Abstract
Platelet integrin αIIbβ3 responds to intracellular signals by binding fibrinogen and triggering cytoskeletal reorganization, but the mechanisms of αIIbβ3 signaling remain poorly understood. To better understand this process, we established conditions to study αIIbβ3 signaling in primary murine megakaryocytes. Unlike platelets, these platelet precursors are amenable to genetic manipulation. Cytokine-stimulated bone marrow cultures produced three arbitrary populations of αIIbβ3-expressing cells with increasing size and DNA ploidy: small progenitors, intermediate-size young megakaryocytes, and large mature megakaryocytes. A majority of the large megakaryocytes bound fibrinogen in response to agonists, while almost none of the smaller cells did. Fibrinogen binding to large megakaryocytes was inhibited by Sindbis virus-mediated expression of isolated β3 integrin cytoplasmic tails. Strikingly, large megakaryocytes from mice deficient in the transcription factor NF-E2 failed to bind fibrinogen in response to agonists, despite normal surface expression of αIIbβ3. Furthermore, while megakaryocytes from wild-type mice spread on immobilized fibrinogen and exhibited filopodia, lamellipodia and Rho-dependent focal adhesions and stress fibers, NF-E2–deficient megakaryocytes adhered poorly. These studies establish that agonist-induced activation of αIIbβ3 is controlled by NF-E2–regulated signaling pathways that mature late in megakaryocyte development and converge at the β3 cytoplasmic tail. Megakaryocytes provide a physiologically relevant and tractable system for analysis of bidirectional αIIbβ3 signaling.
Keywords: αIIbβ3, integrin, megakaryocyte, NF-E2, signaling
Integrin αIIbβ3 functions in platelets as an adhesion receptor for fibrinogen and von Willebrand factor and as a signaling receptor that regulates organization of the cytoskeleton (Fox 1996; Hartwig et al. 1996; Shattil et al. 1998). The adhesive and signaling functions of αIIbβ3 are closely intertwined. For example, ligand binding to αIIbβ3 is regulated by inside out signals that control receptor affinity and avidity (Ginsberg et al. 1992), while outside in signals triggered by integrin ligation influence platelet shape, granule secretion, and clot retraction (Schoenwaelder et al. 1994; Hartwig et al. 1996; Yuan et al. 1997; Jenkins et al. 1998; Leng et al. 1998). Until recently, most studies of αIIbβ3 were conducted using platelets or, alternatively, immortalized cell lines transfected with αIIbβ3 (O'Toole et al. 1994; Qi et al. 1998). These studies have been useful in formulating a conceptual framework for integrin signaling that includes a complicated network of heterotrimeric G proteins, protein and lipid kinases and phosphatases, phospholipases, proteases, and small GTPases (Fox 1996; Rittenhouse 1996; Shattil et al. 1998). However, a detailed understanding of αIIbβ3 signaling has been impeded by the inability to manipulate gene expression in anucleate platelets and by concerns that observations in cell lines may not be entirely relevant to platelets. In this context, it is now possible to study platelets in gene-targeted mice, an advance that is beginning to yield important new insights into the structure and function of αIIbβ3 (Holmbäck et al. 1996; Offermanns et al. 1997; Aszódi et al. 1999; Hauser et al. 1999; Hodivala-Dilke et al. 1999; Law et al. 1999). Nonetheless, further analysis of αIIbβ3 signaling would be facilitated if there were a means to manipulate genes ex vivo in an appropriate primary cell.
Megakaryocytes, the direct precursors of platelets, represent a potential model system to achieve this goal. The development of megakaryocytes from hematopoietic stem cells requires a suitable microenvironment, the action of specific hematopoietic growth factors, including thrombopoietin (TPO), and a hierarchy of transcription factors, including FOG, GATA-1, and NF-E2 (Kaushansky 1995; Long 1998; Vyas et al. 1999). Recombinant growth factors allow for preferential expansion and maturation of murine and human megakaryocyte progenitors ex vivo, enabling biochemical studies to be carried out (Mountford et al. 1999; Rojnuckarin et al. 1999). In fact, human megakaryocytes cultured under such conditions have been shown to bind fibrinogen or a ligand-mimetic anti-αIIbβ3 monoclonal antibody after acute stimulation with TPO, demonstrating the presence of inside out signaling pathways in these cells (Zauli et al. 1997a).
Therefore, we set out to determine whether primary murine megakaryocytes could be used to further characterize αIIbβ3 signaling and, in effect, bridge the gap between in vitro experiments with platelets and clinicopathological analyses of gene-targeted mice. The results validate this approach and establish that agonist-induced inside out signaling and fibrinogen binding to αIIbβ3 require a gene or genes regulated by the transcription factor, NF-E2. Furthermore, these studies show that protein expression can be manipulated in mature megakaryocytes using Sindbis virus vectors, enabling a molecular analysis of αIIbβ3 signaling in the appropriate cellular context.
Materials and Methods
Murine Bone Marrow Cultures
All chemicals and reagents were from Sigma Chemical Co. unless indicated otherwise. Initial studies were carried out with wild-type megakaryocytes derived from BALB/C mice. p45 NF-E2–deficient mice (a generous gift from Drs. Ramesh Shivdasani and Stuart Orkin, Harvard Medical School, Boston, MA) are in a mixed C57Bl/6-129/Sv background (Shivdasani et al. 1995). When their megakaryocytes were studied, wild-type mice with a similarly mixed background were used as controls. There was no obvious difference between wild-type megakaryocytes from these different strains with respect to the αIIbβ3 functions studied. Bone marrow cells were harvested from 8–9-wk-old mice by flushing both sets of femurs and tibias with PBS containing 2% bovine serum albumin, 0.38% trisodium citrate, and 1 U/ml DNAse. Mononuclear cells were isolated over Ficoll Hypaque (1.080 g/ml; Pharmacia) after a 30-min centrifugation at 400 g. Low-density mononuclear cells were cultured for up to 9 d at a starting density of 106 cells/ml in Iscove's Modified Dulbecco's medium (Irvine Scientific) supplemented as described (Zauli et al. 1997a), and including 10 ng/ml murine TPO (a gift from Kirin Brewery Ltd., Japan), murine IL-6 and human IL-11 (Biosource International, Inc.). Cytospins of 20,000–100,000 cells were stained with Wright-Giemsa to analyze cell morphology.
Flow Cytometry
Cell size, antigen expression and DNA ploidy were assessed by flow cytometry. After 0–9 d in culture, bone marrow-derived cells were centrifuged at 200 g for 10 min, washed and resuspended in PBS at 4 × 106/ml. Then 50-μl aliquots were incubated for 30 min at 4°C with one of the following antibodies: 10 μg/ml FITC-conjugated anti-αIIb (rat anti–mouse CD41; PharMingen); biotin anti-mouse GP V (murine clone C24.25.1; a gift from V. Ramakrishnan, Cor Therapeutics, Inc., South San Francisco, CA); FITC-anti-Gr-1, a granulocyte marker (rat anti–mouse; PharMingen); phycoerythrin-anti-Sca-1, a progenitor cell marker (rat anti–mouse; PharMingen); or a 1:1,000 dilution of rabbit anti–mouse GP Ibα (from V. Ramakrishnan). Negative controls included an irrelevant isotype-matched rat or mouse antibody (PharMingen) or normal rabbit serum, as needed. When the primary antibodies were biotin-anti–GP V or rabbit anti-GP Ibα, the cells were washed and subsequently incubated for 15 min at 4°C with 20 μg/ml FITC-streptavidin or 14 μg/ml FITC anti-rabbit immunoglobulin (heavy plus light chains), respectively (Biosource International). Cells were finally resuspended in PBS containing 1 μg/ml propidium iodide and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). DNA ploidy was assessed as described (Williams et al. 1998).
Inside Out Signaling in Megakaryocytes
Purified human fibrinogen (Enzyme Research Laboratories) was labeled with FITC (Shattil et al. 1987). Human fibrinogen was used as a ligand instead of murine fibrinogen because of its wider availability and its capacity to bind to and support the aggregation of activated murine platelets (Law et al. 1999). Furthermore, in preliminary studies, murine and human fibrinogen were found to be equivalent inhibitors of FITC-human fibrinogen binding to agonist-stimulated murine and human platelets. After 6 d in culture, bone marrow cells were sedimented by gravity for 60 min at 37°C in a 50-ml conical polypropylene tube. In addition to achieving modest enrichment of megakaryocytes, gravity sedimentation limited any functional damage that might occur to these fragile cells during centrifugation. When the effects of certain inhibitors were studied (see Results), they or appropriate vehicle control buffer were added at this stage for the final 20 min. Cells were then gently resuspended to 4 × 106/ml in modified Tyrode's buffer containing 1 mM CaCl2 and 1 mM MgCl2 (O'Toole et al. 1994) and then incubated for 30 min at room temperature in the presence of FITC-fibrinogen (250 μg/ml), specific agonists and inhibitors, 10 μg/ml of the non–function-blocking anti-αIIb antibody, and 20 μg/ml phycoerythrin-streptavidin (Molecular Probes). After a 10-fold dilution with buffer containing 1 μg/ml propidium iodide, fibrinogen binding was quantified by flow cytometry (Pampori et al. 1999).
FITC-fibrinogen binding was monitored in the FL1 channel of the flow cytometer on the gated subset of viable cells (e.g., negative for propidium iodide, FL3) that expressed αIIbβ3 (FL2). Preliminary studies indicated that fibrinogen binding was inhibited ≥90% by either 10 mM EDTA (Law et al. 1999), 5 μM kistrin (an RGD-containing disintegrin known to block αIIbβ3 function; Adler et al. 1991), or 20 μg/ml 1B5, a function-blocking hamster monoclonal antibody specific for murine αIIbβ3 (a gift from B. Coller and S. Smyth, Mt. Sinai Medical Center, New York, NY; Lengweiler et al. 1999). Therefore, specific fibrinogen binding was defined as binding that was inhibited by kistrin or EDTA. When it was necessary to compare the binding data between subpopulations of cells that were heterogeneous in size and αIIbβ3 density, specific FITC-fibrinogen binding was expressed as a percent of maximal binding obtained in the presence of 1 mM MnCl2, an activator of integrins (Bazzoni and Hemler 1998).
To determine the effect of isolated integrin cytoplasmic tails on fibrinogen binding to megakaryocytes, cDNA encoding chimeric proteins consisting of the extracellular and transmembrane domains of the Tac subunit of the human IL-2 receptor (CD25) and the human β3 cytoplasmic tail (Tac-β3) or the α5 cytoplasmic tail (Tac-α5; Chen et al. 1994; LaFlamme et al. 1994) were cloned into a Sindbis virus vector (pSinRep5; Invitrogen). As a further control, a tailess Tac construct was also prepared. Linear, capped viral RNA was prepared and pseudovirions were produced in BHK cells using a Sindbis Expression System (Invitrogen). Pseudovirion titers were such that a 1:3,000 dilution of BHK cell supernatant infected virtually all BHK cells in a 100-mm dish, as determined by surface expression of Tac using a phycoerythrin-conjugated antibody to human CD25 (PharMingen). To infect megakaryocytes, day 5 bone marrow cells were suspended in a 1:1.5 dilution of virus for 1 hand then diluted with 7 vol of serum-free medium and cultured for another 24 h. The cells were stained with FITC-fibrinogen, phycoerythrin-anti–human CD25 (PharMingen), and propidium iodide, and fibrinogen binding to large, viable megakaryocytes was quantified by flow cytometry.
Outside In Signaling in Megakaryocytes
Bone marrow-derived cells were cultured for 5 or 6 d, allowed to settle by gravity for 1 h and resuspended to 2 × 105/ml in buffer containing 137 mM NaCl, 2.7 mM KCl, 3.3 mM NaH2PO4, 1 mM MgCl2, and 3.8 mM Hepes, pH 7.35. Cells were then incubated on fibrinogen-coated coverslips for 45 min at 37°C in the presence or absence of 100 nM phorbol myristate acetate, the latter added to activate protein kinase C and enhance cell spreading (Leng et al. 1998). After removing nonadherent cells, the adherent cells were fixed in 3.7% formaldehyde, permeabilized with 0.2% Triton X-100 in PBS, and stained with monoclonal anti-vinculin antibody, FITC-anti-mouse IgG and rhodamine-phalloidin. Cells were then analyzed by confocal laser scanning microscopy and photomicrographs were prepared using Adobe Photoshop 5.0 (Hato et al. 1998; Leng et al. 1998).
In some experiments, megakaryocytes from day 5 cultures were infected with recombinant Sindbis virus expressing either C3 exoenzyme, an inhibitor of Rho GTPase (Aktories et al. 1992; Sindbis/C3), or chloramphenicol acyl transferase (Sindbis/CAT; kind gifts from Drs. C.S. Hahn, University of Virginia and M.A. Schwartz, Scripps Research Institute). Viral stocks were produced and titered in BHK cells as described (Bobak et al. 1997; Hahn et al. 1992). For infection, megakaryocytes were allowed to adhere to fibrinogen-coated coverslips for 30 min at 37°C and incubated with Sindbis/C3 or Sindbis/CAT for 1 h at 37°C at a multiplicity of infection of 50. After washing, the cells were incubated another 2.5 h in buffer, and 100 nM phorbol myristate acetate was added for the final 30 min to enhance spreading. After washing, adherent cells were fixed, permeabilized, stained, and examined by confocal microscopy. In parallel, some cells were infected on fibrinogen-coated plastic dishes, scraped into SDS sample buffer, and 50-μg aliquots were subjected to SDS-PAGE under reducing conditions (Laemmli 1970). After transfer to nitrocellulose, blots were probed with a rabbit polyclonal antibody specific for Sindbis E1 protein (a gift from J.H. Strauss, California Institute of Technology, Pasadena, CA) or with a murine monoclonal antibody to Rho (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed for 0.5–2 min by chemiluminescence (ECL; Amersham Corp.).
Results
αIIbβ3 and Megakaryocyte Development
To obtain primary murine megakaryocytes for studies of αIIbβ3 signaling, a low-density fraction of bone marrow was cultured in serum-free medium containing 10 ng/ml TPO, IL-6, and IL-11. Total cell number decreased progressively by 45, 73, and 89% on days 3, 6, and 9, respectively. On the other hand, Wright-Giemsa staining showed a progressive increase in the proportion of large, polyploid cells, such that by day 6 both small, immature megakaryocytes with basophilic cytoplasm and larger, mature megakaryocytes with granular, eosinophilic cytoplasm were readily apparent (Fig. 1). The vast majority of cells on day zero were small, as assessed by flow cytometric light scatter (Fig. 2 A). However, by day 3 a subpopulation of larger cells of intermediate size was evident, and by day 6 a subpopulation of large cells was present, typically amounting to 5–10% of the total. When the large cells were FACS-sorted and stained, they consisted of mature megakaryocytes (Fig. 1 G). The relative proportion of large megakaryocytes decreased slightly by day 9 in culture, presumably the result of programmed cell death (Fig. 2 A; Zauli et al. 1997b). On day 6, the DNA ploidy of the small cells was 2N, while that of the intermediate-size and large cells ranged from 2-32N and 8-128N, respectively (Fig. 2 B).
Figure 1.
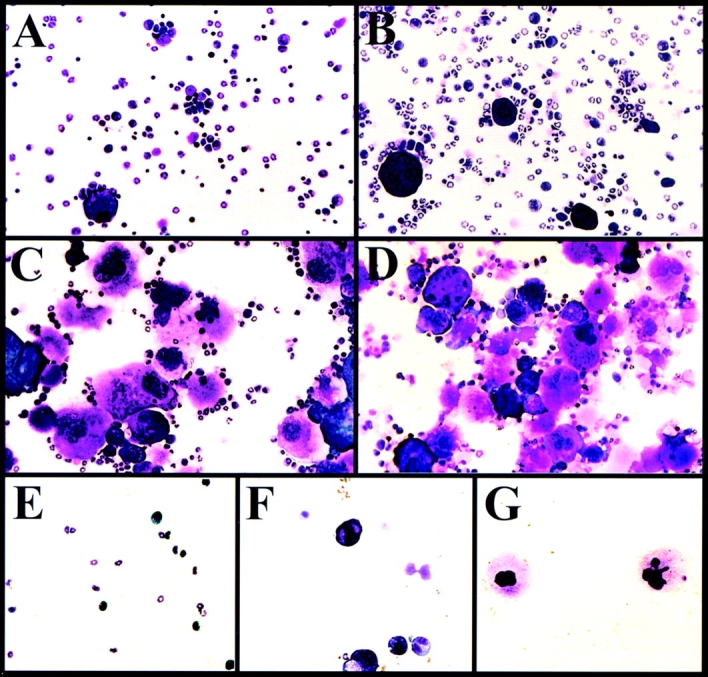
Morphology of murine bone marrow cells. Bone marrow was harvested and maintained in culture as described in Materials and Methods. Wright-Giemsa-stained cytospin preparations after zero (A), 3 (B), 6 (C), and 9 (D) d demonstrated an increase in the proportion of megakaryocytes over time. Day 6 cells were sorted on the basis of size, as determined by flow cytometric light scatter profiles (see Fig. 2 A). E, illustrates the sorted small cells; F, intermediate-size cells; and G, large cells. The objective lens was 20×.
Figure 2.
Size and DNA ploidy of murine bone marrow cells. Cells cultured as in Fig. 1 were characterized by flow cytometry as described in Materials and Methods. A shows dot plots for forward (FSC) and side (SSC) light scatter profiles of 10,000 cells. In each plot, three arbitrary analysis gates have been drawn. The gate at the lower left includes small cells, the middle gate intermediate-size cells, and the gate at the upper right large cells. Note a progressive increase in the proportion of intermediate-size and large cells with time. In B, cells on day 6 of culture were analyzed for DNA content. Note the increase in ploidy with cell size.

Surface antigen expression was characterized by flow cytometry (Table ). The proportion of αIIbβ3-expressing cells increased progressively with time and by day 6 it represented 21% of all small cells, 98% of the intermediate-size cells and >99% of the large cells. On day zero, 15% of the small cells were positive for αIIbβ3, due in part to the presence of platelets. Throughout the culture period, a significant minority of the cells expressed Sca-1 (Table ), and some coexpressed αIIbβ3, suggesting that they were committed megakaryocyte progenitors. Expression of components of the platelet GP Ib-V-IX complex appeared to lag behind expression of αIIbβ3 because not every αIIbβ3-expressing cell was positive for GP Ib or GP V (Table ). Moreover, some cells expressed GP Ib but not GP V, suggesting that GP V is not required for GP Ib expression. On day 6, most of the small cells were positive for the myeloid marker, Gr-1, as were 53% of the intermediate-size cells and 2% of the large cells. Taken together, these results indicate that murine megakaryocytes can undergo progressive expansion and maturation in culture, permitting a detailed analysis of αIIbβ3 signaling during megakaryocyte development.
Table 1.
Expression of Surface Markers during Ex Vivo Expansion of Megakaryocytes
| Day 0 | Day 3 | Day 6 | Day 9 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Small | Small | Medium | Large | Small | Medium | Large | Small | Medium | Large | |
| αIIb | 15.3 | 5.9 | 50.6 | 99.5 | 21.4 | 98.1 | 99.7 | 27.9 | 72.0 | 99.4 |
| GP Ibα | 1.9 | 5.1 | 50.5 | 90.8 | 11.4 | 78.8 | 84.6 | 17.0 | 56.7 | 92.1 |
| GP V | 8.9 | 7.8 | 29.2 | 54.6 | 14.9 | 45.4 | 53.3 | 21.7 | 54.4 | 88.9 |
| Sca-1 | 12.6 | 18.1 | 45.1 | 32.1 | 7.6 | 27.2 | 11.0 | 13.3 | 21.1 | 22.1 |
| Gr-1 | 57.6 | 88.6 | 49.1 | 8.9 | 86.8 | 53.3 | 2.5 | 90.7 | 53.8 | 14.7 |
αIIbβ3 Signaling in Developing Megakaryocytes
Inside out signaling in αIIbβ3-expressing megakaryocyte progenitors and developing megakaryocytes was assessed on day 6 of culture by measuring the agonist-dependent binding of a saturating concentration of FITC-fibrinogen. Large megakaryocytes bound little or no fibrinogen in the absence of an agonist (Fig. 3 A). On the other hand, specific fibrinogen binding was observed after a 30-min incubation of the cells with 10 ng/ml SDF-1α, a CXCR4 receptor agonist (Riviere et al. 1999; Wang et al. 1998) or 100 ng/ml TPO, the agonist for c-Mpl receptors (Kaushansky 1999). Fibrinogen binding was even greater in response to typical platelet agonists, including 10–100 μM ADP plus epinephrine, 0.5 U/ml thrombin, or 2 mM PAR4 thrombin receptor-activating peptide (GYPGKF; Kahn et al. 1998; Fig. 3 A). Although higher concentrations of these agonists did not stimulate additional fibrinogen binding, combinations of three or more agonists markedly increased fibrinogen binding, in some cases equivalent to that obtained with MnCl2 (Fig. 3 A). None of the agonists increased surface expression of αIIbβ3.
Figure 3.
Fibrinogen binding to αIIbβ3-expressing megakaryocyte progenitors and megakaryocytes. Bone marrow cells on day 6 of culture were stimulated with the indicated agonists in the presence of FITC-fibrinogen, a non–function-blocking anti-αIIb antibody (to identify αIIbβ3-expressing cells), and propidium iodide (to exclude dead cells). Agonist concentrations were: SDF-1α, 10 ng/ml; TPO, 100 ng/ml; ADP and Epi(nephrine), 100 μM each; Thrombin, 0.5 U/ml; and PAR4 receptor-activating peptide, 2 mM. The data in A depict specific fibrinogen binding determined by flow cytometry and are expressed as a percent of maximum specific binding obtained in response to MnCl2. Fibrinogen binding in B is expressed as the percent of cells that stained positive for FITC-fibrinogen. Data represent the means ± SEM of five separate experiments.
Fibrinogen binding induced by the combination of PAR4-activating peptide, ADP, and epinephrine was inhibited >80% by preincubation of the cells with 2 μM PGI2 or PGE1, compounds that inhibit platelet aggregation through receptor-mediated stimulation of adenylyl cyclase (Hawiger et al. 1980). In addition, fibrinogen binding was inhibited 48% by 100 nM wortmannin, an inhibitor of certain PI 3-kinases, 40% by 12 μM bisindolylmaleimide, a protein kinase C inhibitor, and 57% by 100 μM BAPTA-AM, a chelator of cytoplasmic free Ca2+. In contrast, preincubation of cells with 1 mM aspirin to inhibit cyclooxygenase had no effect on fibrinogen binding, although it did inhibit the response to arachidonic acid as expected. Collectively, these results indicate that excitatory agonists can stimulate and certain inhibitory agonists can block the ligand binding function of αIIbβ3 in large megakaryocytes, just as in platelets. Furthermore, activation of αIIbβ3 is regulated in a positive fashion by signaling pathways that likely involve PI 3-kinase, protein kinase C, and free Ca2+, and in a negative fashion by cyclic AMP.
Fig. 3 B shows that up to 60% of large megakaryocytes bound fibrinogen in response to agonists. To place this in perspective, ≥90% of agonist-activated blood platelets obtained from the same donor mice bound fibrinogen (not shown). The lack of responsiveness of some large megakaryocytes appeared to be due to impaired signaling to αIIbβ3 because FITC-fibrinogen binding to all of the large cells could be induced with MnCl2. Furthermore, very few intermediate-size megakaryocytes and no small αIIbβ3-expressing cells bound fibrinogen in response to the agonists depicted in Fig. 3 or in response to as much as 500 nM phorbol myristate acetate to activate protein kinase C. The results were similar if fetal hematopoietic tissue from the livers of day 14.5 murine fetuses was used instead of bone marrow as the source of megakaryocyte progenitors (not shown; Lecine et al. 1998a). Overall, these results indicate that inside out signaling pathways responsible for activation of αIIbβ3 become fully developed only late in megakaryocyte development.
Platelet adhesion to an immobilized αIIbβ3 ligand triggers the reorganization of actin filaments, filopodial and lamellipodial extension, and cell spreading (Nachmias and Golla 1991; Yuan et al. 1997; Hagmann et al. 1998; Leng et al. 1998). To determine if megakaryocytes can engage in such outside in signaling, day 6 bone marrow cultures were incubated on fibrinogen-coated coverslips for 45 min. By this time, most of the large megakaryocytes had become adherent, and this response was αIIbβ3-dependent because it could be blocked by 5 μM kistrin. Adherent megakaryocytes ranged in diameter from 10 to >50 μm, and many exhibited filopodia and lamellipodia (Fig. 4A and Fig. C). When 100 nM phorbol myristate acetate was present, the megakaryocytes spread more extensively and most now exhibited focal adhesions and stress fibers (Fig. 4B and Fig. D). Actin filaments were often circumferential and parallel to the cell margins, rather than perpendicular as in fibroblasts, an observation made previously with guinea pig megakaryocytes (Leven and Nachmias 1982). These results indicate that ligand binding to αIIbβ3 can promote cytoskeletal reorganization in megakaryocytes, accompanied by changes in cell shape.
Figure 4.

Distribution of vinculin and F-actin in large megakaryocytes adherent to fibrinogen. Megakaryocytes from day 6 cultures were incubated for 45 min on fibrinogen-coated coverslips in the absence (A and C) or presence (B and D) of 100 nM phorbol myristate acetate. The cells were fixed, permeabilized and stained to visualize vinculin (green) and F-actin (red) by confocal microscopy. Each panel shows a 0.2-μm confocal image taken near the ventral surface of a representative cell. The arrowheads in D point to vinculin-rich focal adhesions, and the arrow points to actin stress fibers. This experiment is representative of three so performed. Bar, 10 μm.
Mechanisms of αIIbβ3 Signaling in Megakaryocytes
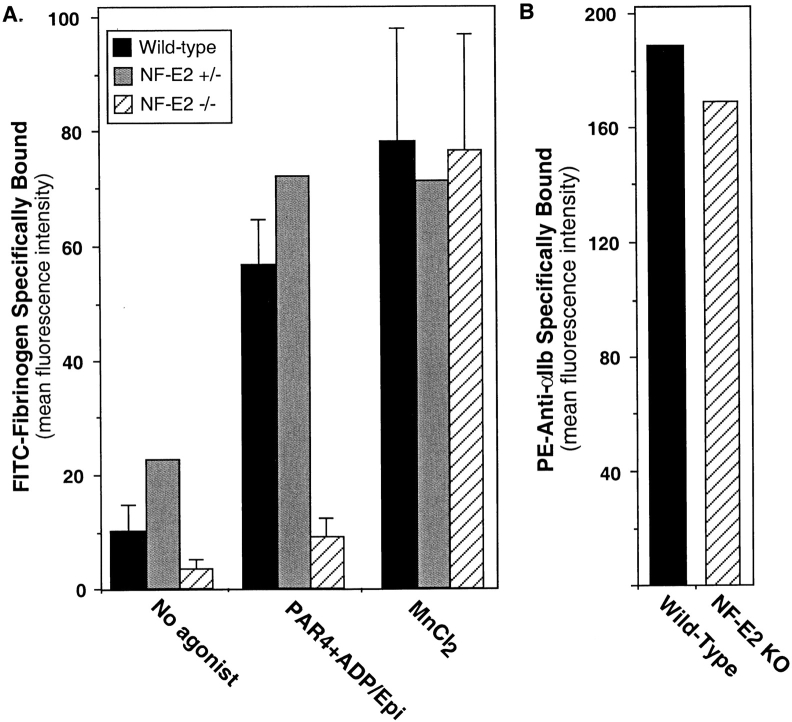
Studies of mice deficient in the p45 subunit of NF-E2 have revealed that this transcription factor is necessary for the final phases of megakaryocyte development and platelet production (Shivdasani et al. 1995; Lecine et al. 1998b). Since inside out signaling to αIIbβ3 was fully developed only in mature megakaryocytes (Fig. 3), we considered whether genes regulated by NF-E2 might be required for αIIbβ3 activation. Accordingly, bone marrow from NF-E2−/− mice was cultured for 6 days with TPO, IL-6, and IL-11, yielding a subpopulation of large megakaryocytes similar to wild-type and NF-E2+/− controls. However, unlike cells from wild-type or NF-E2+/− mice, large NF-E2−/− megakaryocytes displayed a virtual absence of FITC-fibrinogen binding in response to the combination of PAR4 thrombin receptor-activating peptide, epinephrine, and ADP (Fig. 5). Similar results were obtained when the cells were stimulated with as much as 500 nM phorbol myristate acetate. This impairment of fibrinogen binding was due to a defect in inside out signaling because NF-E2−/− megakaryocytes expressed normal amounts of αIIbβ3 on their surfaces, and they bound FITC-fibrinogen as well as wild-type or NF-E2+/− megakaryocytes in response to MnCl2 (Fig. 5). These results indicate that NF-E2, or more likely genes regulated by NF-E2, are required for agonist-induced activation of αIIbβ3.
Figure 5.
Effect of NF-E2 deficiency on agonist-induced fibrinogen binding to large megakaryocytes. In A, fibrinogen binding to large megakaryocytes was assessed as in Fig. 3 and expressed as mean fluorescence intensity. The concentration of PAR4 receptor-activating peptide was 2 mM, ADP and Epi(nephrine) were 100 μM each and MnCl2 was 1 mM. NF-E2−/− and wild-type megakaryocytes were directly compared in 4 separate experiments (means ± SEM), and NF-E2+/− megakaryocytes were also studied on one of these occasions. Note that unlike their wild-type and NF-E2+/− counterparts, the NF-E2−/− megakaryocytes failed to bind fibrinogen in response to agonists, but did so in response to MnCl2. In B, αIIbβ3 expression in wild-type and NF-E2−/− megakaryocytes was compared on one occasion using an anti-αIIb antibody.
In fibroblastic cell lines, the heterologous expression of membrane-tethered β3 integrin cytoplasmic tails (in the form of a human Tac-β3 fusion protein) can reverse the high-affinity state of a constitutively active mutant of αIIbβ3. In contrast, Tac-α5 tails have no such effect (Chen et al. 1994). To determine if chimeric β3 tails could block agonist-induced activation of αIIbβ3, day 5 bone marrow cultures were incubated for 1 h with Sindbis pseudovirions expressing Tac-β3 RNA or, as a control, pseudovirions expressing Tac-α5. 24 h later, flow cytometry showed that the Tac chimeras had been expressed in 5–20% of large megakaryocytes. Under these conditions, Sindbis virus infection did not affect either αIIbβ3 surface expression or megakaryocyte viability. Large megakaryocytes expressing relatively low levels of Tac-β3 or Tac-α5 bound fibrinogen in response to agonists almost as well as noninfected cells. In contrast, megakaryocytes expressing relatively high levels of Tac-β3 bound very little fibrinogen, and significantly less than cells expressing high levels of Tac-α5 (P < 0.01; Fig. 6). The more modest reduction in fibrinogen binding in cells expressing high levels of Tac-α5 was also observed with a tailless Tac construct (not shown), suggesting that it might have been due to viral infection, per se. Since Tac-β3 cannot form a heterodimer with αIIb but may nevertheless interact with cytoplasmic proteins that normally bind to αIIbβ3 (Chen et al. 1994), these results suggest that one or more β3 cytoplasmic tail-binding proteins mediates physiological activation of αIIbβ3.
Figure 6.
Effects of chimeric integrin cytoplasmic tails on agonist-induced fibrinogen binding to large megakaryocytes. After 5 d in culture, megakaryocytes in suspension were infected with Sindbis virus expressing either the Tac-β3 cytoplasmic tail or the Tac-α5 tail, as described in Materials and Methods. 24 h later, Tac expression and FITC-fibrinogen binding to large megakaryocytes were studied by flow cytometry. Arbitrary analysis gates were placed around megakaryocytes that expressed no Tac, relatively low levels of Tac, and relatively high levels of Tac. Data represent the means ± SEM of seven separate experiments.
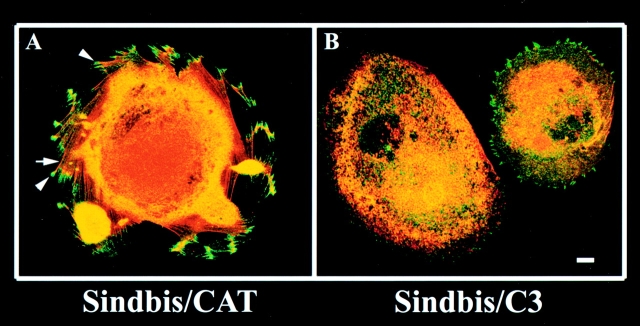
To explore mechanisms of outside in signaling in megakaryocytes, cells adherent to fibrinogen were exposed for 1 h to Sindbis virus encoding C3 exoenzyme, which ADP-ribosylates and inactivates the small GTPase, Rho (Aktories et al. 1992; Moorman et al. 1999). After subsequent incubation in the presence of phorbol myristate acetate to enhance cell spreading, cytoskeletal organization was analyzed. Both Sindbis/C3 and a control virus, Sindbis/CAT, infected adherent cells, as evidenced by the time-dependent expression of the 61-kD viral E1 protein on Western blots of cell lysates (not shown). Infection with Sindbis/C3 was associated with a reduction in the percent of megakaryocytes that contained focal adhesions and stress fibers (Fig. 7A and Fig. B). Whereas 70% of adherent megakaryocytes incubated with Sindbis/CAT displayed focal adhesions and stress fibers, only 30% of cells incubated with Sindbis/C3 did so (Fig. 7 C, P < 0.001). Furthermore, the remaining stress fibers in Sindbis/C3-treated megakaryocytes were often thinner and less densely packed than in the control cells. The effect of Sindbis/C3 on the megakaryocyte cytoskeleton could reasonably be attributed to ADP-ribosylation of Rho, because ∼50% of this protein in cell lysates displayed a retarded electrophoretic mobility characteristic of ribosylation (Fig. 7 C, inset). In contrast to its effect on focal adhesions and stress fibers, Sindbis/C3 did not appear to impair megakaryocyte spreading or the formation of filopodia and lamellipodia. Thus, Rho is responsible for a subset of cytoskeletal responses in megakaryocytes that is triggered, in part, by outside in signaling through αIIbβ3.
Figure 7.
Effect of C3 exoenzyme on outside in signaling through αIIbβ3 in megakaryocytes. After 5 d in culture, fibrinogen-adherent megakaryocytes were infected with Sindbis virus expressing either CAT or C3 exoenzyme as described in Materials and Methods. Cells were then incubated for another 2.5 h, the last 30 min of which 100 nM phorbol myristate acetate was added to enhance spreading. After fixation and staining as in Fig. 4, the cells were analyzed by confocal microscopy. A and B show representative confocal images of cells infected with Sindbis/CAT and Sindbis/C3, respectively. Note the prominent vinculin-rich focal adhesions (arrowheads) and stress fibers (arrow) in A, but not in B. In C, 100 adherent megakaryocytes treated with each virus were scored for focal adhesions and stress fibers by two independent observers. Data are the means ± SEM of three separate experiments. The inset shows a Western blot of cell lysates probed with an antibody against Rho. In three such experiments, the intensity of the slower migrating (ADP-ribosylated) Rho band from Sindbis/C3-infected cells averaged 50% of the total Rho. Bar, 10 μm.
Discussion
A common strategy to characterize intracellular signaling pathways is to express wild-type and mutant gene products and observe the effects on cell function. Unfortunately, this approach cannot be used to characterize integrin signaling in platelets because of inherent difficulties in expressing recombinant proteins in these anucleate cells. Consequently, we hypothesized that primary murine megakaryocytes might serve as a relevant and tractable model system for studies of αIIbβ3 signaling. Culture of murine bone marrow in the presence of TPO, IL-6, and IL-11 led to megakaryocyte expansion and maturation, allowing functional studies of αIIbβ3 and the following conclusions to be drawn. (a) As with platelets, megakaryocytes are capable of inside out signaling to control agonist-induced fibrinogen binding to αIIbβ3. This process becomes fully functional late in megakaryocyte development, requires a gene or genes regulated by transcription factor NF-E2, and converges on the β3 cytoplasmic tail. (b) As with platelets, the adhesion of megakaryocytes to immobilized fibrinogen triggers outside in signals through αIIbβ3 that lead to cell spreading and cytoskeletal reorganization. Adherent megakaryocytes exhibit filopodia, lamellipodia, and Rho-dependent focal adhesions and stress fibers. (c) Sindbis virus vectors can be used to express heterologous proteins in megakaryocytes, enabling αIIbβ3 signaling to be studied in a physiological context in ways not possible with platelets.
Mechanisms of Inside Out Signaling in Megakaryocytes
In both human and murine platelets, ligand binding to αIIbβ3 is regulated by signaling events that modulate integrin affinity and/or avidity (Shattil et al. 1998; Tsakiris et al. 1999). In platelets, the use of selective enzyme inhibitors has implicated protein and lipid kinases, such as PI 3-kinase and protein kinase C, in receptor-mediated activation of αIIbβ3. However, the mechanism by which effectors modulate αIIbβ3 function is unknown. A recent report concluded that human bone marrow-derived megakaryocytes stimulated with TPO adhere to immobilized fibrinogen in a manner dependent on αIIbβ3 and PI 3-kinase (Zauli et al. 1997a). The present studies establish that the binding of soluble fibrinogen to αIIbβ3 is regulated in murine megakaryocytes. Unstimulated megakaryocytes failed to bind soluble fibrinogen, indicating that αIIbβ3 is in a default low affinity/avidity state. Stimulation with agonists, including TPO and substances that interact with G protein-linked receptors (SDF-1α, thrombin, PAR4 receptor-activating peptide, ADP, epinephrine), caused rapid activation of αIIbβ3 and fibrinogen binding to a majority of mature megakaryocytes (Fig. 3). In fact, a combination of agonists caused as much fibrinogen binding as did MnCl2, an activator of integrins (Bazzoni and Hemler 1998). Platelet aggregation inhibitors, such as PGI2 and PGE1 as well as inhibitors of PI 3-kinase and protein kinase C, blocked agonist-induced fibrinogen binding. Thus, αIIbβ3 is likely subject to similar regulatory mechanisms in mature megakaryocytes and in platelets.
A consistent finding in this study was that few intermediate-size megakaryocytes and no small αIIbβ3-expressing progenitors bound fibrinogen in response to agonists (Fig. 3 B). This failure could not be explained by a lack of αIIbβ3 expression, by prior binding of ligands to αIIbβ3 during culture, or by structural alterations in the integrin, since these cells could bind fibrinogen in response to MnCl2. The most likely explanation for these observations is that inside out signaling pathways to αIIbβ3 become fully developed relatively late in megakaryocytopoiesis. Developmental regulation of αIIbβ3 affinity/avidity, as opposed to regulation of αIIbβ3 expression, could have beneficial in vivo consequences by limiting the binding of soluble adhesive ligands to αIIbβ3 on progenitors and young megakaryocytes, thereby preventing their unnecessary aggregation. At the same time, the presence of αIIbβ3 on the surface of these cells, even in a low affinity/avidity state, might enable them to adhere to fibrinogen or other ligands in the extracellular matrix (Savage et al. 1992), and to initiate outside in signals (Haimovich et al. 1993).
The most striking finding in this study was an inability of p45 NF-E2−/− megakaryocytes to bind fibrinogen in response to agonists (Fig. 5). NF-E2 contains a 45-kD subunit that is restricted to hematopoietic cells and a ubiquitous 18-kD subunit (Andrews 1998). Since p45 and p18 hetero-oligomers are required for transcriptional activation, the results with p45-deficient megakaryocytes suggest that one or more genes regulated by NF-E2 are required for full inside out signaling. This discovery would have been difficult to validate and explore in platelets because NF-E2−/− mice have so few circulating platelets (Shivdasani et al. 1995). In addition to highlighting the value of the megakaryocyte model, these results suggest that the identification and characterization of NF-E2–regulated genes should advance our understanding of the mechanisms of inside out regulation of αIIbβ3.
Recently, an in vivo immunoselection strategy was used to identify thromboxane synthase as a p45 NF-E2 target gene in the HEL cell megakaryoblastic cell line (Deveaux et al. 1997). In platelets, this enzyme is responsible for the conversion of prostaglandin endoperoxides to thromboxane A2. Since thromboxane A2 is a platelet agonist, a deficiency of thromboxane synthase in NF-E2−/− megakaryocytes could, in theory, help to explain the inside out signaling defect in these cells. However, this is unlikely for several reasons. First, most investigators including ourselves have found that agonists such as thrombin, ADP, and epinephrine stimulate little or no fibrinogen binding to HEL cells or to other megakaryoblastic cell lines. Thus, although these cells express thromboxane synthase, they appear to lack critical intermediates required for inside out signaling (Jennings et al. 1996; Cichowski et al. 1999). Second, we found that large megakaryocytes from wild-type mice bound fibrinogen in response to PAR4 peptide, ADP, and epinephrine even after treatment with aspirin, which blocks cyclooxygenase and subsequent production of thromboxane A2 (Roth et al. 1975). Third, thromboxane A2 is not involved in the initial activation of αIIbβ3 in platelets, but rather reinforces the responses to other agonists (Thomas et al. 1998). It is now feasible, and may be preferable, to use primary megakaryocytes instead of megakaryoblastic cell lines to identify NF-E2–regulated genes involved in αIIbβ3 signaling.
Sindbis virus-mediated expression of Tac-β3 integrin cytoplasmic tails in megakaryocytes caused dose-dependent inhibition of agonist-induced fibrinogen binding to αIIbβ3 (Fig. 6). Although expressed to the same extent as Tac-β3, Tac-α5 caused significantly less inhibition of agonist-induced fibrinogen binding to megakaryocytes (P < 0.01), and the extent of inhibition was identical to that observed with a tailless Tac construct. The marked inhibitory effect of Tac-β3 is consistent with the suggestion that overexpressed β3 tails may bind and titrate cytoplasmic proteins that otherwise would interact with αIIbβ3 to regulate receptor affinity or avidity (Chen et al. 1994; LaFlamme et al. 1994). Several proteins have been shown to interact with the β3 tail in model systems and by affinity chromatography, including cytoskeletal proteins such as talin, α-actinin, filamin, and myosin, a 14-kD polypeptide called β3-endonexin, and the cytoplasmic tail of αIIb (Otey et al. 1993; Haas and Plow 1996; Hemler 1998; Jenkins et al. 1998; Pfaff et al. 1998). In principle, any or all of these interactions with the β3 tail might influence the conformation or oligomerization state of αIIbβ3 and, therefore, its ligand binding properties (Kucik et al. 1996; Kashiwagi et al. 1997; Sampath et al. 1998; Bennett et al. 1999).
Mechanisms of Outside In Signaling in Megakaryocytes
The adhesion of platelets to surfaces coated with fibrinogen or von Willebrand factor triggers tyrosine phosphorylation of numerous proteins, cell spreading, and actin rearrangements (Haimovich et al. 1993; Heemskerk et al. 1997; Yuan et al. 1997; Leng et al. 1998). In the current study, we found that murine megakaryocytes also adhere to fibrinogen via αIIbβ3 and undergo spreading and cytoskeletal reorganization in an αIIbβ3-dependent fashion, particularly in the presence of a costimulus like phorbol myristate acetate (Fig. 4). This response is similar in some respects to the morphological changes and actin rearrangements in fibroblasts that are triggered by the combination of cell adhesion via β1 integrins and stimulation by growth factors (Burridge and Chrzanowska-Wodnicka 1996). β1 integrins (α4β1 and α5β1) have been demonstrated to play key roles in hematopoietic cell development, presumably by mediating outside in signaling (Verfaillie 1998). In addition, β1 integrins in mature human or guinea pig megakaryocytes can support attachment and spreading on fibronectin and focal adhesion formation (Berthier et al. 1998; Schick et al. 1998). In contrast, other than regulation of fibrinogen uptake into α-granules, very little is known about the function of αIIbβ3 in megakaryocytes (Handagama et al. 1993).
αIIbβ3 signaling cannot be essential for megakaryocytopoiesis or thrombopoiesis because platelet numbers are normal in mice and humans deficient in αIIbβ3 (Hodivala-Dilke et al. 1999). This is not to say, however, that αIIbβ3 plays no role in these processes. For example, proplatelet and platelet formation may involve a series of membrane protrusive events which could involve integrin-triggered cytoskeletal reorganization (Choi et al. 1995; Lecine et al. 1998b). In support of this, certain anti-αIIbβ3 antibodies can inhibit proplatelet formation by cultured megakaryocytes (Takahashi et al. 1999). The formation of filopodia, lamellipodia, and focal adhesions in fibrinogen-adherent megakaryocytes (Fig. 4) suggests that signals from αIIbβ3 may activate Rho family GTPases, including cdc42, Rac, and Rho (Hall 1998). Rho in particular is implicated by the reduction in focal adhesions and stress fibers in megakaryocytes transduced with C3 exoenzyme (Fig. 7). Since Rho GTPases may become activated during integrin-mediated cell adhesion (Ren et al. 1999) and signal to the cytoskeleton and nucleus (Hall 1998; Treisman et al. 1998), we suggest that signals downstream of αIIbβ3 may play some adjunctive role in megakaryocyte or platelet development. In this context, antibody or peptide blockade of ligand binding to αIIbβ3 has been shown to impair human megakaryocyte colony formation in a fibrin gel (Zauli et al. 1997a), and treatment of a human megakaryoblastic cell line with C3 exoenzyme caused an increase in DNA ploidy (Takada et al. 1996). Further studies of the role of αIIbβ3 signaling in megakaryocytes are warranted.
Retroviruses and lentiviruses are being evaluated as transfer vectors for gene therapy, and they also hold promise for studies of megakaryocyte function (Burstein et al. 1999; Miyoshi et al. 1999; Murphy and Leavitt 1999; Wilcox et al. 1999). The present experiments show that Sindbis virus vectors provide an alternative way to achieve transient expression of recombinant proteins in terminally differentiated hematopoietic cells like megakaryocytes. Transient expression may be particularly useful in situations where long-term expression of a recombinant protein might interfere with cell growth or differentiation, as with C3 exoenzyme and Tac-β3 (Henning et al. 1997; Lukashev et al. 1994). Taken together with new strategies to achieve megakaryocyte-specific gene expression in vivo (Murphy and Leavitt 1999), several complementary approaches are now available to manipulate gene expression in megakaryocytes, establishing these cells as an ideal system for determining the molecular basis of αIIbβ3 signaling.
Acknowledgments
The authors are grateful to Dr. Kenneth Kaushansky and his laboratory for generous help in setting up the megakaryocyte culture system. We thank Drs. Ramesh Shivdasani and Stuart Orkin for the NF-E2−/− mice, Drs. Takashi Kato and Yoshihiro Tanaka for TPO, Dr. V. Ramakrishnan for the anti-GP Ib and GP V antibodies, Drs. Barry Coller and Susan Smyth for antibody 1B5, Dr. James H. Strauss for antibody to Sindbis E1, and Drs. Chang S. Hahn and Martin A. Schwartz for the Sindbis/CAT and Sindbis C3 vectors.
These studies were supported by research grants from the National Institutes of Health (HL 56595, HL 48728, HL 54476).
Footnotes
The first two authors contributed equally to this work.
Abbreviations used in this paper: CAT, chloramphenicol acyltransferase; TPO, thrombopoietin.
References
- Adler M., Lazarus R.A., Dennis M.S., Wagner G. Solution structure of kistrin, a potent platelet aggregation inhibitor and GP IIb-IIIa antagonist. Science. 1991;253:445–448. doi: 10.1126/science.1862345. [DOI] [PubMed] [Google Scholar]
- Aktories K., Mohr C., Koch G. Clostridium botulinum C3 ADP-ribosyltransferase. Curr. Topics Microbiol. Immunol. 1992;175:115–131. doi: 10.1007/978-3-642-76966-5_6. [DOI] [PubMed] [Google Scholar]
- Andrews N.C. The NF-E2 transcription factor. Int. J. Biochem. Cell Biol. 1998;30:429–432. doi: 10.1016/s1357-2725(97)00135-0. [DOI] [PubMed] [Google Scholar]
- Aszódi A., Pfeifer A., Ahmad M., Glauner M., Zhou X.H., Ny L., Andersson K.E., Kehrel B., Offermanns S., Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G., Hemler M.E. Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- Bennett J.S., Zigmond S., Vilaire G., Cunningham M., Bednar B. The platelet cytoskeleton regulates the affinity of the integrin αIIbβ3 for fibrinogen. J. Biol. Chem. 1999;274:25301–25307. doi: 10.1074/jbc.274.36.25301. [DOI] [PubMed] [Google Scholar]
- Berthier R., Jacquier-Sarlin M., Schweitzer A., Block M.R., Molla A. Adhesion of mature polyploid megakaryocytes to fibronectin is mediated by beta 1 integrins and leads to cell damage. Exp. Cell Res. 1998;242:315–327. doi: 10.1006/excr.1998.4119. [DOI] [PubMed] [Google Scholar]
- Bobak D., Moorman J., Guanzon A., Gilmer L., Hahn C. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene. 1997;15:2179–2189. doi: 10.1038/sj.onc.1201396. [DOI] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burstein S.A., Dubart A., Norol F., Debili N., Friese P., Downs T., Yu X., Kincade P.W., Villeval J.L., Vainchenker W. Expression of a foreign protein in human megakaryocytes and platelets by retrovirally mediated gene transfer. Exp. Hematol. 1999;27:110–116. doi: 10.1016/s0301-472x(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Chen Y.-P., O'Toole T.E., Shipley T., Forsyth J., LaFlamme S.E., Yamada K.M., Shattil S.J., Ginsberg M.H. “Inside-out” signal transduction inhibited by isolated integrin cytoplasmic domains. J. Biol. Chem. 1994;269:18307–18310. [PubMed] [Google Scholar]
- Choi E.S., Nichol J.L., Hokom M.M., Hornkohl A.C., Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85:402–413. [PubMed] [Google Scholar]
- Cichowski K., Orsini M.J., Brass L.F. PAR1 activation initiates integrin engagement and outside-in signalling in megakaryoblastic CHRF-288 cells. Biochim. Biophys. Acta Mol. Cell Res. 1999;1450:265–276. doi: 10.1016/s0167-4889(99)00065-8. [DOI] [PubMed] [Google Scholar]
- Deveaux S., Cohen-Kaminsky S., Shivdasani R.A., Andrews N.C., Filipe A., Kuzniak I., Orkin S.H., Romeo P.H., Mignotte V. p45 NF-E2 regulates expression of thromboxane synthase in megakaryocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5654–5661. doi: 10.1093/emboj/16.18.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.E.B. Platelet activationnew aspects Haemostasis 26Suppl.1996. 102 131 [DOI] [PubMed] [Google Scholar]
- Ginsberg M.H., Du X., Plow E.F. Inside-out integrin signalling. Curr. Opin. Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Haas T.A., Plow E.F. The cytoplasmic domain of αIIbβ3. A ternary complex of the integrin alpha and beta subunits and a divalent cation. J. Biol. Chem. 1996;271:6017–6026. doi: 10.1074/jbc.271.11.6017. [DOI] [PubMed] [Google Scholar]
- Hagmann J., Grob M., Welman A., van Willigen G., Burger M.M. Recruitment of the LIM protein hic-5 to focal contacts of human platelets. J. Cell Sci. 1998;111:2181–2188. doi: 10.1242/jcs.111.15.2181. [DOI] [PubMed] [Google Scholar]
- Hahn C.S., Hahn Y.S., Braciale T.J., Rice C.M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc. Natl. Acad. Sci. USA. 1992;89:2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Lipfert L., Brugge J.S., Shattil S.J. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J. Biol. Chem. 1993;268:15868–15877. [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Handagama P., Scarborough R.M., Shuman M.A., Bainton D.F. Endocytosis of fibrinogen into megakaryocyte and platelet α-granules is mediated by αIIbβ3 (glycoprotein IIb-IIIa) Blood. 1993;82:135–138. [PubMed] [Google Scholar]
- Hartwig J.H., Kung S., Kovacsovics T., Janmey P.A., Cantley L.C., Stossel T.P., Toker A. D3 phosphoinositides and outside-in integrin signaling by glycoprotein IIb-IIIa mediate platelet actin assembly and filopodial extension induced by phorbol 12-myristate 13-acetate. J. Biol. Chem. 1996;271:32986–32993. doi: 10.1074/jbc.271.51.32986. [DOI] [PubMed] [Google Scholar]
- Hato T., Pampori N., Shattil S.J. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin αIIbβ3 . J. Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W., Knobeloch K.P., Eigenthaler M., Gambaryan S., Krenn V., Geiger J., Glazova M., Rohde E., Horak I., Walter U., Zimmer M. Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:8120–8125. doi: 10.1073/pnas.96.14.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J., Parkinson S., Timmons S. Prostacyclin inhibits mobilisation of fibrinogen-binding sites on human ADP- and thrombin-treated platelets. Nature. 1980;283:195–197. doi: 10.1038/283195a0. [DOI] [PubMed] [Google Scholar]
- Heemskerk J.W.M., Vuist W.M.J., Feijge M.A.H., Reutelingsperger C.P.M., Lindhout T. Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent plateletsevidence for regulation by protein tyrosine kinase-dependent Ca2+ responses. Blood. 1997;90:2615–2625. [PubMed] [Google Scholar]
- Hemler M.E. Integrin associated proteins. Curr. Opin. Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Henning S.W., Galandrini R., Hall A., Cantrell D.A. The GTPase Rho has a critical regulatory role in thymus development. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke K.M., McHugh K.P., Tsakiris D.A., Rayburn H., Crowley D., Ullman-Cullere M., Ross F.P., Coller B.S., Teitelbaum S., Hynes R.O. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbäck K., Danton M.J.S., Suh T.T., Daugherty C.C., Degen J.L. Impaired platelet aggregation and sustained bleeding in mice lacking the fibrinogen motif bound by integrin αIIbβ3 . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:5760–5771. [PMC free article] [PubMed] [Google Scholar]
- Jenkins A.L., Nannizzi-Alaimo L., Silver D., Sellers J.R., Ginsberg M.H., Law D.A., Phillips D.R. Tyrosine phosphorylation of the β3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J. Biol. Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- Jennings L.K., Slack S.M., Wall C.D., Mondoro T.H. Immunological comparisons of integrin alpha IIb beta 3 (GPIIb-IIIa) expressed on platelets and human erythroleukemia cellsevidence for cell specific differences. Blood Cells Mol. Dis. 1996;22:23–35. doi: 10.1006/bcmd.1996.0005. [DOI] [PubMed] [Google Scholar]
- Kahn M.L., Zheng Y.W., Huang W., Bigornia V., Zeng D.W., Moff S., Farese R.V., Jr., Tam C., Coughlin S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kashiwagi H., Schwartz M.A., Eigenthaler M.A., Davis K.A., Ginsberg M.H., Shattil S.J. Affinity modulation of platelet integrin αIIbβ3 by β3-endonexin, a selective binding partner of the β3 integrin cytoplasmic tail. J. Cell Biol. 1997;137:1433–1443. doi: 10.1083/jcb.137.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K. ThrombopoietinThe primary regulator of platelet production. Blood. 1995;86:419–431. [PubMed] [Google Scholar]
- Kaushansky K. The enigmatic megakaryocyte gradually reveals its secrets. Bioessays. 1999;21:353–360. doi: 10.1002/(SICI)1521-1878(199904)21:4<353::AID-BIES12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kucik D.F., Dustin M.L., Miller J.M., Brown E.J. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J. Clin. Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaFlamme S.E., Thomas L.A., Yamada S.S., Yamada K.M. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J. Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D.A., Nannizzi-Alaimo L., Ministri K., Hughes P., Forsyth J., Turner M., Shattil S.J., Ginsberg M.H., Tybulewicz V., Phillips D.R. Genetic and pharmacological analyses of Syk function in αIIbβ3 signaling in platelets. Blood. 1999;93:2645–2652. [PubMed] [Google Scholar]
- Lecine P., Blank V., Shivdasani R. Characterization of the hematopoietic transcription factor NF-E2 in primary murine megakaryocytes J. Biol. Chem. 273 1998. 7572 7578a [DOI] [PubMed] [Google Scholar]
- Lecine P., Villeval J.L., Vyas P., Swencki B., Xu Y., Shivdasani R.A. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes Blood 92 1998. 1608 1616b [PubMed] [Google Scholar]
- Leng L., Kashiwagi H., Ren X.-D., Shattil S.J. RhoA and the function of platelet integrin αIIbβ3 . Blood. 1998;91:4206–4215. [PubMed] [Google Scholar]
- Lengweiler S., Smyth S.S., Jirouskova M., Scudder L.E., Park H., Moran T., Coller B.S. Preparation of monoclonal antibodies to murine platelet glycoprotein IIb/IIIa (alphaIIbbeta3) and other proteins from hamster-mouse interspecies hybridomas. Biochem. Biophys. Res. Commun. 1999;262:167–173. doi: 10.1006/bbrc.1999.1172. [DOI] [PubMed] [Google Scholar]
- Leven R.M., Nachmias V.T. Cultured megakaryocyteschanges in the cytoskeleton after adenosine diphosphate-induced spreading. J. Cell Biol. 1982;92:313–323. doi: 10.1083/jcb.92.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M.W. Megakaryocyte differentiation events. Semin. Hematology. 1998;35:192–199. [PubMed] [Google Scholar]
- Lukashev M.E., Sheppard D., Pytela R. Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed β1 integrin cytoplasmic domain. J. Biol. Chem. 1994;269:18311–18314. [PubMed] [Google Scholar]
- Miyoshi H., Smith K.A., Mosier D.E., Verma I.M., Torbett B.E. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- Moorman J.P., Luu D., Wickham J., Bobak D.A., Hahn C.S. A balance of signaling by Rho family small GTPases RhoA, Rac1 and Cdc42 coordinates cytoskeletal morphology but not cell survival. Oncogene. 1999;18:47–57. doi: 10.1038/sj.onc.1202262. [DOI] [PubMed] [Google Scholar]
- Mountford J.C., Melford S.K., Bunce C.M., Gibbons J., Watson S.P. Collagen or collagen-related peptide cause [Ca2+]i elevation and increased tyrosine phosphorylation in human megakaryocytes. Thromb Haemost. 1999;82:1153–1159. [PubMed] [Google Scholar]
- Murphy G.J., Leavitt A.D. A model for studying megakaryocyte development and biology. Proc. Natl. Acad. Sci. USA. 1999;96:3065–3070. doi: 10.1073/pnas.96.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias V.T., Golla R. Vinculin in relation to stress fibers in spread platelets. Cell Motil. Cytoskel. 1991;20:190–202. doi: 10.1002/cm.970200303. [DOI] [PubMed] [Google Scholar]
- O'Toole T.E., Katagiri Y., Faull R.J., Peter K., Tamura R., Quaranta V., Loftus J.C., Shattil S.J., Ginsberg M.H. Integrin cytoplasmic domains mediate inside out signaling. J. Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S., Toombs C.F., Hu Y.H., Simon M.I. Defective platelet activation in Gαq-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- Otey C.A., Vasquez G.B., Burridge K., Erickson B.W. Mapping of the α-actinin binding site within the β1 integrin cytoplasmic domain. J. Biol. Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- Pampori N., Hato T., Stupack D.G., Aidoudi S., Cheresh D.A., Nemerow G.R., Shattil S.J. Mechanisms and consequences of affinity modulation of integrin αvβ3 detected with a novel patch-engineered monovalent ligand. J. Biol. Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Liu S., Erle D.J., Ginsberg M.H. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- Qi W., Loh E., Vilaire G., Bennett J.S. Regulation of alphaIIb beta3 function in human B lymphocytes. J. Biol. Chem. 1998;273:15271–15278. doi: 10.1074/jbc.273.24.15271. [DOI] [PubMed] [Google Scholar]
- Ren X.D., Kiosses W.B., Schwartz M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S.E. Phosphoinositide 3-kinase activation and platelet function. Blood. 1996;88:4401–4414. [PubMed] [Google Scholar]
- Riviere C., Subra F., Cohen-Solal K., Cordette-Lagarde V., Letestu R., Auclair C., Vainchenker W., Louache F. Phenotypic and functional evidence for the expression of CXCR4 receptor during megakaryocytopoiesis. Blood. 1999;93:1511–1523. [PubMed] [Google Scholar]
- Rojnuckarin P., Drachman J.G., Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytesrole in endomitosis. Blood. 1999;94:1273–1282. [PubMed] [Google Scholar]
- Roth G.J., Stanford N., Majerus P.W. Acetylation of prostaglandin synthase by aspirin. Proc. Natl. Acad. Sci. USA. 1975;72:3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath R., Gallagher P.J., Pavalko F.M. Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail—activation-dependent regulation of associations with talin and α-actinin. J. Biol. Chem. 1998;273:33588–33594. doi: 10.1074/jbc.273.50.33588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage B., Shattil S.J., Ruggeri Z.M. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin αIIbβ3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J. Biol. Chem. 1992;267:11300–11306. [PubMed] [Google Scholar]
- Schick P.K., Wojenski C.M., He X., Walker J., Marcinkiewicz C., Niewiarowski S. Integrins involved in the adhesion of megakaryocytes to fibronectin and fibrinogen. Blood. 1998;92:2650–2656. [PubMed] [Google Scholar]
- Schoenwaelder S.M., Jackson S.P., Yuan Y., Teasdale M.S., Salem H.H., Mitchell C.A. Tyrosine kinases regulate the cytoskeletal attachment of integrin αIIbβ3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin polymers. J. Biol. Chem. 1994;269:32479–32487. [PubMed] [Google Scholar]
- Shattil S.J., Cunningham M., Hoxie J.A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987;70:307–315. [PubMed] [Google Scholar]
- Shattil S.J., Kashiwagi H., Pampori N. Integrin signalingthe platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- Shivdasani R.A., Rosenblatt M.F., Zucker-Franklin D., Jackson C.W., Hunt P., Saris C.J., Orkin S.H. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- Takada M., Morii N., Kumagai S., Ryo R. The involvement of the rho gene product, a small molecular weight GTP-binding protein, in polyploidization of a human megakaryocytic cell line, CMK. Exp. Hematol. 1996;24:524–530. [PubMed] [Google Scholar]
- Takahashi R., Sekine N., Nakatake T. Influence of monoclonal antiplatelet glycoprotein antibodies on in vitro human megakaryocyte colony formation and proplatelet formation. Blood. 1999;93:1951–1958. [PubMed] [Google Scholar]
- Thomas D.W., Mannon R.B., Mannon P.J., Latour A., Oliver J.A., Hoffman M., Smithies O., Koller B.H., Coffman T.M. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2 . J. Clin. Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Alberts A.S., Sahai E. Regulation of SRF activity by Rho family GTPases. Cold Spring Harbor Symp. Quant. Biol. 1998;63:643–651. doi: 10.1101/sqb.1998.63.643. [DOI] [PubMed] [Google Scholar]
- Tsakiris D.A., Scudder L., Hodivala-Dilke K., Hynes R.O., Coller B.S. Hemostasis in the mouse (Mus musculus)a review. Thromb. Haemost. 1999;81:177–188. [PubMed] [Google Scholar]
- Verfaillie C.M. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92:2609–2612. [PubMed] [Google Scholar]
- Vyas P., Ault K., Jackson C.W., Orkin S.H., Shivdasani R.A. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93:2867–2875. [PubMed] [Google Scholar]
- Wang J.F., Liu Z.Y., Groopman J.E. The α-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–764. [PubMed] [Google Scholar]
- Wilcox D.A., Olsen J.C., Ishizawa L., Griffith M., White G.C., II. Integrin alphaIIb promoter-targeted expression of gene products in megakaryocytes derived from retrovirus-transduced human hematopoietic cells. Proc. Natl. Acad. Sci. USA. 1999;96:9654–9659. doi: 10.1073/pnas.96.17.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.L., Pipia G.G., Datta N.S., Long M.W. Thrombopoietin requires additional megakaryocyte-active cytokines for optimal ex vivo expansion of megakaryocyte precursor cells. Blood. 1998;91:4118–4126. [PubMed] [Google Scholar]
- Yuan Y., Dopheide S.M., Ivanidis C., Salem H.H., Jackson S.P. Calpain regulation of cytoskeletal signaling complexes in von Willebrand factor-stimulated platelets. Distinct roles for glycoprotein Ib-V-IX and glycoprotein IIb-IIIa (integrin alphaIIbbeta3) in von Willebrand factor-induced signal transduction. J. Biol. Chem. 1997;272:21847–21854. doi: 10.1074/jbc.272.35.21847. [DOI] [PubMed] [Google Scholar]
- Zauli G., Bassini A., Vitale M., Gibellini D., Celeghini C., Caramelli E., Pierpaoli S., Guidotti L., Capitani S. Thrombopoietin enhances the αIIbβ3-dependent adhesion of megakaryocytic cells to fibrinogen or fibronectin through PI 3 kinase Blood 89 1997. 883 895a [PubMed] [Google Scholar]
- Zauli G., Vitale M., Falcieri E., Gibellini D., Bassini A., Celeghini C., Columbaro M., Capitani S. In vitro senescence and apoptotic cell death of human megakaryocytes Blood 90 1997. 2234 2243b [PubMed] [Google Scholar]