Abstract
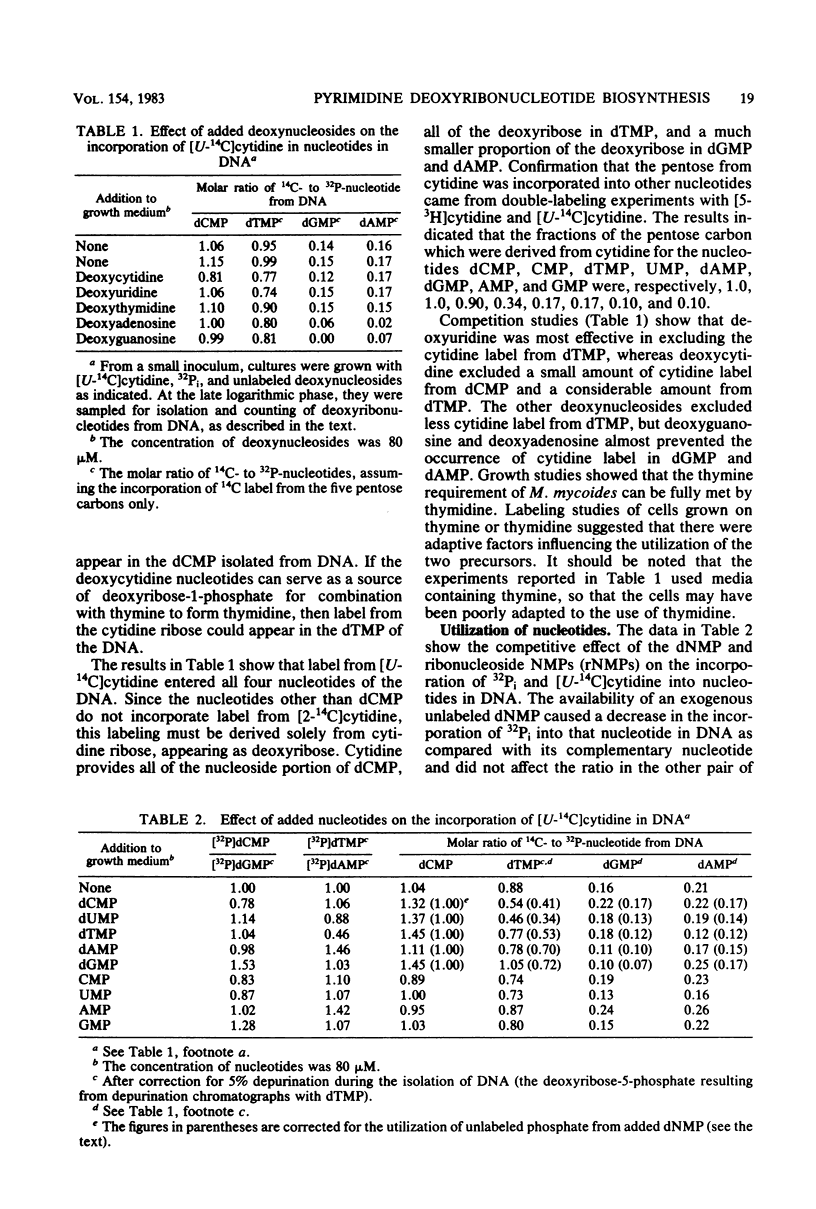
By measuring the specific activity of deoxyribonucleotides isolated from DNA after the incorporation of 14C-labeled precursors with and without competition from other nucleotide precursors, we defined the major pathways of pyrimidine deoxyribonucleotide synthesis in Mycoplasma mycoides subsp. mycoides. Uracil, guanine, and thymine are required for the synthesis of nucleotides. Cytidine competed effectively with uracil to provide all of the deoxycytidine nucleotide, as well as most of the deoxyribose-1-phosphate, for the synthesis of thymidylate from thymine via thymidine phosphorylase. Each of dUMP, dCMP, and dTMP competed with cytidine for incorporation into DNA thymidylate. Appreciable incorporation of exogenous deoxyribonucleoside 5'-monophosphates into DNA without prior dephosphorylation was observed. Dephosphorylation also occurred since the added deoxyribonucleotide provided phosphate for the synthesis of the other nucleotides in DNA in competition with the 32Pi in the growth medium. Hydroxyurea inhibited cell growth and decreased the intracellular level of dATP, consistent with the action of a ribonucleoside diphosphate reductase with regulatory properties similar to those of the Escherichia coli enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnara A. S., Finch L. R. Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal Biochem. 1972 Jan;45(1):24–34. doi: 10.1016/0003-2697(72)90004-8. [DOI] [PubMed] [Google Scholar]
- Elford H. L. Effect of hydroxyurea on ribonucleotide reductase. Biochem Biophys Res Commun. 1968 Oct 10;33(1):129–135. doi: 10.1016/0006-291x(68)90266-0. [DOI] [PubMed] [Google Scholar]
- Kramer G., Wiegers U., Hilz H. mRNA turnover studies applying labeled uridine require an evaluation of specific radioactivities of UTP and RNA-U. Biochem Biophys Res Commun. 1973 Nov 16;55(2):273–281. doi: 10.1016/0006-291x(73)91084-x. [DOI] [PubMed] [Google Scholar]
- Mitchell A., Finch L. R. Enzymes of pyrimidine metabolism in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1979 Mar;137(3):1073–1080. doi: 10.1128/jb.137.3.1073-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A., Finch L. R. Pathways of nucleotide biosynthesis in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1977 Jun;130(3):1047–1054. doi: 10.1128/jb.130.3.1047-1054.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A., Sin I. L., Finch L. R. Enzymes of purine metabolism in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1978 Jun;134(3):706–712. doi: 10.1128/jb.134.3.706-712.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale G. A., Mitchell A., Finch L. R. Formylation of methionyl-transfer ribonucleic acid in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1981 May;146(2):816–818. doi: 10.1128/jb.146.2.816-818.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Deoxycytidine triphosphate deaminase: identification and function in Salmonella typhimurium. J Bacteriol. 1971 Feb;105(2):657–665. doi: 10.1128/jb.105.2.657-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Edlin G., Fuchs J. A., Neuhard J., Thomassen E. Deoxycytidine triphosphate deaminase: characterization of an Escherichia coli mutant deficient in the enzyme. J Bacteriol. 1971 Feb;105(2):666–672. doi: 10.1128/jb.105.2.666-672.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODWELL A. W. Nutrition and metabolism of Mycoplasma mycoides var. mycoides. Ann N Y Acad Sci. 1960 Jan 15;79:499–507. doi: 10.1111/j.1749-6632.1960.tb42716.x. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- Reynolds E. C., Finch L. R. Estimation of [32P]deoxyribonucleoside triphosphates in cell extracts using periodate treatment. Anal Biochem. 1977 Oct;82(2):591–595. doi: 10.1016/0003-2697(77)90200-7. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell A. W. A defined medium for Mycoplasma strain Y. J Gen Microbiol. 1969 Sep;58(1):39–47. doi: 10.1099/00221287-58-1-39. [DOI] [PubMed] [Google Scholar]


