Abstract
The multiprotein complex, dynactin, is an integral part of the cytoplasmic dynein motor and is required for dynein-based motility in vitro and in vivo. In living cells, perturbation of the dynein–dynactin interaction profoundly blocks mitotic spindle assembly, and inhibition or depletion of dynein or dynactin from meiotic or mitotic cell extracts prevents microtubules from focusing into spindles. In interphase cells, perturbation of the dynein–dynactin complex is correlated with an inhibition of ER-to-Golgi movement and reorganization of the Golgi apparatus and the endosome–lysosome system, but the effects on microtubule organization have not previously been defined. To explore this question, we overexpressed a variety of dynactin subunits in cultured fibroblasts. Subunits implicated in dynein binding have effects on both microtubule organization and centrosome integrity. Microtubules are reorganized into unfocused arrays. The pericentriolar components, γ tubulin and dynactin, are lost from centrosomes, but pericentrin localization persists. Microtubule nucleation from centrosomes proceeds relatively normally, but microtubules become disorganized soon thereafter. Overexpression of some, but not all, dynactin subunits also affects endomembrane localization. These data indicate that dynein and dynactin play important roles in microtubule organization at centrosomes in fibroblastic cells and provide new insights into dynactin–cargo interactions.
Keywords: dynein, dynactin, centrosomes, γ tubulin, cytoarchitecture
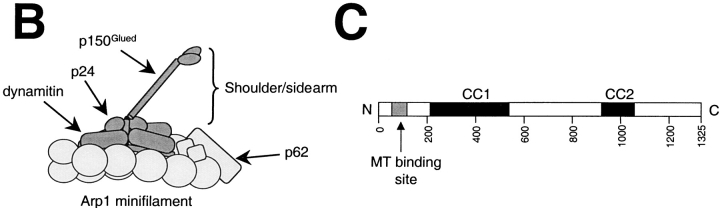
Cytoplasmic dynein is the predominant minus end–directed microtubule motor in eukaryotic cells. This large, multisubunit enzyme works in conjunction with a second multiprotein complex, dynactin, which was first discovered as a factor that could activate cytoplasmic dynein-driven vesicle movement in vitro (Gill et al. 1991; Schroer and Sheetz 1991). Dynactin is generally believed to function as an adapter that allows dynein to bind cargo. Dynactin has two distinct structural domains, an actin-like minifilament backbone and a flexible projecting sidearm (Schafer et al. 1994; Allan 1996; Schroer 1996; see Fig. 1). Dynein is thought to bind the dynactin sidearm subunit, p150Glued (Karki and Holzbaur 1995; Vaughan and Vallee 1995). The distal end of the p150Glued sidearm also contains a pair of microtubule binding sites (one per p150Glued subunit; Waterman-Storer et al. 1995) whose functions are not completely understood. Transient microtubule binding by dynactin may stabilize the dynein–microtubule interaction and allow the dynein motor to move more processively (King, S.J., and T.A. Schroer, manuscript submitted for publication). As seen for its homologue, CLIP-170 (Pierre et al. 1992), p150Glued microtubule binding activity may be regulated to allow for stable, high-affinity binding under some circumstances. p150Glued, along with the dynamitin and p24 subunits, forms a stable subcomplex in dynactin that is referred to as the shoulder/sidearm (Eckley et al. 1999). This protein complex can be released from dynactin by chaotropic salts or an excess of dynamitin (Echeverri et al. 1996; Karki et al. 1998; Eckley et al. 1999). Cells overexpressing dynamitin thus contain free shoulder/sidearm that is no longer attached to the actin-like backbone. It is believed that dynein can still bind the shoulder/sidearm, but now lacks a mechanism for binding cargo, which leads to a wide variety of motility defects.
Figure 1.
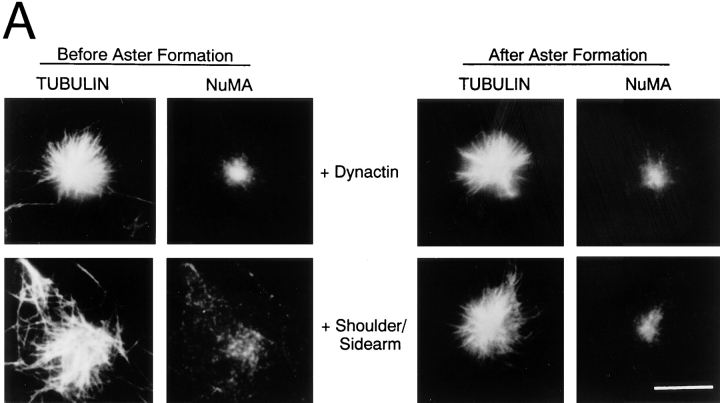
(A) Effect of shoulder/sidearm on dynein-dependent microtubule focusing in vitro. Excess dynactin (top) or purified dynactin shoulder/sidearm (bottom) was added to the cell extract before (left) or after (right) aster formation. The samples were fixed and stained with antibodies to tubulin and NuMA. Bar, 10 μm. (B) Schematic representation of dynactin structure. Shoulder/sidearm components are indicated by dark shading. (C) Schematic depicting the organization of chicken p150Glued. The gray boxes indicate the positions of the predicted coiled-coil 1 (CC1) and coiled-coil 2 (CC2). The cDNA (GenBank/EMBL/DDBJ accession number AF191146, see Materials and Methods) encodes a protein that contains the NH2-terminal microtubule binding domain and is largely homologous to rat p150Glued (accession number X62160; Holzbaur et al. 1991) and our original chicken p150Glued clone (accession number X62773; Gill et al. 1991).
The dynein/dynactin motor has been proposed to drive a variety of motile events in mitosis and meiosis (Karki and Holzbaur 1999). Much attention has focused on spindle poles, where dynein and dynactin are proposed to play multiple roles (Compton 1998). In living cells, perturbation of either protein results in defective spindle pole separation and a general loss of pole integrity (Vaisberg et al. 1993; Echeverri et al. 1996). In in vitro systems that reconstitute spindle or aster formation, depletion or inhibition of either dynein, or dynactin results in unfocused, aberrant microtubule arrays (Verde et al. 1991; Gaglio et al. 1996; Heald et al. 1996; Merdes et al. 1996). Dynein is thought to provide a focusing activity that retains loosely associated microtubule minus ends at the spindle pole and counterbalances the opposing forces of centrosome-associated plus end–directed motors of the BimC family.
Although it is well-established that dynein and dynactin provide a critical microtubule focusing activity at spindle poles, little is known about their contributions to centrosome function in nonmitotic cells. Centrosomes are the primary site of microtubule nucleation, but once assembled, microtubules can have multiple fates. In fibroblasts, most appear to project radially from a single spot, the microtubule organizing center, suggesting that they remain tightly associated with the centrosome. In neurons and polarized epithelial cells, in contrast, many microtubules are released from centrosomes and become reorganized into nonradial arrays that project into neurites or away from the apical face of the cell. Here, dynein may promote microtubule release from centrosomes (Keating et al. 1997; Ahmad et al. 1998). That microtubule release commonly occurs in nonfibroblastic cells and in all cells during mitosis suggests that it may also occur in interphase fibroblasts. In this case, dynein and dynactin might be expected to promote microtubule focusing as in spindles. In support of this hypothesis, overexpression of a mutant dynein heavy chain in Dictyostelium is found to result in aberrant microtubule organization (Koonce and Samso 1996). Moreover, dynactin is highly concentrated at centrosomes in fibroblasts (Gill et al. 1991; Clark and Meyer 1992; Paschal et al. 1993), suggesting that it may recruit dynein to this organelle or otherwise contribute to centrosome function.
Centrosome assembly and duplication require intact microtubules (Kuriyama 1982), which suggests that newly synthesized centrosome components may be actively transported toward the parent centrosome via a dynein/dynactin-dependent mechanism. When the cell and centrosome cycles are decoupled by pharmacological treatment, new centrosomes continue to be formed (Balczon et al. 1995). If microtubules are depolymerized, pericentriolar proteins no longer assemble into new centrosomes, but instead remain dispersed throughout cytoplasm (Balczon et al. 1999). These proteins bind microtubules in a dynactin-dependent manner, consistent with the hypothesis that the dynein/dynactin motor complex drives transport of centrosome precursors to the growing centrosome. Thus, dynein and dynactin may contribute in additional ways to centrosome function.
In the present study, we have examined the role played by dynactin in microtubule organization in vivo and in vitro. In an in vitro assay for mitotic aster formation (Gaglio et al. 1996), addition of excess free shoulder/sidearm, but not intact dynactin, inhibits mitotic aster formation. Overexpression in fibroblasts of any of the three shoulder/sidearm subunits, as well as fragments of the dynein-binding subunit p150Glued, causes the normal radial microtubule array to lose focus and become disorganized. Microtubule regrowth after depolymerization is delayed, suggesting a loss of nucleating activity from centrosomes. Consistent with this, γ tubulin appears in ectopic foci, while pericentrin, another centrosomal protein, is not affected. Regrowing microtubules form a radial array at first, but within a matter of hours the array becomes disorganized. Overexpression of most shoulder/sidearm components does not detectably alter dynactin structure, suggesting that these proteins act in a dominant negative fashion, perhaps by serving as competitive inhibitors of the dynein–dynactin interaction. Our results provide the first evidence that, in nonmitotic fibroblasts, dynactin is a major contributor to microtubule organization and centrosome integrity.
Materials and Methods
Mitotic Aster Assembly Assay
Mitotic asters were assembled in HeLa cell lysates as previously described (Gaglio et al. 1995). In brief, synchronized cells were homogenized and a postnuclear supernatant was prepared. Endogenous microtubules were stabilized by addition of taxol. Purified shoulder/sidearm (see below) or intact dynactin was added to the extract at a concentration approximately equal to the endogenous dynactin concentration, as estimated from immunoblots for p150Glued (D.A. Compton, unpublished observations).
Purification of Dynactin Shoulder/Sidearm Complex
Purified bovine brain dynactin was prepared as described (Bingham et al. 1998) and shoulder/sidearm isolated as described (Eckley et al. 1999). In brief, 10 mg of dynactin was dissociated by adding 0.7 M potassium iodide, incubated on ice for 30 min, and then dynactin subcomplexes and subunits were separated by gel filtration chromatography on a Superose12 column (Pharmacia LKB Biotechnology, Inc.). Fractions of interest were dialyzed, and then sedimented into a 5–20% sucrose gradient. Shoulder/sidearm complex purified by this method was cryoprotected by addition of 1.25 M sucrose, snap frozen in small aliquots, and stored at −80°C for later use.
Expression Constructs
A full-length chicken p150Glued cDNA was obtained by screening a λgt10 library (gift of B. Ranscht, Scripps Laboratories Inc.) with the original p150Glued clone, p150A (Gill et al. 1991). The insert was subcloned into the EcoRI site of pGW1-CMV (Compton and Cleveland 1993). Constructs encoding the predicted coiled-coil regions (CC1 and CC2; see Fig. 1 C) of p150Glued were engineered using PCR from p150A (Gill et al. 1991). CC1 (amino acids 217–548) was made using the primers CGTGCCATGGAGGAAGAAAATCTGCGTTCC (upstream) and CCGGGATCCTTACTGCTGCTGCTTCTCTGC (downstream). CC2 (amino acids 926–1049) was made using primers CGTGCCATGGCCGAGCTGCGGGCAGCTGC (upstream) and CCGGGATCCTTACCCCTCGATGGTCCGCTTGG (downstream). Both PCR products were ligated into pTA (Invitrogen Corp.), subcloned into the NcoI and BamHI sites of pET-3c (Novagen, Inc.), subcloned again into pVEX using XbaI and EcoRI, and then finally into pGW1-CMV using NdeI and BamHI. The mouse p24 gene was characterized by sequencing EST AA002440 completely on both strands. It contained a single conservative amino acid substitution (E131–Q131) when compared with a previously published mouse p24 gene (Pfister et al. 1998). p24-green fluorescent protein (GFP)1 was engineered by subcloning the entire p24 cDNA into the EcoRI site of pEGFP-C2 (Clontech). Orientation was determined by diagnostic digests and the fusion open reading frame was confirmed by sequencing. Dynamitin-HA in pCB6 was a gift from C. Valetti (Valetti et al. 1999). Dynamitin-GFP in pcDNA3 was a gift from E. Vaisberg (University of Colorado, Boulder, CO). In fixed cells, GFP-tagged proteins were detected by their intrinsic fluorescence; Abs were used on blots.
Antibodies
p150Glued: mAb 150.1 (Steuer et al. 1990), mAb 150B (Gaglio et al. 1996; Blocker et al. 1997), pAb UP502 (gift from E.L.F. Holzbaur, University of Pennsylvania, State College, PA). Arp1: mAb 45A (Schafer et al. 1994), rabbit antibody to recombinant human Arp1 (gift from J. Lees-Miller, Cold Spring Harbor Laboratories, Cold Spring Harbor, NY). p62: mAb 62B (Schafer et al. 1994). p24: affinity-purified rabbit antibody R5700 (Pfister et al. 1998). Tubulin: α tubulin mAb DM1A (Sigma Chemical Co.), rabbit antibody white-wall Tyr (w2; Gundersen et al. 1984), affinity-purified rabbit antibody against peptide KVEGEGEEEGEEY (gift from E. Karsenti, EMBL). γ Tubulin: mAb GTU 88 (Sigma Chemical Co.), rabbit antiserum pAb (Sigma Chemical Co.) against peptide EEFATEGTDRKDVFFYK. Pericentrin: rabbit antibody pAb 4b (Doxsey et al. 1994). Mannosidase II: rabbit antibody from K. Moremen (University of Georgia, Athens, GA). HA: anti–HA epitope mAb (Daro et al. 1996). β Galactosidase: mAb from Promega. GFP: pAb from Molecular Probes, Inc. FITC- and Texas red–conjugated horse anti–mouse and –rabbit (Vector Laboratories, Inc.) and Cy5-conjugated donkey anti–rabbit (Jackson ImmunoResearch Laboratories Inc.) were used as secondary antibodies.
Cell Culture
Cos-7 and L cells were grown in DMEM (GIBCO-BRL, Life Technologies, Inc.), supplemented with 10% FCS (Summit Technologies). For transient transfections, cells were grown to 70–90% confluency, harvested with 0.05% trypsin-EDTA, and then 1–2 × 107 cells were resuspended in 0.5 ml OPTI-MEM (GIBCO-BRL) and electroporated with 10 μg DNA at 230–240 V using an electro cell manipulator 600 (BTX). Cells were seeded on 22-mm2 coverslips (2 × 105 cells/coverslip) in six-well dishes and grown for 14–24 h before being processed for immunofluorescence. Transfection efficiencies of 60–80% (Cos7) or 20–50% (L) were routinely obtained.
Immunofluorescence
Cells were rinsed with D-PBS and then fixed in −20°C MeOH for 10 min. Coverslips were then blocked in TTBS (TBS, 0.1% Tween-20, and 2% BSA) incubated for 30 min in primary antibody, washed in TTBS (3 × 5 min), and incubated in secondary antibody for 15 min, all at room temperature. Samples were washed again and mounted on slides in 3:1 Mowiol 4–88 (Calbiochem Corp.): n-propyl gallate (Sigma Chemical Co.) in PBS plus 50% glycerol. For each overexpressed protein, at least 200 overexpressing cells on multiple coverslips were analyzed in two or more independent experiments.
Overexpressed p150Glued and CC1 were detected using mAb 150.1, which recognizes an epitope within CC1 and not the COOH terminus as reported earlier (Schafer et al. 1994). mAb 150.1 does not react with mammalian p150Glued. Overexpressed CC2 was detected using mAb 150B. Endogenous p150Glued was detected with rabbit antibody UP502. Arp1 was detected with a pAb against human Arp1.
Microscopy
Immunofluorescence microscopy was performed using an Axiovert 35 microscope (Carl Zeiss Inc.). Images were recorded on TMAX-400 film (Eastman-Kodak Co.), and digitized using a ScanMaker III scanner (Microtek). Additional images were recorded on a DeltaVision deconvolving microscope system (Applied Precision, Inc.). All images were imported into Adobe Photoshop® v3.0 (Adobe Systems, Inc.) for contrast manipulation and figure assembly.
Microtubule Regrowth Assay
Cells were transfected, seeded on coverslips, and grown 14–24 h as described above. Microtubules were depolymerized in 33 μM nocodazole (Sigma Chemical Co.) in DMEM for 30 min on ice, and then washed three times with room temperature DMEM and incubated at room temperature to allow regrowth. Coverslips were fixed at timed intervals in −20°C MeOH and processed for immunofluorescence as described above.
Sedimentation Analysis and Immunoblotting
Transfected cells were harvested, lysed, and sedimented as described in Echeverri et al. 1996, except that 4 × 10 cm2 dishes were used. Sucrose gradients (SW-50 rotor) were fractionated (400-μl fractions) and analyzed by immunoblotting on Immobilon-P membrane (Millipore Corp.). Blots were incubated with antibodies to dynactin subunits and the overexpressed protein, and then with alkaline phosphatase–conjugated goat anti–rabbit or –mouse IgG for detection using the Western-Light system (Tropix). Endogenous p150Glued and Arp1 were detected with mAbs 150B and 45A. Overexpressed p150Glued and CC1 were detected with mAb 150.1; CC2 was detected with mAb 150B.
Results
Excess Dynactin Shoulder/Sidearm Interferes with Microtubule Self-Focusing In Vitro
Cells overexpressing the dynactin subunit, dynamitin, show a wide variety of motility defects (Echeverri et al. 1996; Burkhardt et al. 1997; Ahmad et al. 1998; Valetti et al. 1999), all of which are thought to be due to the decoupling of dynactin's dynein- and cargo-binding functions. In these cells, the dynein-binding p150Glued subunit released by excess dynamitin is assumed to continue to bind dynein. To explore this possibility, we used an assay for mitotic aster assembly (Gaglio et al. 1995) to determine the effects of purified dynactin shoulder/sidearm (Fig. 1) on dynein activity in vitro. Aster formation requires dynein and dynactin function; asters do not form in extracts immunodepleted of either protein, and activity can be restored by readdition of purified dynein or dynactin. Dynactin, and a small amount of dynein, is incorporated into the asters (Gaglio et al. 1996). The shoulder/sidearm of dynactin was added to mitotic HeLa cell extracts before or after aster formation. When added at a concentration approximately equal to endogenous dynactin, shoulder/sidearm inhibited aster formation (Fig. 1 A, left). Once asters were formed, however, excess shoulder/sidearm had no effect (Fig. 1 A, right). Addition of equimolar dynactin did not inhibit aster formation under either condition. These findings support the hypothesis that free dynactin shoulder/sidearm can interact with dynein and prevent it from performing its normal functions. It also appears that the dynactin that incorporates into asters during assembly is adequate to maintain aster integrity, suggesting a relatively stable association with the aster core.
Perturbation of Microtubule Organization in Cells Overexpressing Dynactin Shoulder/Sidearm Subunits
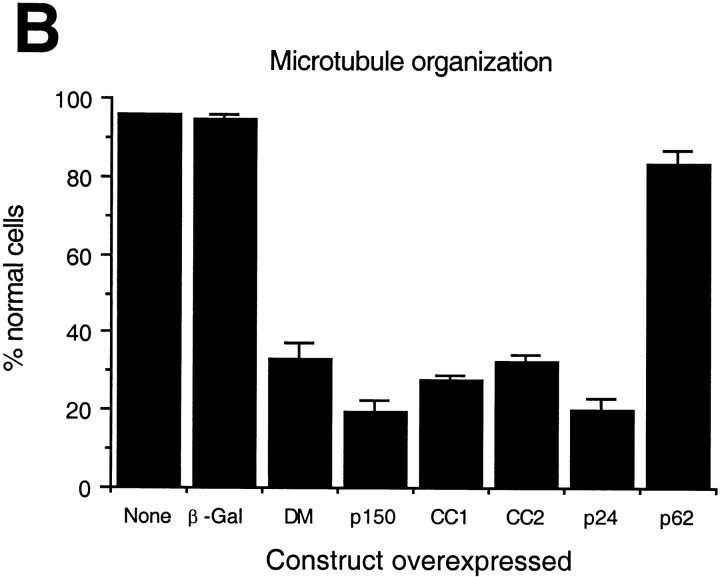
We then performed a series of experiments to determine how excess shoulder/sidearm subunits might affect microtubule organization in living cells. In all this work, protein overexpression was driven by the cytomegalovirus promoter. We only analyzed cells that contained evenly distributed (i.e., soluble) recombinant proteins, and not those that contained large protein aggregates (seen in some cells overexpressing p24 or p62). We first determined the effects of chicken dynamitin overexpression on the interphase microtubule array. In a previous study (Burkhardt et al. 1997), dynamitin was reported to have no effect on interphase microtubule organization in HeLa cells, which are an epithelial cell line that contains a broad microtubule organizing zone rather than a single, well-defined focus. Cos7 fibroblasts overexpressing dynamitin, in contrast, were reported to contain microtubules that were less well-focused than normal. We extended this observation by evaluating microtubule organization in Cos7 cells using immunofluorescence microscopy (Fig. 2). Determination of the percentage of cells that contained normal or abnormal microtubule arrays (Fig. 2 B and Table ) revealed that most dynamitin overexpressing cells contained large numbers of microtubules, but that these were no longer organized into a tightly focused, radial array.
Figure 2.
Effects of dynactin subunit overexpression on microtubule organization in Cos7 cells. (A) Representative images of cells double labeled with Abs to the transfected proteins (or imaged by GFP; left) and tubulin (right). CC2 was occasionally found to accumulate in the nucleus. Bar, 10 μm. (B) Cells overexpressing each protein were scored as normal if they had a radially focused microtubule array. None, cells in the transiently transfected population that were not overexpressing protein; DM, dynamitin. Error bars indicate SD.
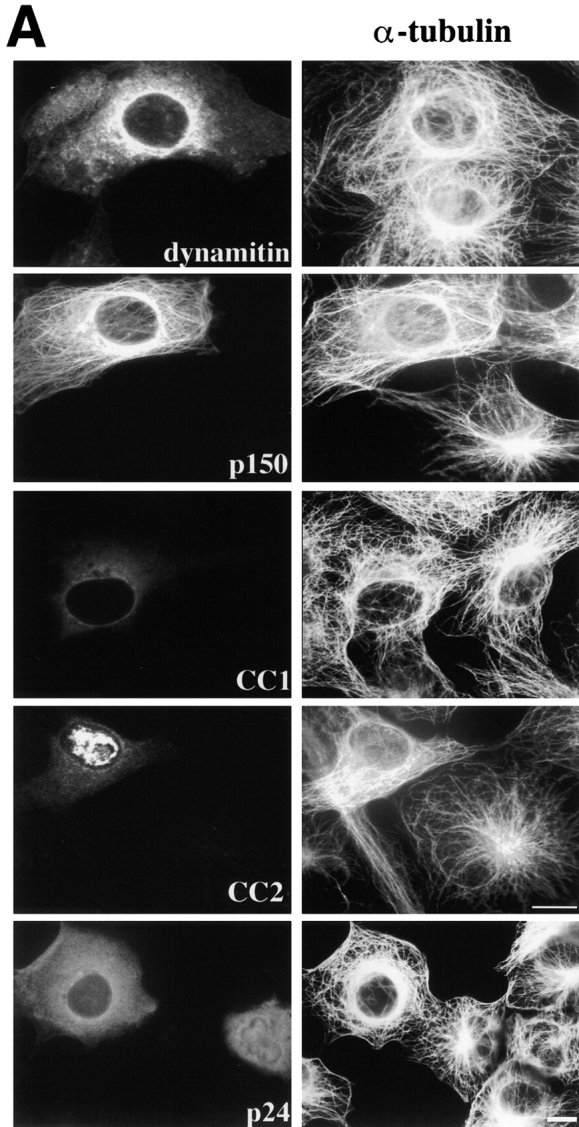
Table 1.
Summary of Effects of Dynactin Subunit Overexpression on Subcellular Organization
| Class | Overexpressed protein | Microtubule array | Centrosomal p150 | γ Tubulin | Centrosomal Arp1 | Golgi | Dynactin structure | Pericentrin |
|---|---|---|---|---|---|---|---|---|
| — | Control (β-Gal) | 95 | 93 | 89 | 84 | 85 | Normal | 93 |
| A | Dynamitin | 33 | 19 | 54 | 40 | 5 | Disrupted | 93 |
| B | p150 | 20 | ND | 48 | 14 | 15 | Normal | 94 |
| CC1 | 28 | 22 | 46 | 30 | 7 | Normal | 95 | |
| C | CC2 | 33 | 37 | 49 | 89 | 88 | Normal | 95 |
| p24-GFP | 20 | 39 | 47 | 85 | 80 | Normal | 94 | |
| — | p62 | 83 | 87 | 85 | 81 | 63 | Normal | 95 |
Cells were scored as described in Fig. 2 (microtubule array), 3 (dynactin structure), 4 (Golgi), 5 (γ tubulin) and 6 (centrosomal Arp1 and p150), or for a pericentriolar focus of pericentrin (right-most column). The percentage of cells showing a normal phenotype is given for each overexpression condition; standard deviations are provided in the figures. Dynactin structure was analyzed on sucrose gradients (Fig. 3) and was scored as normal if endogenous p150Glued, p62, Arp1, and p24 cosedimented in a single peak at 20S. Dynactin shoulder/sidearm subunits are grouped into phenotypic classes (A, B, and C) as described in Discussion.
Dynamitin overexpression causes release of dynactin shoulder/sidearm subunits that are hypothesized to competitively inhibit dynein-cargo binding. We reasoned that overexpression of just the dynein-binding subunit, p150Glued, might mimic the effects of dynamitin. As previously reported for rat p150Glued (Waterman-Storer et al. 1995), overexpressed chicken p150Glued bound microtubules along their length (Fig. 2 A) and, in some cells, induced microtubule bundling (data not shown). In addition, the overall organization of the microtubule cytoskeleton was perturbed and microtubules no longer appeared to radiate from a single perinuclear focus.
The microtubule binding and bundling seen with overexpressed p150Glued made it difficult to draw any clear conclusions about its effects on microtubule organization (see also Waterman-Storer et al. 1995). We therefore engineered two p150Glued expression vectors (Fig. 1 C) that lacked the NH2-terminal microtubule binding domain. Coiled-coil 1 (CC1; amino acids 217–548) is a 39,021-D fragment that corresponds to the central predicted coiled coil. This part of the protein binds dynein intermediate chain in vitro (Karki and Holzbaur 1995; Vaughan and Vallee 1995) and is thus thought to be dynactin's dynein-binding domain. Within the dynactin molecule, coiled-coil 2 (CC2; amino acids 926–1049; 14,093 D) is thought to lie near the Arp1 filament (Schroer 1996), where it may bind Arp1 directly (Waterman-Storer et al. 1995). Circular dichroism analysis revealed CC1 and CC2 to be α helices (data not shown), as predicted from their sequences. When overexpressed, neither CC1 nor CC2 bound microtubules, but overexpressing cells had disorganized, unfocused microtubule arrays similar to those seen previously (Fig. 2). This suggested that the microtubule disorganization seen in cells overexpressing full-length p150Glued was not simply due to its microtubule binding activity.
Finally, we examined microtubule organization in cells overexpressing p24, the third shoulder/sidearm subunit, tagged with green fluorescent protein. Again, we saw disorganized microtubules and, in some cells, p24-GFP appeared to accumulate at centrosomes (Fig. 2). Myc-tagged p24 had similar effects (data not shown), suggesting that the GFP tag did not affect function.
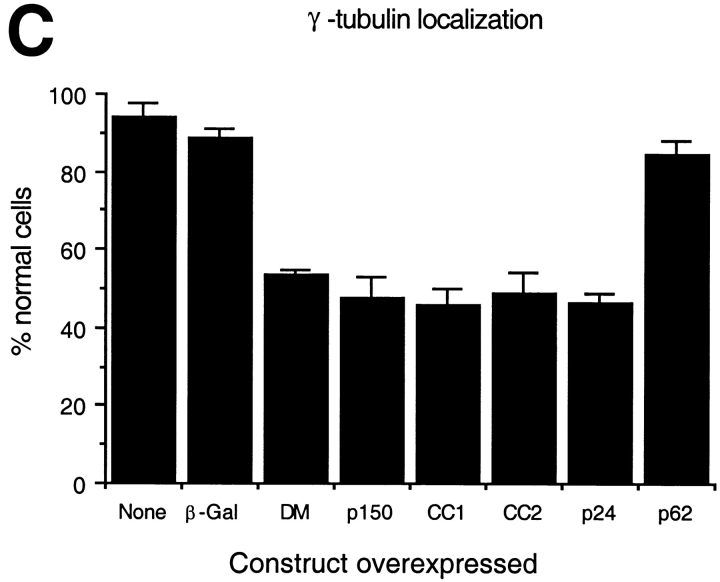
Several controls were performed (Fig. 2 B and Table ) to verify the significance of our results. Nearly all (95%) cells present on the same coverslip that were not overexpressing the protein of interest had radially focused microtubules. Normal microtubule organization was also seen in cells overexpressing a control protein, β galactosidase (β-Gal). Cells overexpressing p62, a component of dynactin's Arp1 backbone (Schafer et al. 1994; Eckley et al. 1999) had a slightly higher incidence of microtubule disorganization than controls, but significantly fewer cells were affected than with shoulder/sidearm subunit overexpression. We conclude that overexpression of dynactin shoulder/sidearm subunits specifically induces microtubule disorganization.
Effects on Dynactin Structure and Mitosis
Because overexpression of p150Glued, CC1, CC2, or p24 all had similar effects on microtubule organization to dynamitin, we determined whether interphase cells showed other perturbations characteristic of the “dynamitin effect.” Dynamitin overexpression disrupts dynactin structure (Echeverri et al. 1996; Karki et al. 1998), presumably because dynamitin is the linker that binds shoulder/sidearm subunits to the Arp1 minifilament backbone. The disruptive effects of other shoulder/sidearm subunits on microtubule organization led us to ask whether any of these proteins also disrupted dynactin structure. To address this question, we determined whether or not dynactin remained a single complex that sedimented at ≈20S (Fig. 3). Cells transfected with the different expression constructs were treated with detergent and the cell lysates were sedimented into sucrose gradients. Gradient fractions were then analyzed on immunoblots to determine the distribution of endogenous p150Glued, p62, Arp1, and p24, as well as the overexpressed proteins (Fig. 3). In samples prepared from cells overexpressing dynamitin, we observed two overlapping pools of p150Glued and p24, one at ≈17–18S and one at ≈9S, as expected from previous studies (Echeverri et al. 1996; Karki et al. 1998; Valetti et al. 1999). No other overexpressed dynactin subunit had a detectable effect on dynactin's sedimentation behavior. Most of the overexpressed proteins sedimented between 4–11S, the expected position of monomers or dimers, but a small portion of overexpressed p150Glued and p24-GFP cosedimented at 20S with other dynactin subunits, suggesting that they were able to incorporate into dynactin. Apparently, overexpression of shoulder/sidearm components can disrupt microtubule organization without detectably altering dynactin structure. This suggests that the free subunits are acting independently of the whole molecule.
Figure 3.
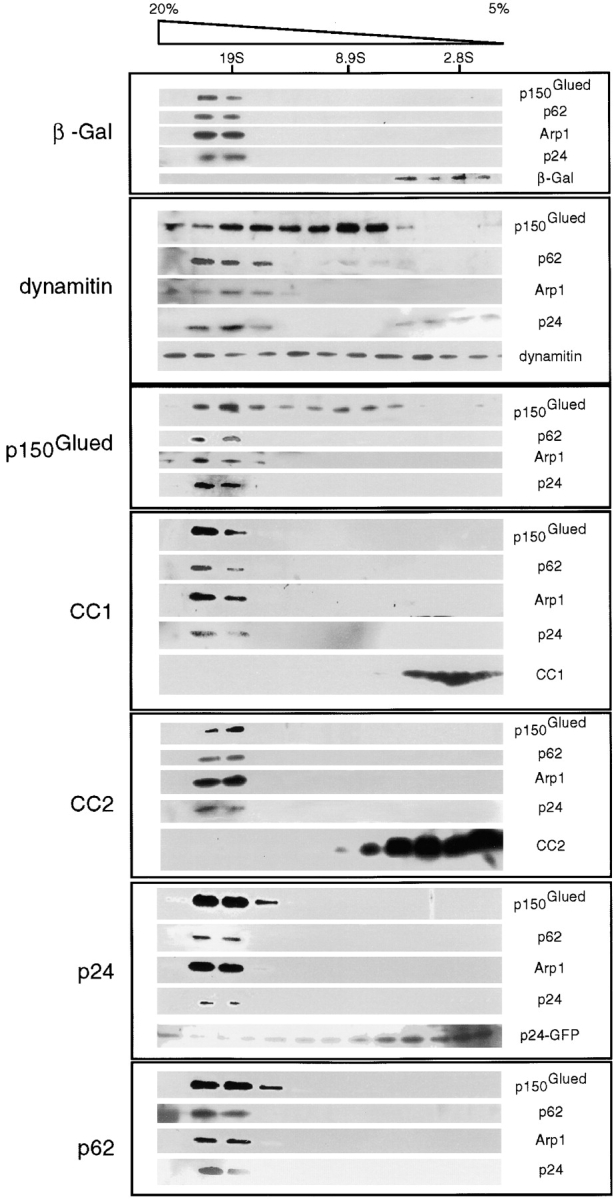
Effects of dynactin subunit overexpression on dynactin structure. Detergent lysates of transfected cells were sedimented into 5–20% sucrose gradients as described in Materials and Methods. Transfection efficiency was at least 65%, as determined by including, in each dish, a coverslip that was fixed and stained at harvest. Individual gradient fractions were analyzed by immunoblotting for endogenous dynactin subunits using mAbs 150B (except cells transfected with p150Glued), 62B and 45A, plus a pAb against p24; overexpressed proteins were detected using mAb150.1 (p150Glued and CC1), 150B (CC2), 62B (p62), anti–HA (HA-dynamitin), or pAb GFP (p24-GFP). The sucrose gradient and positions of sedimentation standards are indicated at the top.
Dynamitin overexpression causes cells to arrest in pseudoprometaphase owing to a variety of spindle defects (Echeverri et al. 1996). We therefore determined whether other shoulder/sidearm subunits had the same effect. As seen for dynamitin, most mitotic cells overexpressing CC1 had uni- or multipolar spindles (data not shown). Cells overexpressing p24-GFP or p24-myc died 20–24 h after transfection and mitotic cells were never observed, so we could not assess spindle morphology or mitotic progression. However, cells overexpressing CC2 were seen in all stages of mitosis and their spindles appeared normal (data not shown), indicating that mitosis was not affected.
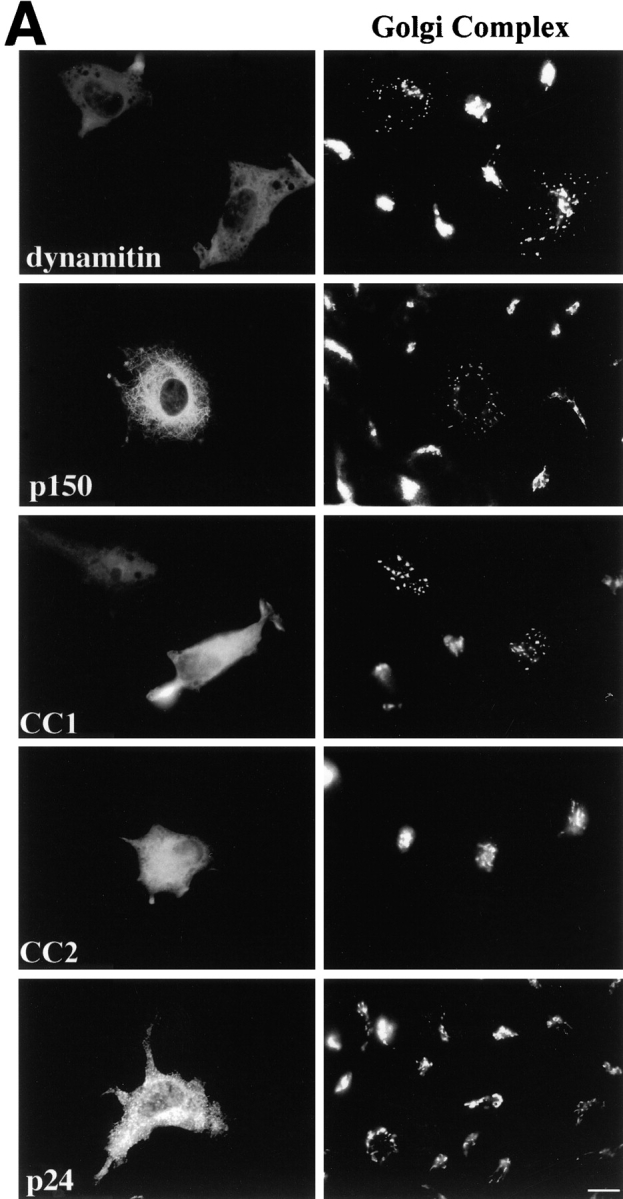
Effects on Golgi Complex Morphology
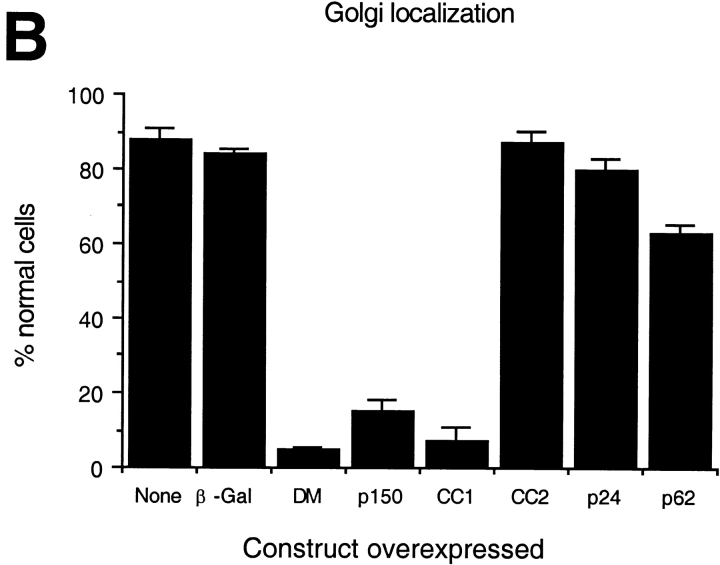
Another hallmark of dynamitin overexpression is disruption of the Golgi complex into small stacks dispersed throughout the cytoplasm (Burkhardt et al. 1997). We therefore determined the extent of Golgi complex fragmentation in cells overexpressing shoulder/sidearm components (Fig. 4 and Table ). Mouse L cells transfected with the different expression constructs were stained with antibodies to the medial Golgi enzyme mannosidase II. Most cells overexpressing either p150Glued or CC1 contained fragmented Golgi complexes similar to those seen in dynamitin overexpressing cells, while most cells overexpressing CC2, p24-GFP, or the control protein β-Gal contained Golgi complexes with the typical juxtanuclear localization and ribbon-like morphology. An intermediate number of cells overexpressing the dynactin backbone subunit, p62, had disrupted Golgi complexes.
Figure 4.
Effects of dynactin subunit overexpression on Golgi complex organization in L cells. (A) Representative images of cells double labeled with Abs to the transfected proteins (or imaged for GFP; left) and mannosidase II (right). L cells transfected less efficiently than Cos7. Bar, 10 μm. (B) Cells overexpressing the protein of interest were scored as normal if they had a single juxtanuclear Golgi structure. None, cells in the transiently transfected population that were not overexpressing protein; DM, dynamitin. Error bars indicate SD.
Shoulder/Sidearm Subunit Overexpression Leads to a Loss of Pericentriolar Components
Full-length p150Glued and CC1 disrupted microtubule (Fig. 2) and Golgi complex (Fig. 4) organization, but did so without detectably altering dynactin structure (Fig. 3). Biochemical studies indicate that the NH2-terminal half of p150Glued can bind dynein directly (Karki and Holzbaur 1995; Vaughan and Vallee 1995), which may explain our results. In living cells, overexpression of p150Glued or CC1 might interfere with dynein-based motility via the same basic mechanism as dynamitin. Excess dynamitin causes release of shoulder/sidearm that is thought to bind dynein, while free p150Glued and CC1 may bind dynein directly and compete for its interactions with intact dynactin. In both cases, the net effect would be that dynein can no longer interact with cargo. All three proteins would thus be expected to have similar effects on dynein-based motility. What this model does not explain, however, is how overexpressed CC2 and p24 interfere with dynein function, as they perturb microtubule organization but do not appear to have an effect on Golgi complex structure.
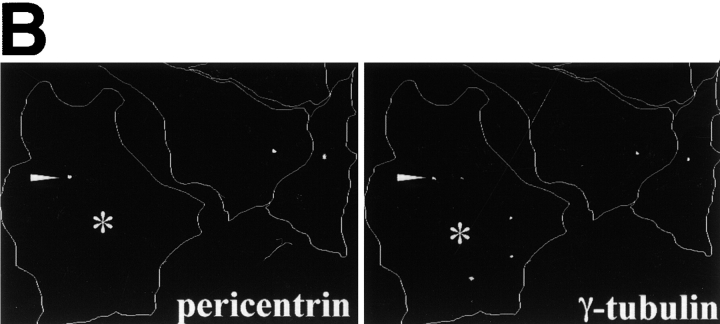
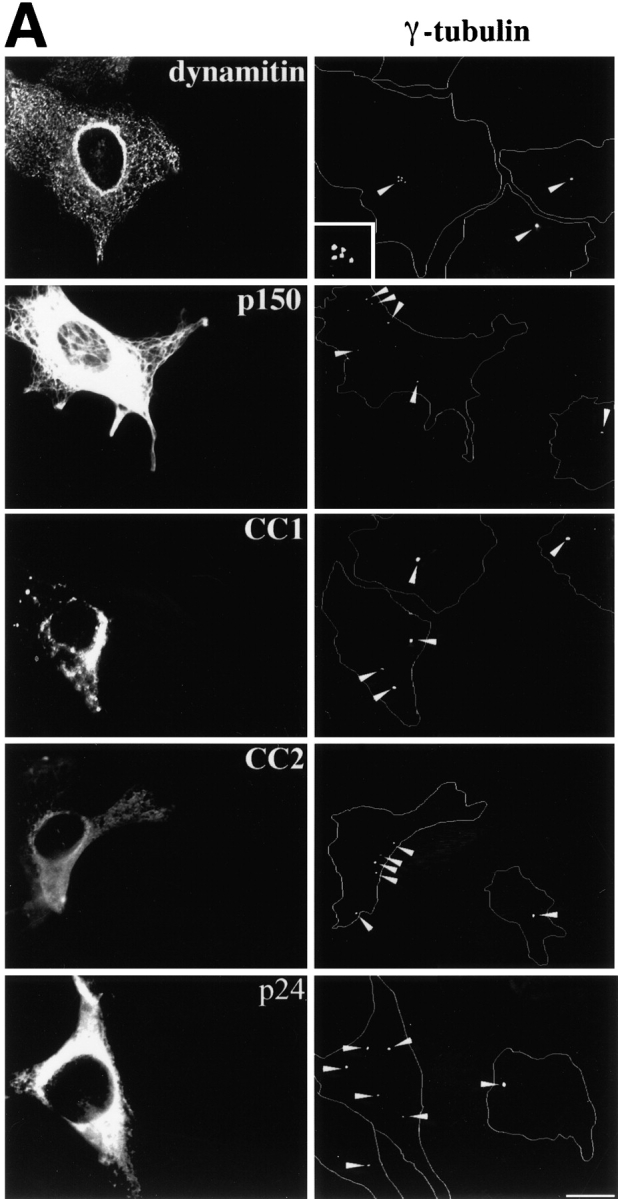
To learn more about the underlying basis of the microtubule perturbations we saw, we examined centrosome structure and function in cells overexpressing dynactin shoulder/sidearm subunits. Cells were stained with antibodies to the centrosomal proteins γ tubulin or pericentrin. In the vast majority of untransfected cells or control-transfected cells expressing β-gal or p62, γ tubulin and pericentrin both localized to a single focus or paired foci near the nucleus. Pericentrin staining was not affected by overexpression of any dynactin subunit (Table ). In contrast, γ tubulin localization was altered in about half the cells overexpressing dynactin shoulder/sidearm subunits (Fig. 5 C and Table ). Multiple γ tubulin foci were present (Fig. 5 A), in addition to a single perinuclear focus that also stained for pericentrin (Fig. 5 B). Two patterns of γ tubulin foci were seen: individual foci scattered throughout the cell and clusters of foci near the nucleus. Cells overexpressing shoulder/sidearm subunits commonly had four or more foci (in addition to the parent centrosome), while controls contained at most two foci that were always perinuclear. As many as nine widely spread foci could be detected per cell, while up to 12 foci were seen per cluster. Scattered foci were more common than clusters (≈3:1). All shoulder/sidearm subunits had similar effects on γ tubulin localization.
Figure 5.
Effects of dynactin subunit overexpression on γ tubulin distribution in Cos7 cells. (A) Representative images of cells double labeled with Abs to the transfected proteins (or imaged for GFP; left) and γ tubulin (right). The inset shows a cluster of γ tubulin foci enlarged 3×. Bar, 10 μm. (B) Pericentrin (left) and γ tubulin (right) staining in a cell overexpressing dynamitin (*). Arrowheads in A and B mark γ tubulin or pericentrin foci. (C) Cells overexpressing the protein of interest were scored as normal if they had one or two perinuclear γ tubulin foci. None, cells in the transiently transfected population that were not overexpressing protein; DM, dynamitin. Error bars indicate SD.
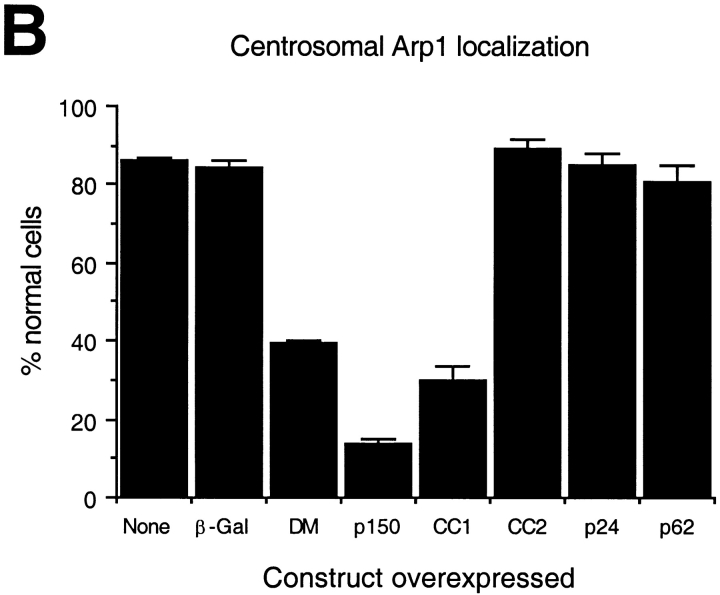
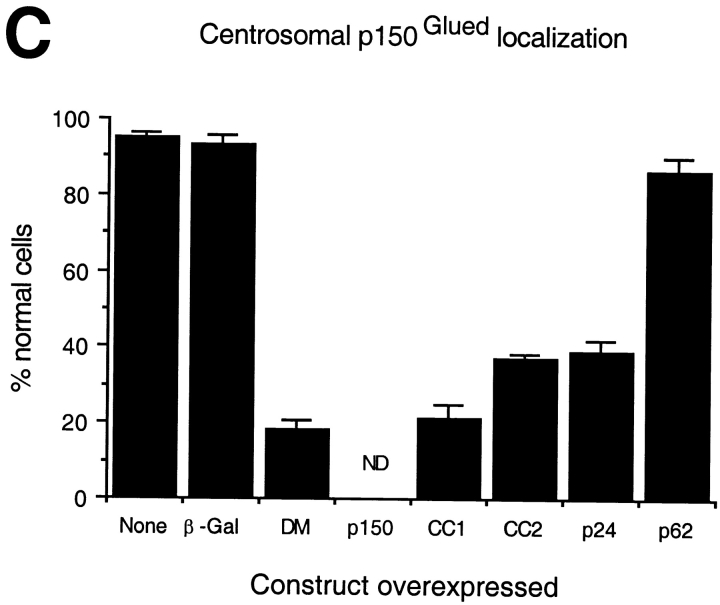
Dynactin itself is associated with centrosomes, both in vivo (Gill et al. 1991; Clark and Meyer 1992; Paschal et al. 1993; Waterman-Storer et al. 1995) and in vitro (Clark and Meyer 1992). However, centrosomal localization requires intact cytoplasmic microtubules (Paschal et al. 1993; Schroer, T.A., unpublished observations), suggesting that dynactin is not a bona fide centrosomal protein. Because shoulder/sidearm subunit overexpression affected γ tubulin distribution, it seemed possible that centrosomal dynactin localization might also be altered. To test this hypothesis, control and dynactin subunit overexpressing cells were stained with antibodies to Arp1, the major component of the dynactin backbone (Fig. 6). Most control cells contained a single bright spot of Arp1 that colocalized with γ tubulin (data not shown). The same result was seen in cells overexpressing p24-GFP, CC2, or p62. In contrast, most cells overexpressing dynamitin, p150Glued, or CC1 did not contain a detectable Arp1 focus. These are the same subunits whose overexpression correlates with Golgi complex dispersion and mitotic arrest.
Figure 6.
Effects of dynactin subunit overexpression on centrosomal dynactin subunits in Cos7 cells. (A) Representative images of cells labeled with Abs to Arp1 (left) or p150Glued (right). Cells overexpressing protein were identified using Abs to the transfected proteins (or imaged for GFP) and are marked (*). The images in the two columns are from different experiments. Bar, 10 μm. (B and C) Cells overexpressing the protein of interest were scored as normal if they had a perinuclear focus of Arp1 (B) or p150Glued (C). None, cells in the transiently transfected population that were not overexpressing protein; DM, dynamitin. Error bars indicate SD.
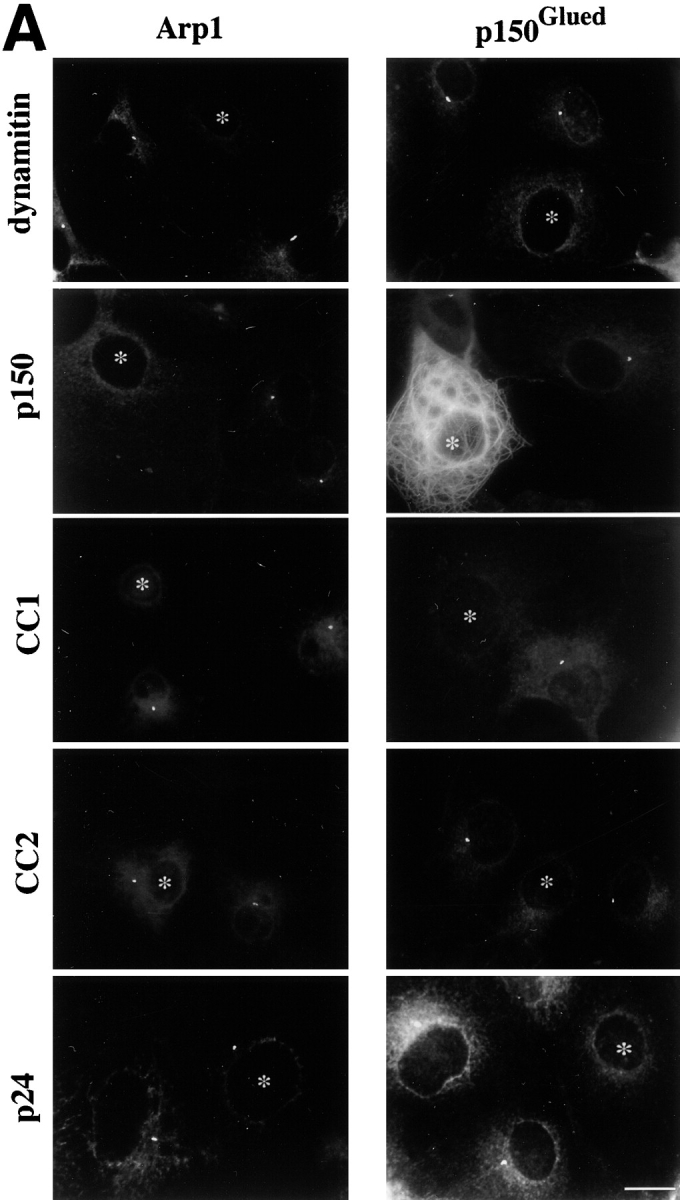
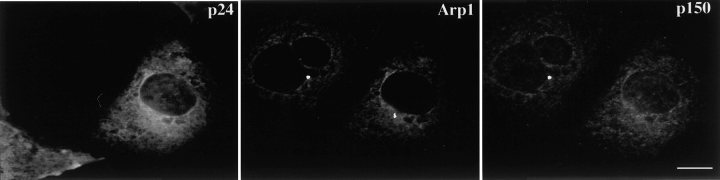
Overexpression of all shoulder/sidearm subunits had an effect on microtubule organization and γ tubulin localization, suggesting that the loss of microtubule focus might be correlated with centrosome integrity. Consistent with this, centrosomes in cells overexpressing dynamitin, p150Glued or CC1 also appeared to lack dynactin, as judged by Arp1 staining. However, if microtubule disorganization is due to massive disruption of pericentriolar material, the two phenomena should correlate directly. This is not what we observed, since centrosomes in cells overexpressing CC2 and p24 still appeared to contain Arp1. To better characterize the centrosome-associated dynactin pool in these cells, they were stained for the shoulder/sidearm component p150Glued (Fig. 6a and Fig. c). Most control cells contained a single centrosomal focus of p150Glued, similar to what was seen for Arp1. Overexpressed subunits that caused a loss of Arp1 from centrosomes (i.e., dynamitin and CC1) also caused a loss of p150Glued. Most cells overexpressing CC2 or p24-GFP also did not have a perinuclear focus of p150Glued. Double labeling for Arp1 and p150Glued revealed that most cells overexpressing p24-GFP had perinuclear Arp1 foci that were not associated with p150Glued (Fig. 7 and Table ). Thus, overexpression of p24-GFP appears to selectively release p150Glued from Arp1 at centrosomes. This occurs in the absence of a detectable effect on the bulk dynactin pool (Fig. 3).
Figure 7.
p24 overexpression induces the separation of p150Glued from Arp1 at centrosomes. (A) Representative image of a p24-GFP–overexpressing cell (left) double labeled with antibodies to Arp1 (center) and p150Glued (right). Bar, 10 μm.
Table 2.
Loss of p150Glued from Centrosomes in Cells Overexpressing p24
| Overexpressed protein | p150Glued + | p150Glued − |
|---|---|---|
| None (control) | 99.0 | 1.0 |
| p24-GFP | 36.3 | 63.7 |
Cells overexpressing p24-GFP, or nonexpressing cells on the same coverslip, were double labeled for Arp1 and p150Glued. Cells containing a perinuclear focus of Arp1 (≈90% of the cells, see Table ) were scored for the presence (p150Glued +) or absence (p150Glued −) of a perinuclear focus of p150Glued. The numbers given are the percent-age of cells showing each phenotype. n, 180 cells for p24-GFP and 215 cells for con-trol.
Effects on Microtubule Nucleation and Retention at Centrosomes
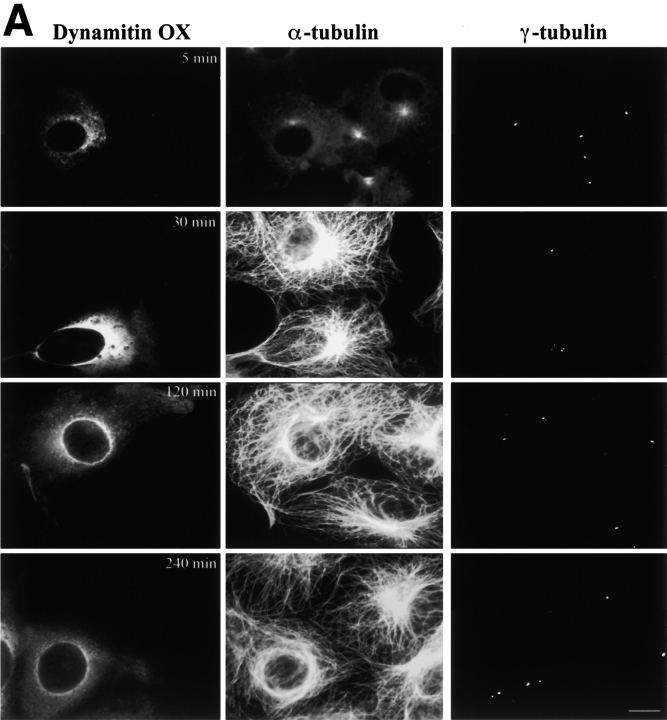
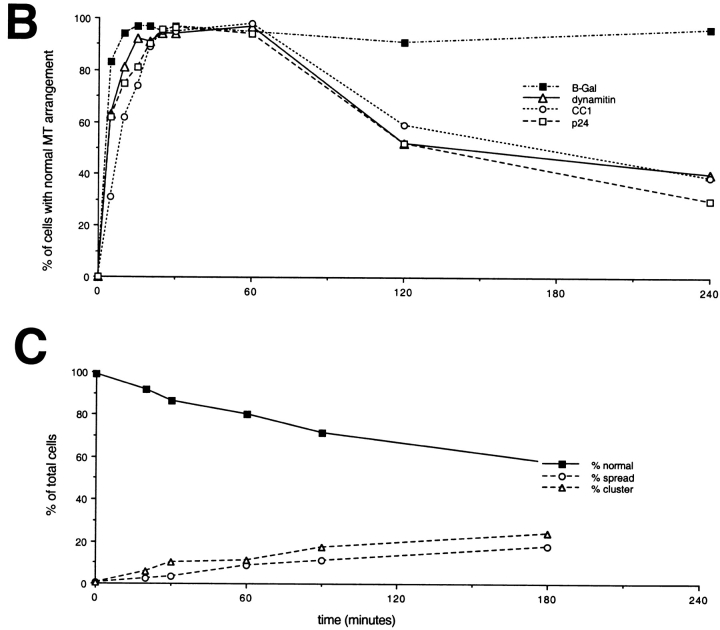
Since centrosome organization was clearly altered by shoulder/sidearm subunit overexpression, we next examined effects on centrosome function. Many overexpressing cells contained ectopic γ tubulin foci, suggesting that noncentrosomal microtubule nucleation might at least partially account for the altered microtubule array seen in most cells. To test this hypothesis, we determined the pattern of microtubule regrowth after cold and nocodazole-induced depolymerization (Fig. 8). After increasing intervals of regrowth (0 min to 6 h), cells were fixed and stained for α and γ tubulin. In untransfected control cells, single microtubule asters were seen at 5 min regrowth and, by 30 min, a robust, radial array had developed. By 1 h, the microtubule distribution appeared the same as at steady state (e.g., Fig. 2), and remained unchanged for the rest of the experiment. Similar results were obtained in cells overexpressing β-Gal or the dynactin p62 subunit. Immediately after microtubule depolymerization, cells overexpressing dynamitin, CC1, CC2, or p24-GFP contained a single detectable γ tubulin focus rather than multiple spots. The focus was near the nucleus, stained for pericentrin (data not shown), and colocalized with the site of microtubule aster formation. This suggested it was the centrosome. Although this perinuclear structure could nucleate microtubules, a more careful analysis revealed that microtubule regrowth was not completely normal. Little if any microtubule regrowth was detected at 5 min and, at 10 min, only small asters were observed, suggesting that microtubule nucleation was delayed. However, growth continued steadily and at the end of 1 h, each cell had a well-developed, single radial microtubule array (Fig. 8 B). Although we saw only a single aster during this time, multiple γ tubulin foci became apparent. These were first detected at 20 min of regrowth and became more abundant with time (Fig. 8a and Fig. C). Peripheral γ tubulin foci and perinuclear clusters were observed, although the latter were more prevalent. Cells that contained multiple γ tubulin foci appeared to contain only a single microtubule aster, suggesting that nucleation was not occurring at ectopic foci. This implies that the aberrant microtubule arrays seen at steady state were not the result of noncentrosomal nucleation. Although Fig. 8a and Fig. C, shows only the behavior of cells overexpressing dynamitin-GFP, similar results were obtained in cells overexpressing p24-GFP.
Figure 8.
Effects of shoulder/sidearm subunit overexpression on microtubule nucleation and centrosome assembly. Dynamitin-GFP–transfected cells were treated with cold and nocodazole to promote microtubule disassembly, and then washed and warmed to room temperature to allow microtubule regrowth. At the times indicated, the cells were fixed and double labeled with Abs to α and γ tubulin. Representative images are shown in A. Bar, 10 μm. (B) Time course of microtubule aster regrowth (0–60 min) and defocusing (60–240 min) for representative expression constructs (β-Gal, dynamitin-GFP, CC1, p24-GFP). (C) Time course of the disappearance of a single γ tubulin focus (▪) and appearance of multiple γ tubulin foci in cells overexpressing dynamitin-GFP. Immediately after removal of nocodazole, all cells had a single predominant γ tubulin spot (e.g., A, top right). (▵) Percentage of cells with closely clustered γ tubulin foci (see 30- and 120-min time points in A). (○) Percentage of cells containing widely spread γ tubulin foci (see 240-min time point in A).
We were surprised to find that the pattern of microtubule regrowth was relatively normal in these cells since, at steady state, microtubule and centrosomal protein distributions were so clearly perturbed. This result suggested that cells containing overexpressed shoulder/sidearm components still nucleated microtubules at the centrosome, but that the newly assembled microtubules were no longer retained at this site. To test this hypothesis, we examined microtubule distribution in cells at later times of regrowth (Fig. 8a and Fig. B). Disorganized, unfocused microtubules were detected in some cells at 2 h, and by 6 h the cells had returned to the steady state condition (60–80% abnormal). Analysis of the distribution of γ tubulin in cells overexpressing dynamitin-GFP revealed that the number of noncentrosomal γ tubulin foci also increased with time. At 20 min, most cells with multiple foci contained six foci or fewer but, by 3 h, as many as 12 foci were detected in some cells (data not shown). At all time points, both perinuclear clusters and widely spread foci were seen (Fig. 8 C), suggesting that the two arose in parallel.
Discussion
The present study extends significantly our understanding of dynein and dynactin function in interphase cells and provides new insight into mechanisms of microtubule anchoring at centrosomes. Our findings suggest that dynein and dynactin play key roles in microtubule organization, centrosome integrity, and centrosome assembly. The use of multiple dynactin subunits and subunit fragments has allowed us to selectively explore the function of the dynein- and microtubule-binding dynactin subunit, p150Glued. Our results lend strong support to the idea that dynein function requires binding to dynactin via p150Glued. Our data also indicate that dynactin provides a previously undescribed microtubule anchoring function at centrosomes.
The overexpressed proteins used in this study can be grouped into three classes based on the severity of the phenotype they elicit when overexpressed in cultured fibroblasts (Table ). Dynamitin (class A) has the broadest range of effects, perturbing dynactin structure and centrosome integrity, and interfering with endomembrane motility, microtubule organization, and mitosis. Dynamitin overexpression is thought to act by disassembling the entire cellular pool of dynactin and leaving, in its place, decoupled dynein- and cargo-binding elements. Neither piece can function independently, leading to an inhibition of all dynein-based motile events. In addition to the previously reported effects of dynamitin overexpression on mitotic progression (Echeverri et al. 1996) and membrane localization (Burkhardt et al. 1997), we find that microtubule focusing and localization of pericentriolar components to centrosomes are perturbed in interphase cells.
Full length p150Glued and CC1 (class B) also affect a variety of functions but, unlike dynamitin, they do so without having a detectable effect on dynactin structure or stability. Class B agents most likely act by providing the cell with an excess of free dynein-binding polypeptides that competitively inhibit the interaction of dynein with intact dynactin. This inhibits all dynein-based motility in cells that still contain normal concentrations of dynactin. Antibodies such as mAb 70.1 (Heald et al. 1996; Burkhardt et al. 1997; Gaglio et al. 1997) or 74.1 (Steffen et al. 1997) can also be considered class B agents, as they bind dynein intermediate chain and interfere sterically with the dynein–dynactin interaction.
The dynactin p24 subunit and CC2 (class C) are significantly more selective in their effects than are class A or B. They do not interfere with dynactin structure or stability and do not disrupt the organization or localization of the Golgi complex. In contrast to cells overexpressing dynamitin in which movement is abolished (Valetti et al. 1999), cells overexpressing p24-GFP also show normal levels and patterns of endosome motility (Schroer, T.A., and N.J. Quintyne, unpublished observations). In addition, CC2 has no obvious effect on mitotic events. These findings indicate that class C agents do not interfere with cytosolic dynein activity, yet they have profound effects on interphase microtubule and centrosome organization. This suggests they interfere with dynactin function in a way that does not directly relate to its interaction with dynein.
Overexpression of the dynactin p62 subunit affects only some dynein-dependent phenomena, and always to a lesser extent than the other subunits tested. Microtubule organization was altered in only a small population of cells. Centrosomal p150Glued, Arp1, and γ tubulin localization appeared completely normal. In contrast, nearly 40% of cells (as compared with 10% of controls) contained disrupted Golgi complexes. These results suggest that p62, and possibly other components of dynactin's tetrameric pointed-end complex (Eckley et al. 1999), may contribute to the interactions of dynactin with membranes, but not with centrosomes.
Dynein and Microtubule Organization
Our understanding of dynein's contributions to microtubule organization in interphase are strongly influenced by what has been learned from in vitro studies in mitotic and meiotic systems (reviewed in Compton 1998). Dynein is essential for the formation and stability of asters or spindle poles. Its primary role is to transport microtubule minus ends (Verde et al. 1991; Heald et al. 1996) and pole components (Gaglio et al. 1996; Merdes et al. 1996) to a common site, thereby driving pole formation and focusing. Ongoing dynein activity is also required for pole maintenance (Gaglio et al. 1997; Heald et al. 1997), suggesting that it helps keep microtubules in place. It is not clear that dynactin is required for microtubule–microtubule sliding events, although it may facilitate dynein–microtubule interactions. However, dynactin itself is actively transported to the pole, perhaps in a complex with other matrix components such as NuMA (Merdes et al. 1996). Dynactin that has accumulated at poles is not affected by exogenous free shoulder/sidearm (Fig. 1), suggesting that it is incorporated into a relatively stable structure.
Dynein, in contrast, does not appear to be stably associated with spindle poles (Gaglio et al. 1996, Gaglio et al. 1997). This makes sense, since dynein acting at the pole will cause microtubules that are not well anchored to be ejected (Gaglio et al. 1996). Microtubule retention is proposed to involve microtubule-binding activities such as NuMA, as well as an opposing BimC family motor (Gaglio et al. 1996). Dynactin may also provide a microtubule binding function via its p150Glued subunit.
The perturbations of microtubule organization that occur in interphase fibroblasts are highly reminiscent of what is seen when dynein or dynactin function is inhibited in vitro. In both cases, microtubules are not focused into radial arrays, and dynactin subunits do not accumulate at microtubule minus ends. Whether microtubules are formed artificially or nucleated from centrosomes, our results suggest that two principles underlie microtubule organization throughout the cell cycle. First, to maintain a uni- or bipolar radial array, microtubules that are released from centrosomes must be retrieved, most likely by dynein. Second, in both interphase and mitosis, dynein appears to transport pericentriolar components to the centrosome. These include dynactin, γ tubulin, and perhaps pericentrin during interphase, and dynactin and NuMA during mitosis.
Dynactin Functions at Centrosomes
The interactions between dynein, dynactin, microtubules, and centrosome components are complex. Dynactin is required for dynein to bind cargo, yet in some cases is cargo itself. Pericentriolar material serves as a docking site for dynactin, but dynein and dynactin are required for it to be recruited to centrosomes. Despite these interwoven relationships, our data allow some simple conclusions to be drawn. Disorganization of the interphase microtubule array is tightly correlated with the loss of p150Glued from centrosomes (Table ), suggesting that this dynactin subunit contributes a key microtubule anchoring function. That unfocused microtubules are seen in cells that contain centrosomal foci of γ tubulin or Arp1 indicates that neither protein is sufficient to maintain the radial microtubule array. We propose that dynein-mediated transport is required for targeting and delivery of dynactin to centrosomes. Dynein may also translocate free shoulder/sidearm, p150Glued, or CC1 toward the centrosome, but no accumulation is observed, suggesting that the Arp1 minifilament is required to bind dynactin to pericentriolar material. We propose that centrosomal p150Glued binds microtubules tightly, countering outward-directed pulling or ejection forces. The link between p150Glued and Arp1 is therefore under constant tension, which may render centrosomal dynactin susceptible to disassembly when excess p24 or CC2 is present. CC2 may displace shoulder/sidearm by binding Arp1 directly (Waterman-Storer et al. 1995).
According to this model, class C agents should induce dynactin disassembly whenever the p150Glued-Arp1 link is under tension, which might be expected to occur whenever cargo is moved. Yet overexpression of p24 or CC2 does not correlate with membrane localization or motility defects, and the bulk pool of cytosolic dynactin is not affected (Fig. 3). The forces exerted on dynactin at centrosomes are likely to be significant since they involve large numbers of motor molecules operating on the entire microtubule cytoskeleton. This may not be true for endomembranes, particularly discrete tubulovesicular structures such as late endosomes and ER-to-Golgi transport complexes. Moreover, dynein can bind membranes via multiple dynactin-independent mechanisms. Binding can be mediated by transmembrane protein “receptors,” such as rhodopsin (Tai et al. 1999), as well as membrane lipids themselves (Lacey and Haimo 1994). Multiple attachment mechanisms would reduce the net tension on the p150Glued sidearm and prevent dynactin disassembly. Finally, individual dynactin molecules that are acting as dynein cargo are not expected to be under significant tension and would therefore remain intact.
Effects on Other Pericentriolar Components
We find that defects in microtubule organization are much more prevalent than γ tubulin dispersion, suggesting that the two phenomena arise independently. Noncentrosomal aggregates of γ tubulin may form in two ways. First, γ tubulin fragmentation could be linked in some way to microtubule disorganization, as suggested by the widely spread, peripheral aggregates seen in cells overexpressing class A, B, or C agents. Outward-directed forces acting on microtubules may cause entire pieces of pericentriolar material to be torn away from the centrosome in conjunction with microtubules. Second, the noncentrosomal γ tubulin foci we observe might correspond to newly synthesized proteins that have accumulated in the periphery, perhaps in association with microtubules, but cannot be transported inward in the absence of dynein activity. The results of our microtubule regrowth experiments suggest that the latter can occur. Multiple γ tubulin foci are seen in cells that appear to contain single microtubule asters, indicating that γ tubulin dispersion can precede microtubule disorganization. While it seems very likely that pericentriolar components are transported to centrosomes in a microtubule- and dynein-dependent manner (Kuriyama 1982; Dictenberg et al. 1998; Balczon et al. 1999), the possibility that some γ tubulin foci arise by centrosome fragmentation cannot be rigorously excluded.
Future Directions
Cells overexpressing class C agents share a number of superficial similarities with cultured epithelia. Such cells nucleate microtubules from centrosomes, and then release them to yield an unfocused array (Keating et al. 1997), and some epithelia contain noncentrosomal pools of γ tubulin (Meads and Schroer 1995). Cultured epithelial cells contain a single, perinuclear Golgi complex (Schweizer et al. 1988; Bacallao et al. 1989; Ihrke et al. 1998) and are mitotically active. The radial microtubule array present in fibroblasts is obviously not required for normal cellular function, leading to the question of why such an arrangement exists. It is possible that the organization of microtubules into a single, radial array facilitates movement of the microtubule cytoskeleton as a whole. Precedent is seen in mitotic epithelial and embryonic cells, where the entire spindle rotates in a process that is thought to involve cortically anchored dynein and dynactin (Busson et al. 1998; Skop and White 1998). A similar mechanism may underlie the movement of the entire interphase microtubule array in amoebae, migrating fibroblasts, macrophages, and T cells. Whatever the underlying reason for a radial array, our results suggest that the different microtubule organizations seen in fibroblasts, epithelia, and neurons may be profoundly influenced by the activities and subcellular localizations of dynein and dynactin.
Acknowledgments
We thank Kristen Harwick Poland and Mary A. Dionne for technical assistance, Drs. John Carra and Peter Privalov for the CD studies, and Dr. Barbara Ranscht for the chick embryo cDNA library. Thanks also go to the Schroer lab for helpful comments on the manuscript and to Jim Bingham for dynactin shoulder/sidearm. We are grateful to Drs. J.C. Bulinski, S. Doxsey, G. Gundersen, E. Holzbaur, E. Karsenti, S.M. King, J. Lees-Miller, and K. Moremen for antibodies.
N.J. Quintyne was supported by a National Institutes of Health (NIH) training grant to the Department of Biology, Johns Hopkins University. D.A. Compton was supported by grants from the American Cancer Society (RPG-95-010-04-CSM) and NIH (GM51542). T.A. Schroer was supported by the David and Lucile Packard Fellowship for Science and Engineering and the NIH (GM44589 and DK44375).
Footnotes
1.used in this paper: β-Gal, β galactosidase; GFP, green fluorescent protein
Dr. Gill's current address is The Institute for Genomic Research, Rockville, MD 20850.
References
- Ahmad F.J., Echeverri C.J., Vallee R.B., Baas P.W. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J. Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan V. Motor proteinsa dynamic duo. Curr. Biol. 1996;6:630–633. doi: 10.1016/s0960-9822(09)00434-5. [DOI] [PubMed] [Google Scholar]
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E.H.K., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J. Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon R., Bao L., Zimmer W.E., Brown K., Zinkowski R.P., Brinkley B.R. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon R., Varden C.E., Schroer T.A. A role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell. Motil. Cytoskelet. 1999;42:60–72. doi: 10.1002/(SICI)1097-0169(1999)42:1<60::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bingham J.B., King S.J., Schroer T.A. Purification of dynein and dynactin from brain tissue. Methods Enzymol. 1998;298:171–184. doi: 10.1016/s0076-6879(98)98017-x. [DOI] [PubMed] [Google Scholar]
- Blocker A., Severin F.F., Burkhardt J.K., Bingham J.B., Yu H., Olivo J.-C., Schroer T.A., Hyman A.A., Griffiths G. Molecular requirements for bi-directional movement of phagosomes along microtubules. J. Cell Biol. 1997;137:113–129. doi: 10.1083/jcb.137.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J.K., Echeverri C.J., Nilsson T., Vallee R.B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson S., Dujardin D., Moreau A., Dompierre J., De Mey J.R. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Clark S.W., Meyer D.I. Centractin is an actin homologue associated with the centrosome. Nature. 1992;359:246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- Compton D.A. Focusing on spindle poles. J. Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- Compton D.A., Cleveland D.W. NuMA is required for the proper completion of mitosis. J. Cell Biol. 1993;120:947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro E., van der Sluijs P., Galli T., Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci. USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg J.B., Zimmerman W., Sparks C.A., Young A., Vidair C., Zheng Y., Carrington W., Fay F.S., Doxsey S.J. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S.J., Stein P., Evans L., Calarco P.D., Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley D.M., Gill S.R., Melkonian K.A., Bingham J.B., Goodson H.V., Heuser J.E., Schroer T.A. Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the Arp1 minifilament pointed end. J. Cell Biol. 1999;147:307–319. doi: 10.1083/jcb.147.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Dionne M.A., Compton D.A. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Saredi A., Bingham J.B., Hasbani J., Gill S.R., Schroer T.A., Compton D.A. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Saredi A., Compton D.A. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J. Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.R., Schroer T.A., Szilak I., Steuer E.R., Sheetz M.P., Cleveland D.W. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G.G., Kalnoski M.H., Bulinski J.C. Distinct populations of microtubulestyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E.L.F., Hammarback J.A., Paschal B.M., Kravit N.G., Pfister K.K., Vallee R.B. Homology of a 150 K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued . Nature. 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Ihrke G., Martin G.V., Shanks M.R., Schrader M., Schroer T.A., Hubbard A.L. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J. Cell Biol. 1998;141:115–133. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S., Holzbaur E.L. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E.L.F. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Karki S., LaMonte B., Holzbaur E.L.F. Characterization of the p22 subunit of dynactin reveals the localization of cytoplasmic dynein and dynactin to the midbody of dividing cells. J. Cell Biol. 1998;142:1023–1034. doi: 10.1083/jcb.142.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating T.J., Peloquin J.G., Rodionov V.I., Momcilovic D., Borisy G.G. Microtubule release from the centrosome. Proc. Natl. Acad. Sci. USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce M.P., Samso M. Overexpression of cytoplasmic dynein's globular head causes a collapse of the interphase microtubule network in Dictyostelium . Mol. Biol. Cell. 1996;7:935–948. doi: 10.1091/mbc.7.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R. The effect of colcemid on the centriole cycle in Chinese hamster ovary cells. J. Cell Biol. 1982;53:155–171. doi: 10.1242/jcs.53.1.155. [DOI] [PubMed] [Google Scholar]
- Lacey M.L., Haimo L.T. Cytoplasmic dynein binds to phospholipid vesicles. Cell. Motil. Cytoskelet. 1994;28:205–212. doi: 10.1002/cm.970280304. [DOI] [PubMed] [Google Scholar]
- Meads T., Schroer T.A. Polarity and nucleation of microtubules in polarized epithelial cells. Cell. Motil. Cytoskelet. 1995;32:273–288. doi: 10.1002/cm.970320404. [DOI] [PubMed] [Google Scholar]
- Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Paschal B.M., Holzbaur E.L.F., Pfister K.K., Clark S., Meyer D.I., Vallee R.B. Characterization of a 50-kDa polypeptide in cytoplasmic dynein preparations reveals a complex with p150GLUED and a novel actin. J. Biol. Chem. 1993;268:15318–15323. [PubMed] [Google Scholar]
- Pfister K.K., Benashski S.E., Dillman J.F., III, Patel-King R.S., King S.M. Identification and molecular characterization of the p24 dynactin light chain. Cell. Motil. Cytoskelet. 1998;41:154–167. doi: 10.1002/(SICI)1097-0169(1998)41:2<154::AID-CM6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Pierre P., Scheel J., Rickard J., Kreis T.E. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Schafer D.A., Gill S.R., Cooper J.A., Heuser J.E., Schroer T.A. Ultrastructural analysis of the dynactin complexan actin-related protein is a component of a filament that resembles f-actin. J. Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T.A. Structure and function of dynactin. Semin. Cell Biol. 1996;7:321–328. [Google Scholar]
- Schroer T.A., Sheetz M.P. Two activators of microtubule-based vesicle transport. J. Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Fransen J.A.M., Bachi T., Ginsel L., Hauri H.-P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop A., White J. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr. Biol. 1998;8:1110–1116. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen W., Karki S., Vaughan K.T., Vallee R.B., Holzbaur E.L., Weiss D.G., Kuznetsov S.A. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E.R., Schroer T.A., Wordeman L., Sheetz M.P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Tai A.W., Chuang J.-Z., Bode C., Wolfrum U., Sung C.-H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Vaisberg E.A., Koonce M.P., McIntosh J.R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C., Wetzel D.M., Schrader M., Hasbani M.J., Gill S.R., Kreis T.E., Schroer T.A. Role of dynactin in endocytic trafficeffects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell. 1999;In press doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan K.T., Vallee R.B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued . J. Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Berrez J.-M., Antony C., Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggsrequirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer C.M., Karki S., Holzbaur E.L. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc. Natl. Acad. Sci. USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]