Abstract
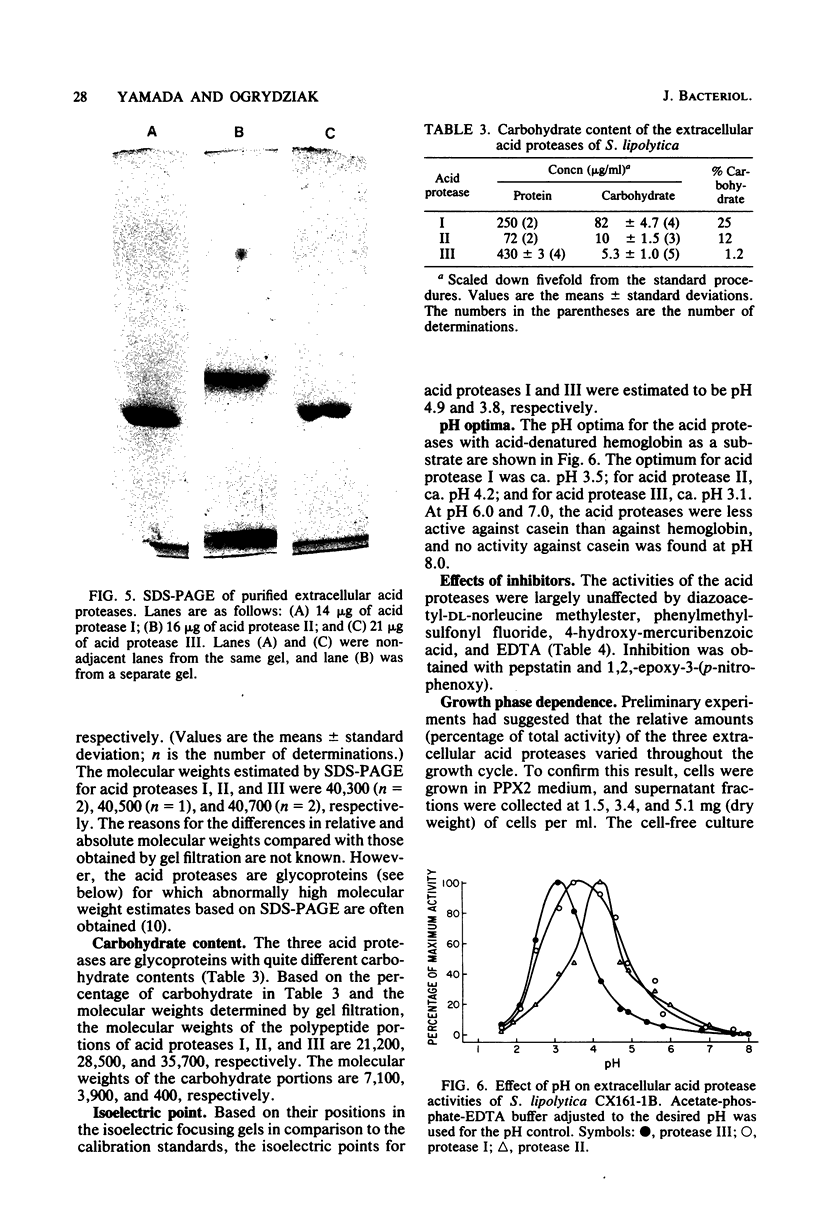
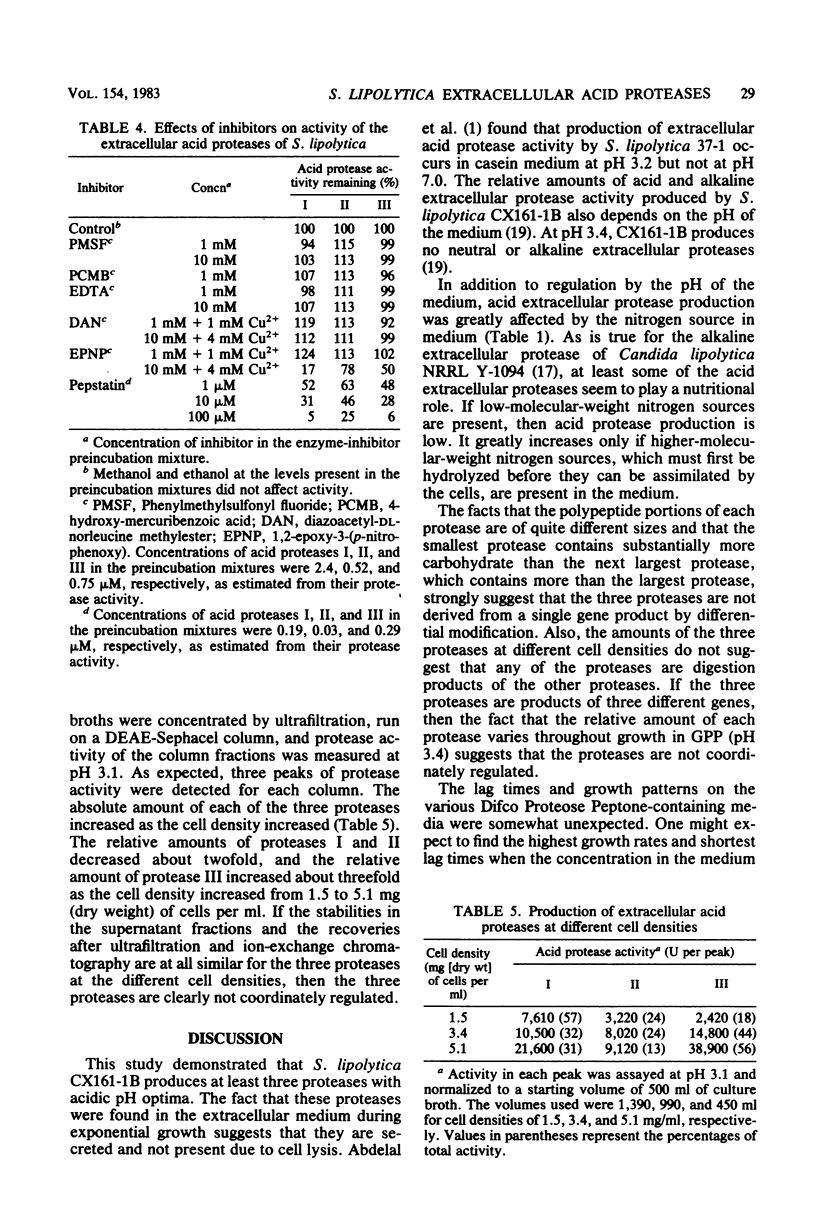
Saccharomycopsis lipolytica CX161-1B produced at least three extracellular acid proteases during exponential growth in medium containing glycerol, Difco Proteose Peptone, and mineral salts at pH 3.4 (Difco Laboratories, Detroit, Mich.). Little extracellular acid protease activity was produced with glutamic acid as the sole nitrogen source, somewhat higher levels were obtained with peptone, and much higher levels were obtained with Difco Proteose Peptone. The relative amounts of the three proteases varied during growth on Difco Proteose Peptone, which suggested that the proteases were not coordinately regulated. The proteases were purified to near homogeneity (as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) by use of ultrafiltration, gel filtration, and DEAE-Sephacel and hydroxylapatite chromatography. Protease I had a molecular weight near 28,000, an isoelectric point of pH 4.9, and a pH optimum of 3.5. Protease II had a molecular weight near 32,000 and a pH optimum of 4.2. Protease III had a molecular weight near 36,000, an isoelectric point of 3.8, and a pH optimum of 3.1. All three proteases were glycoproteins; proteases I, II, and III contained 25, 12, and 1.2% carbohydrate, respectively. The proteases were inhibited by pepstatin and 1,2-epoxy-3-(4-nitrophenoxy) propane and were largely insensitive to diazoacetyl-DL-norleucine methylester and to compounds which inhibit the serine, sulfhydryl, or metallo-proteases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Kennedy E. H., Ahearn D. G. Purification and characterization of a neutral protease from Saccharomycopsis lipolytica. J Bacteriol. 1977 Jun;130(3):1125–1129. doi: 10.1128/jb.130.3.1125-1129.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearn D. G., Meyers S. P., Nichols R. A. Extracellular proteinases of yeasts and yeastlike fungi. Appl Microbiol. 1968 Sep;16(9):1370–1374. doi: 10.1128/am.16.9.1370-1374.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Maddox I. S., Hough J. S. Proteolytic enzymes of Saccharomyces carlsbergensis. Biochem J. 1970 May;117(5):843–852. doi: 10.1042/bj1170843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meussdoerffer F., Tortora P., Holzer H. Purification and properties of proteinase A from yeast. J Biol Chem. 1980 Dec 25;255(24):12087–12093. [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Ogrydziak D. M., Demain A. L., Tannenbaum S. R. Regulation of extracellular protease production in Candida lipolytica. Biochim Biophys Acta. 1977 Apr 27;497(2):525–538. doi: 10.1016/0304-4165(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Ogrydziak D. M., Mortimer R. K. Genetics of Extracellular Protease Production in SACCHAROMYCOPSIS LIPOLYTICA. Genetics. 1977 Dec;87(4):621–632. doi: 10.1093/genetics/87.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrydziak D. M., Scharf S. J. Alkaline extracellular protease produced by Saccharomycopsis lipolytica CX161-1B. J Gen Microbiol. 1982 Jun;128(6):1225–1234. doi: 10.1099/00221287-128-6-1225. [DOI] [PubMed] [Google Scholar]
- Remold H., Fasold H., Staib F. Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta. 1968 Oct 8;167(2):399–406. doi: 10.1016/0005-2744(68)90219-2. [DOI] [PubMed] [Google Scholar]
- Tang J. Evolution in the structure and function of carboxyl proteases. Mol Cell Biochem. 1979 Jul 31;26(2):93–109. doi: 10.1007/BF00232887. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]