 1996) and proteasomal inhibitors (Easwaran et al. 1999) have been correlated with increased levels of β-catenin protein. In our in vitro assay system, there was no detectable increase in β-catenin levels (total or nonglycoprotein-associated) in response to activating levels of added lithium (Fig. 6A and Fig. B). However, it should be noted that the lithium induction of Siamois (approximately twofold) is relatively weak, compared with maximal induction brought about by β-catenin protein (greater than tenfold). Based on our titration of β-catenin protein (see Fig. 3), a response similar to that seen with lithium would require an approximate twofold increase over levels of cytoplasmic β-catenin. Thus, while we detect no such changes in levels in our experiments, it may be difficult to detect minor changes in β-catenin protein levels.
Figure 6.
Lithium does not affect the levels of endogenous or exogenous β-catenin. A, An anti–β-catenin probed Western blot shows levels of endogenous total or non-ConA precipitable (post-ConA) β-catenin, with or without lithium induction. B, Addition of lithium did not affect the stability of small, nonsignaling amounts of added recombinant β-catenin. 7.5 ng of β-catenin protein was added to assay tubes in the presence or absence of added lithium. Total 1% NP-40 lysate was analyzed by Western blot using antibodies against β-catenin. Exogenous and endogenous β-catenin are distinguished by their electrophoretic mobility, as marked.
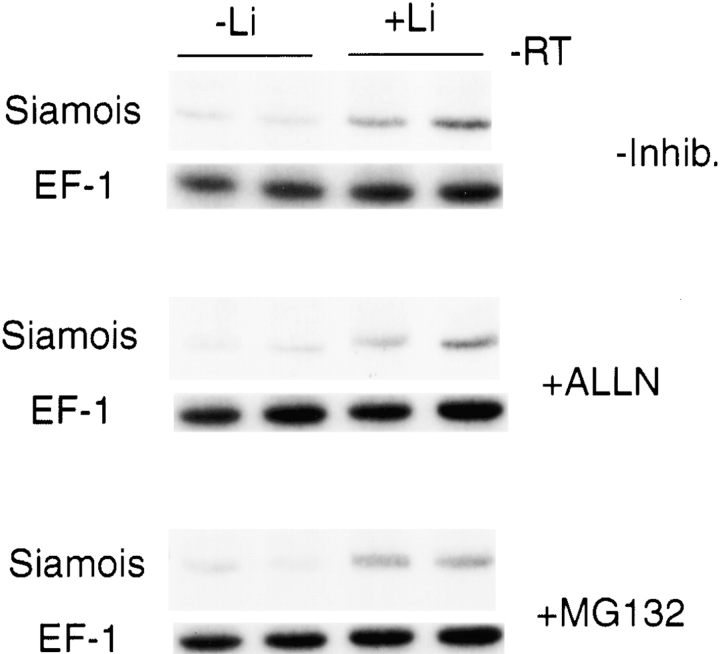
The addition of proteasome inhibitors ALLN or MG132, like lithium, did not detectably alter β-catenin levels in our assay system (data not shown). In addition, inclusion of proteasome inhibitors did not lead to an overall increase in polyubiquitinated proteins, as assessed by Western blots using antiubiquitin antibodies (data not shown), suggesting that proteasomal degradation machinery may not be strongly active in our assay system. Furthermore, proteasome inhibitors did not activate Siamois, nor did they have any effect on lithium-induced activation of Siamois (Fig. 7). Taken together, these results suggest that activation of β-catenin signaling by lithium likely involves mechanisms other than modulation of levels through regulation of proteasomal degradation.
Figure 7.
Effects of proteasome inhibitors on Siamois induction. Samples treated with lithium and/or the proteasome inhibitors ALLN or MG132 were evaluated by RT-PCR using Siamois and EF1α primer sets. Proteasome inhibitors (25 μM), introduced upon crushing the embryos, did not induce Siamois and did not affect lithium induction of Siamois.
Discussion
We have developed a novel cell-free assay system that recapitulates β-catenin–mediated signaling events occurring in the Xenopus embryo. From a developmental standpoint, this assay system has general utility in identification of transcriptional targets of early developmental signaling. In addition, this assay provides a potentially useful new tool for molecular analysis of the complex regulatory mechanisms that control β-catenin signaling.
This cell-free assay system offers several clear advantages over cleaving embryos as a system for identification of direct transcriptional target genes. First, in the cell-free assay system, signaling occurs in a single, uniform cytoplasm in the absence of any intercellular communication, which could lead to secondary inductions. Secondly, as signaling components in the assay are introduced at a time when the embryo is able to respond transcriptionally, the assay allows for a precise timing of inductive events. This is in contrast with RNA injection into cleaving embryos, which requires injection before the onset of zygotic transcription. Because of these advantages, the cell-free assay holds promise not just for studies of β-catenin signaling, but may also be adapted for other signaling pathways in the early embryo.
To confirm the efficacy of our assay system, we first evaluated the ability of β-catenin to induce expected target genes. Siamois, XTwin, and Xnr3 have all been reported to be direct targets of β-catenin signaling, and were all found to be specifically induced in the cell-free assay system. Thus, all required events for induction of target genes, including nuclear localization and interaction with TCF family members, can occur in the cell-free system.
In addition to known target genes, the assay allowed us to identify Cerberus as an additional direct target of β-catenin signaling. Previous reports have shown that activation of the β-catenin signaling pathway results in induction of Cerberus. These whole embryo injection studies suggested that Cerberus may be induced through the secondary inductive effects of the Siamois gene product, rather than direct activation by β-catenin (Darras et al. 1997). It is clear in our assay, however, that induction of Siamois or Twin proteins is not required for the Cerberus response to β-catenin, as the Cerberus induction is protein synthesis independent. There are two possible explanations for these differing results. First, as there is some basal level of expression of Siamois and Twin in our assay, it is possible that β-catenin/TCF and Siamois and/or Twin protein cooperate at the Cerberus promoter. Alternatively, in the embryo, it is possible that Siamois may activate β-catenin signaling, which then directly activates Cerberus. Indeed, there is evidence that Siamois induces itself by a feedback mechanism (Fan et al. 1998) and, given the strong direct inductive effects of β-catenin on Siamois, it is not unreasonable to consider that this may occur indirectly via β-catenin.
While we observed a number of direct inductions of dorsal genes, we found no evidence for secondary inductions (that is, inductions that require intervening cell-cell communications or protein synthesis) under the conditions used for the assay. Particularly notable, in this regard, is the absence of Goosecoid induction. Goosecoid has been reported to involve wnt/β-catenin responsive elements, as well as activin/TGFβ responsive elements (Crease et al. 1998). Wnt/β-catenin responsiveness has been attributed to direct binding of Xtwn (and likely Siamois) at the Goosecoid promoter (Laurent et al. 1997). Since XTwin mRNA expression is strongly induced in the cell-free assay system, the absence of Goosecoid induction is of interest. While we cannot rule out technical reasons for the absence of Goosecoid induction (i.e., insufficient time for secondary inductions or inappropriate cytoplasmic environment), it is possible that induction at the Goosecoid promoter normally requires additional input that in normal embryos would derive from a Nieuwkoop center signaling activity. In addition, none of the other early dorsal marker genes we evaluated were induced by β-catenin, either in the presence or absence of protein synthesis. This would suggest that intervening cell–cell communication or synthesis steps that are required for activation of these genes do not occur in the cell-free assay system.
In addition to identification of target genes, we also used the cell-free assay to examine the source of endogenous signaling pools of β-catenin after addition of lithium. While lithium is known to exert effects on phosphoinositol metabolism in a number of systems, more recent evidence suggests that the dorsalizing effect of lithium on early Xenopus embryos occurs primarily through lithium's inhibitory effects on GSK-3 (Stambolic et al. 1996; Hedgepeth et al. 1997). According to the current turnover model for regulation of β-catenin signaling (Peifer 1996), the downregulation of GSK-3 activity results in the stabilization of free cytoplasmic pools of β-catenin in dorsal cytoplasm, and subsequent activation of dorsal genes. In Drosophila, activation of the Wnt pathway can lead to a net increase in β-catenin in cells, presumably through accumulation of newly synthesized protein (Peifer et al. 1994).
The addition of lithium in our assay system results in an induction of Siamois expression. This induction requires the participation of free pools of β-catenin, as is indicated by the sensitivity of this response to added C-cadherin intracellular domain protein. These observations indicate that, in the cell-free assay system, lithium is capable of generating an endogenous signaling pool of β-catenin. In addition, the insensitivity of the inductive response to protein synthesis inhibition suggests that accumulation of newly synthesized β-catenin is not required for β-catenin signaling activity. Rather, it appears that actively signaling β-catenin protein can be wholly derived from a preexisting, nonsignaling pool (for instance, pools associated with cadherins or cytoplasmic molecules, such as axin and APC), with no requirement for newly synthesized protein.
In these studies, lithium activates endogenous β-catenin signaling in the absence of an input of protein from new synthesis and without detectable increases in cytoplasmic levels of β-catenin. Furthermore, while in some systems proteasomal inhibitors have been shown to activate β-catenin signaling (Easwaran et al. 1999), proteasomal inhibitors in our assay had no effect on levels of β-catenin and did not influence the activation of the signaling pathway with lithium. Thus, signaling in this assay does not appear to rely on the accumulation of cytoplasmic β-catenin brought about by regulation of the proteasomal degradation pathway. Rather, these results could be explained by an alteration in the intrinsic signaling activity of cytoplasmic β-catenin. Indeed, recent studies in our laboratory have indicated that putative GSK-3 phosphorylation sites in the NH2 terminus of β-catenin may regulate its signaling activity independent of levels (Gumbiner, B.M., manuscript submitted for publication), even in the whole embryo. These findings suggest the possibility of the existence of an alternative mechanism of activation that may act independently or in parallel with regulation of cytoplasmic levels of β-catenin.
By allowing direct access to the cytoplasm, the described cell-free assay makes possible a wide range of potential biochemical manipulations in addition to the applications presented here. For example, conditions of this assay could be adapted to allow for testing the effects of immunodepletion of associated components of the β-catenin signaling pathway. Also, the assay is well-suited to allow for assessing the signaling activity of exogenously derived cytoplasm, or for testing cytoplasmic fractions or various purified components for regulatory activities.
Acknowledgments
We thank Dr. Carien Niessen for providing the recombinant C-cadherin intracellular domain proteins used in these studies.
This work was supported by a National Institutes of Health grant (GM37432) awarded to B.M. Gumbiner, by the Dewitt Wallace Fund for Memorial Sloan-Kettering Cancer Center, and by a Cancer Center Support grant NCI-P30-CA-08784. R.W. Nelson is a recipient of a National Research Service Award postdoctoral fellowship (GM17948-02) from the National Institutes of Health.
Footnotes
1.used in this paper: APC, adenomatous polyposis coli; GSK-3, glycogen synthase kinase 3; RT, reverse transcriptase
Richard W. Nelson's current address is Biology Department, Wabash College, Crawfordsville, IN 47933.
References
- Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. Beta-catenin is a target for the ubiquitin–proteasome pathway. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshima H., Iio H., Kobayashi S. Li+ uptake into Xenopus and Cynops oocytes injected with exogenous mRNA, observed by flame emission spectroscopy. Anal. Biochem. 1986;156:257–262. doi: 10.1016/0003-2697(86)90181-8. [DOI] [PubMed] [Google Scholar]
- Behrens J., von Kries J.P., Kuhl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Behrens J., Jerchow B.A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T., Kim S., Sasai Y., Lu B., De Robertis E.M. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Brannon M., Gomperts M., Sumoy L., Moon R.T., Kimelman D. A beta-catenin/XTcf-3 complex binds to the Siamois promoter to regulate dorsal axis specification in Xenopus . Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullions L.C., Levine A.J. The role of beta-catenin in cell adhesion, signal transduction, and cancer. Curr. Opin. Oncol. 1998;10:81–87. doi: 10.1097/00001622-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Busa W.B., Gimlich R.L. Lithium-induced teratogenesis in frog embryos prevented by a polyphosphoinositide cycle intermediate or a diacylglycerol analog. Dev. Biol. 1989;132:315–324. doi: 10.1016/0012-1606(89)90228-5. [DOI] [PubMed] [Google Scholar]
- Crease D.J., Dyson S., Gurdon J.B. Cooperation between the activin and Wnt pathways in the spatial control of organizer gene expression. Proc. Natl. Acad. Sci. USA. 1998;95:4398–4403. doi: 10.1073/pnas.95.8.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras S., Marikawa Y., Elinson R.P., Lemaire P. Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinantsimplications for the patterning of the organiser. Development. 1997;124:4275–4286. doi: 10.1242/dev.124.21.4275. [DOI] [PubMed] [Google Scholar]
- Easwaran V., Song V., Polakis P., Byers S. The ubiquitin proteasome pathway and serine kinase activity modulate adenomatous polyposis coli protein-mediated regulation of β-catenin-lymphocyte enhancer-binding factor signaling. J. Biol. Chem. 1999;274:16641–16645. doi: 10.1074/jbc.274.23.16641. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Gluck U., Gumbiner B.M. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr. Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- Fan M.J., Gruning W., Walz G., Sokol S.Y. Wnt signaling and transcriptional control of Siamois in Xenopus embryos Proc. Natl. Acad. Sci. USA. 95101998. 5626 5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N., Fagotto F., McCrea P., Gumbiner B.M. Embryonic axis induction by the armadillo repeat domain of beta-cateninevidence for intracellular signaling. J. Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarre M., Semenov M.V., Brown A.M. Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann. NY Acad. Sci. 1998;857:43–55. doi: 10.1111/j.1749-6632.1998.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Guger K.A., Gumbiner B.M. Beta-catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal–ventral patterning. Dev. Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. Carcinogenesisa balance between beta-catenin and APC. Curr. Biol. 1997;7:R443–R446. doi: 10.1016/s0960-9822(06)00214-4. [DOI] [PubMed] [Google Scholar]
- Hart M.J., de los Santos R., Albert I.N., Rubinfeld B., Polakis P. Downregulation of beta-catenin by human axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr. Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- He T.C., Sparks A.B., Rago C., Hermeking H., Zawel L., da Costa L.T., Morin P.J., Vogelstein B., Kinzler K.W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford A., Goldstone K., Garner-Hamrick P., Gumbiner B., McCrea P., Kintner C., Noro C.Y., Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hedgepeth C.M., Conrad L.J., Zhang J., Huang H.C., Lee V.M., Klein P.S. Activation of the Wnt signaling pathwaya molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- Huber O., Korn R., McLaughlin J., Ohsugi M., Herrmann B.G., Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech. Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Krupnik V.E., Sokol S.Y. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr. Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- Jiang J., Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Kao K.R., Elinson R.P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kishida S., Yamamoto H., Ikeda S., Kishida M., Sakamoto I., Koyama S., Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- Kodjabachian L., Lemaire P. Is the Nieuwkoop centre a useful concept? Curr. Biol. 1998;8:R918–R921. doi: 10.1016/s0960-9822(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Laurent M.N., Blitz I.L., Hashimoto C., Rothbacher U., Cho K.W. The Xenopus homeobox gene Twin mediates Wnt induction of goosecoid in establishment of Spemann's organizer. Development. 1997;124:4905–4916. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- Maniatis T. A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- Marikawa Y., Elinson R.P. Beta-TrCP is a negative regulator of Wnt/beta-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech. Dev. 1998;77:75–80. doi: 10.1016/s0925-4773(98)00134-8. [DOI] [PubMed] [Google Scholar]
- McCrea P.D., Brieher W.M., Gumbiner B.M. Induction of a secondary body axis in Xenopus by antibodies to β-catenin. J. Cell Biol. 1993;123:477–484. doi: 10.1083/jcb.123.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendry R., Hsu S.C., Harland R.M., Grosschedl R. LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev. Biol. 1997;192:420–431. doi: 10.1006/dbio.1997.8797. [DOI] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., Roose J., Destree O., Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Moon R.T., Kimelman D. From cortical rotation to organizer gene expressiontoward a molecular explanation of axis specification in Xenopus . Bioessays. 1998;20:536–545. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Hamada F., Ishidate T., Anai K., Kawahara K., Toyoshima K., Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- Nelson R.W., Gumbiner B.M. β-catenin directly induces expression of the Siamois gene, and can initiate signaling indirectly via a membrane-tethered form. Ann. NY Acad. Sci. 1998;857:86–98. doi: 10.1111/j.1749-6632.1998.tb10109.x. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos. I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D. Origin and establishment of embryonic polar axes in amphibian development. Curr. Top. Dev. Biol. 1977;11:115–132. doi: 10.1016/s0070-2153(08)60744-9. [DOI] [PubMed] [Google Scholar]
- Orford K., Crockett C., Jensen J.P., Weissman A.M., Byers S.W. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Peifer M. Regulating cell proliferationas easy as APC. Science. 1996;272:974–975. doi: 10.1126/science.272.5264.974. [DOI] [PubMed] [Google Scholar]
- Peifer M., Rauskolb C., Williams M., Riggleman B., Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development. 1991;111:1029–1043. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- Peifer M., Sweeton D., Casey M., Wieschaus E. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Riese J., Yu X., Munnerlyn A., Eresh S., Hsu S.C., Grosschedl R., Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Muller O., Chamberlain S.H., Masiarz F.R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R., Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V., Ruel L., Woodgett J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells [published erratum appears in Curr. Biol. 1997. 7:196] Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Tetsu O., McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Thorpe C.J., Schlesinger A., Carter J.C., Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es J., Loureiro J., Ypma A., Hursh D., Jones T., Bejsovec A. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF . Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Wilson P.A., Melton D.A. Mesodermal patterning by an inducer gradient depends on secondary cell–cell communication. Curr. Biol. 1994;4:676–686. doi: 10.1016/s0960-9822(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Winston J.T., Strack P., Beer-Romero P., Chu C.Y., Elledge S.J., Harper J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie C., Kofron M., Payne C., Anderson R., Hosobuchi M., Joseph E., Heasman J. Maternal beta-catenin establishes a “dorsal signal” in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Kishida S., Uochi T., Ikeda S., Koyama S., Asashima M., Kikuchi A. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos. Mol. Cell. Biol. 1998;18:2867–2875. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C., Torres M., Miller J.R., Huang E., Kimelman D., Moon R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Young C.S., Kitamura M., Hardy S., Kitajewski J. Wnt-1 induces growth, cytosolic β-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol. Cell. Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Fagotto F., Zhang T., Hsu W., Vasicek T.J., Perry W.L., 3rd, Lee J.J., Tilghman S.M., Gumbiner B.M., Costantini F. The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]