Abstract
A therapeutic effect of vitamin A supplementation on the course of photoreceptor degeneration, previously reported for patients with retinitis pigmentosa, was tested in two transgenic mouse models of this disease, each carrying a dominant rhodopsin mutation. The threonine-17 → methionine (T17M) mutation is a class II rhodopsin mutation, characterized by a thermal instability/folding defect and minimal regeneration with the chromophore. The proline-347 → serine (P347S) mutation belongs to class I, comprised of a smaller number of mutations that exhibit no recognized biochemical abnormality in vitro. In the present study, each of the two mouse models was fed a diet containing 2.5 mg of vitamin A palmitate (control) or 102.5 mg of vitamin A palmitate (high vitamin A) per kilogram of diet. Dark-adapted, full-field electroretinograms showed that the high vitamin A diet significantly reduced the rate of decline of a-wave and b-wave amplitudes in the T17M mice but had no significant effect on the decline of electroretinogram amplitude in the P347S mice. Correspondingly, histologic evaluation revealed that the treatment was associated with significantly longer photoreceptor inner and outer segments and a thicker outer nuclear layer in the T17M mice but had no effect on photoreceptor morphology in the P347S mice. In a separate series of experiments, the instability defect of the T17M mutant opsin expressed in vitro was partially alleviated by inclusion of 11-cis-retinal in the culture media. These results show that vitamin A supplementation slows the rate of photoreceptor degeneration caused by a class II rhodopsin mutation. Vitamin A supplementation may confer therapeutic benefit by stabilizing mutant opsins through increased availability of the chromophore.
Retinitis pigmentosa is the name applied to a group of hereditary photoreceptor degenerations. This disease affects about 1.5 million people worldwide (1). A randomized, controlled, double-masked trial concluded that oral vitamin A supplementation slowed, on average, the rate of retinal degeneration in adult patients with the common forms of retinitis pigmentosa (2). The conclusion was based on measuring the rate of electroretinogram (ERG) amplitude decline. In the present study, we monitored the course of photoreceptor degeneration in two murine models of retinitis pigmentosa fed a diet containing either a normal or a higher amount of vitamin A. One objective of this study was to assess the effect of vitamin A supplementation not only on the rate of ERG amplitude decline but also on the histologic appearance of the photoreceptors.
As murine models of retinitis pigmentosa, we chose transgenic mice with the rhodopsin threonine-17 → methionine (T17M) mutation and mice with the rhodopsin proline-347 → serine (P347S) mutation. Over 70 rhodopsin mutations have been documented to cause retinitis pigmentosa (3). On the basis of studies in cultured embryonic kidney cell lines, dominant rhodopsin mutations have been divided into class I and class II (4–6). The T17M mutation was selected in the present study as an example of class II mutant opsins, which are defective in thermal stability/folding, lack full regenerability with the chromophore 11-cis-retinal, and fail to reach the plasma membrane. The P347S mutation was chosen to be representative of class I mutant opsins, which are indistinguishable from wild-type (wt) opsin in all in vitro assays, including formation of photopigment and efficient transport to the plasma membrane. In vivo, the P347S mutation appears to cause aberrant transport of rhodopsin, possibly by disrupting a signal sequence that normally directs the vectorial transport of rhodopsin to the outer segments (7–9).
The selection of a class I and class II mutant for comparison underscores a second objective of the present study, i.e., to test the hypothesis that class II mutants are more likely to respond to vitamin A supplementation than are class I mutants. One putative mechanism through which vitamin A supplementation may slow photoreceptor degeneration is by increasing the availability of the chromophore, 11-cis-retinal. Since class II mutants have decreased stability and since chromophore binding increases the thermal stability of wt opsin (10), higher levels of chromophore might lead to a higher proportion of chromophore-bound, and thus stabilized, mutant rhodopsin. In contrast, photoreceptors expressing a class I mutation may benefit little or not at all because the mutant rhodopsin retains wt-like thermal stability under normal supply of the chromophore.
MATERIALS AND METHODS
Transgenic Mice.
The T17M mice were generated by using a 17-kb human rhodopsin gene fragment with approximately 4.8 kb of 5′ and 6.2 kb of 3′ flanking sequences, in which a single base substitution was introduced to change codon 17 from threonine to methionine. The line used in this study, designated “V,” shows complete photoreceptor degeneration over 8 mo. The P347S mice were from the “A” line that has been previously described (8). By 4 mo of age, P347S A line mice are near the end stage of degeneration, with 1–2 rows of photoreceptor nuclei remaining.
Mice were reared under 65–140 lux white fluorescent cyclic (8:00 a.m. light to 8:00 p.m. dark) light. When the mice were 20–22 days of age, we recorded baseline ERGs and randomized mice with a given mutation to a control or a high vitamin A diet (Table 1), so that the two dietary groups included comparable numbers by litter and gender and were approximately matched for mean weight and baseline ERG amplitude. ERGs were recorded at 2 and 4 mo following randomization for the T17M mice and at 1 and 2 mo following randomization for the P347S mice, to allow for approximately equal ERG amplitude declines for each murine model fed the control diet (see Results). After the second follow-up ERG, mice were sacrificed for analysis of plasma and liver vitamin A levels and for light and electron microscopic examination of the retina. Thirty transgenic mice heterozygous for the human T17M mutation and 25 transgenic mice heterozygous for the human P347S mutation were followed over the course of this study. The ERGs and histologic evaluations were performed by examiners masked to the treatment group assignment. A pilot study in normal mice (n = 12) showed that the high vitamin A diet given for 4 mo did not cause liver toxicity, as monitored by serum liver function profile (Anilytics, Gaithersburg, MD).
Table 1.
Contents of the study diets
| Ingredient | Content*
|
|
|---|---|---|
| Control | High vitamin A | |
| Casein | 100 | 100 |
| Soy protein isolate | 100 | 100 |
| Sucrose | 268 | 268 |
| Cornstarch | 271 | 271 |
| Cholesterol-reduced-milkfat | 26 | 26 |
| Canola oil | 16 | 16 |
| Corn oil | 8 | 8 |
| Cellulose | 100 | 100 |
| Wheat bran | 50 | 50 |
| Mineral mix | 46 | 46 |
| Vitamin mix† | 12 | 12 |
| Choline chloride | 3 | 3 |
| Vitamin A palmitate (mg/kg) | — | 100 |
Units are in g/kg unless otherwise noted.
Control diet includes 2.5 mg of vitamin A per kg of diet.
ERGs.
Following overnight dark adaptation, mice were anesthetized and their pupils were dilated. Full-field ERGs were elicited with 10-μsec flashes of white light (1.37 × 105 cd/m2) presented at intervals of 1 min in darkness; this condition elicits from normal mice an a-wave, generated by photoreceptors, and a b-wave, generated by secondary neurons. Responses were monitored with a chlorided silver wire loop placed on the cornea, which had been anesthetized with 0.5% proparacaine hydrochloride; a saline cotton wick was placed in the mouth as reference electrode and a subdermal electrode was placed in the neck as ground. Responses were differentially amplified at a gain of 5,000 (−3 dB at 2 Hz and 300 Hz), digitized at a sampling rate of 1302 Hz and summed (n = 2–4) with custom software. a-wave amplitudes were quantified from baseline to the peak of the cornea-negative deflection; b-wave amplitudes were quantified from the latter to the major cornea-positive peak. Under these test conditions, responses <5 μV could not be distinguished from noise. Baseline amplitudes ranged from 48 to 298 μV (a-wave) and 448 to 1854 μV (b-wave) for the T17M mice and from 40 to 213 μV (a-wave) and 192 to 831 μV (b-wave) for the P347 mice. Normal lower limits are 188 μV for the a-wave and 580 μV for the b-wave, based on 45 wt pigmented mice.
Plasma and Liver Vitamin A.
Plasma and liver samples were collected and analyzed for retinol (11) and retinyl palmitate (12), respectively, by reverse-phase HPLC. Standards for all-trans-retinol and all-trans-retinyl acetate were obtained from Hoffman–La Roche. The crystalline retinyl palmitate standard was purchased from Sigma.
Light and Electron Microscopy.
Eyes were fixed and embedded in Epon (Tousimis, Rockville, MD). For light microscopy, 1-μm-thick sections were cut along the horizontal meridian at a level through the optic nerve head. Quantitative measurements were made along the horizontal meridian in three 100-μm lengths (each separated by approximately 300 μm) on each side of the optic nerve head and beginning approximately 300 μm from the optic nerve head. For electron microscopy, 0.1-μm sections were stained with uranyl acetate and lead citrate and were viewed on a JEOL 100-C electron microscope.
Statistical Analyses.
The “extreme studentized deviate” statistic was calculated for each distribution of data to identify statistical outliers (13). If extreme (P < 0.01) outliers were detected, they were deleted; if borderline (0.05 ≥ P ≥ 0.01) outliers were detected, analyses were performed both before and after their exclusion for comparison. The effects of diet on plasma retinol and liver retinyl palmitate were assessed by full-factorial analyses of variance to determine whether there were significant effects of diet and whether these effects depended on the murine model. Assuming an exponential rate of decline, as seen in patients with retinitis pigmentosa, we converted ERG amplitudes to natural logarithms (2) and then to the daily log change (Δ log μV/day) by linear regression. The effects of diet on the daily changes in log a-wave and b-wave amplitudes and on the thickness of the layer of inner/outer segments and of the outer nuclear layer were assessed by analysis of covariance controlling for significant differences because of baseline factors unrelated to the diet (i.e., litter, weight, and/or ERG amplitude). In addition, the thickness of the outer nuclear layer was regressed on ERG a-wave or b-wave amplitude from data obtained at the last recording session to determine whether the ERG was an indicator of photoreceptor cell number, irrespective of diet. Analyses were performed with jmp, version 3.2 (SAS Institute, Cary, NC), on a Macintosh computer.
In Vitro Expression of the T17M and P347S Mutant Opsins.
To ask whether binding to 11-cis-retinal stabilizes the T17M mutant opsin, we studied the mutant opsin expressed in vitro in the presence of all-trans-retinal (control) or 11-cis-retinal. The stable mutant P347S was also included in these experiments for comparison and control. The mutant expression plasmids driven by the cytomegalovirus early promoter/enhancer (4, 5) were gifts from Ching-Hwa Sung (Cornell University, Ithaca, NY). The plasmid DNAs were transfected into 293 cells by using the LipofectAmine Plus reagent (Life Technologies, Gaithersburg, MD). 11-cis-Retinal was provided by Rosalie Crouch (Medical University of South Carolina, Charleston, SC). All-trans- and 9-cis-retinals were from Sigma. Liposomes were used as a carrier for delivery of retinals to cells and were prepared according to Jones et al. (14), except that phosphatidylcholine was used at 5 mg/ml. All experiments involving retinals were conducted under dim red light or in the dark. Bulk concentration of 11-cis-retinal in liposome suspension was estimated by OD at 380 nm after dissolving in ethanol (15) and was adjusted to 5 mM. All-trans- and 9-cis-retinal were prepared at the same concentration. To express mutant opsins for spectral measurement, 11-cis- or all-trans-retinal was added to cells at 125 μl per 150-mm dish at 3 h after transfection and a second aliquot was added 12 h later. A second control received liposome only. Cells were harvested 48 h after transfection by using a procedure adapted from a published method (16); one notable difference was that the opsins were regenerated with 11-cis-retinal before solubilization because the unstable mutant opsin might not survive detergent solubilization. After regeneration, the membranes were solubilized in 1% dodecyl maltoside. The solution was scanned between 250 and 650 nm in a model 940 Uvicon spectrophotometer (Kontron Instruments, Milan, Italy) before and after photobleaching. Aliquots were also analyzed for total protein by the Bradford assay and for opsin expression by immunoblotting. Poly(A) RNA was isolated from transfected cells cultured in the presence of 11-cis- or all-trans-retinal and assayed for opsin mRNA levels by Northern blotting. For immunocytochemistry, cells were plated on chamber slides (Nunc) and cultured in the presence of all-trans-, 11-cis-, or 9-cis-retinal. 9-cis-Retinal has similar properties to 11-cis-retinal in generating a photopigment with opsin in vitro and was included in these experiments for comparison. Cells were fixed in ice-cold methanol for 5 min at 24 h after transfection and stained with anti-rhodopsin antibodies rho-1D4 (C-terminal specific) or rho-4D2 (N-terminal specific) (17), followed by Cy3-conjugated goat anti-mouse IgG. The P347S mutant was recognized by 4D2 but not by 1D4, presumably because of the proline 347 substitution near the C terminus. 1D4 gave a much stronger signal on the T17M mutant than did 4D2.
RESULTS
Plasma and Liver Vitamin A.
Plasma retinol was significantly higher (P = 0.017) for the vitamin A-supplemented mice (mean, 31 μg/dl) than for the mice on the control diet (mean, 19 μg/dl). The percentage increase was higher for the P347S mice than for the T17M mice, although the difference was not significant (P = 0.087). Liver retinyl palmitate levels averaged about 15 times higher for the supplemented mice (mean, 6054 μg/g) than for the mice on the control diet (mean, 442 μg/g) (P < 0.001). Again, the increase was not significantly different for the two murine models (P = 0.36). At the final follow-up, mice fed the high vitamin A diet had a healthy appearance and a mean weight (23.1 g) that was 98% of that of the mice that were fed the control diet (23.4 g).
ERGs.
The ERGs of the T17M mice were about twice the amplitude at baseline and declined about half as fast over time, compared with the ERGs of the P347S mice. Vitamin A supplementation significantly slowed the mean daily rates of decline for both a-wave and b-wave amplitudes in the T17M mice (Table 2 and Fig. 1). Vitamin A supplementation reduced the mean rate of decline in a-wave amplitude by 45% (P = 0.005) and the mean rate of decline in b-wave amplitude by 42% (P = 0.034). A significant effect (P = 0.048) was found for the a-wave even after excluding the values for two mice fed the control diet with fast rates of decline that were borderline outliers (Fig. 1, arrows).
Table 2.
Daily decline in ERG amplitude by diet
| ERG | T17M mice
|
P347S mice
|
||||
|---|---|---|---|---|---|---|
| Control (n = 15) | Vitamin A (n = 15) | P value* | Control (n = 13) | Vitamin A (n = 12) | P value† | |
| a-wave | 0.013 ± 0.001 (1.32%) | 0.007 ± 0.001 (0.72%) | 0.005 | 0.027 ± 0.002 (2.66%) | 0.025 ± 0.002 (2.42%) | 0.35 |
| b-wave | 0.010 ± 0.001 (1.00%) | 0.006 ± 0.001 (0.58%) | 0.034 | 0.019 ± 0.002 (1.93%) | 0.018 ± 0.002 (1.82%) | 0.67 |
Data are presented as mean daily natural log ERG amplitude decline ± SEM, adjusted for significant covariates. Numbers in parentheses give the adjusted mean daily percentage decline in amplitude.
Controlling for litter.
Controlling for baseline amplitude.
Figure 1.
Scatter diagrams and mean ± 1 standard error values for the daily percent change in a-wave and b-wave amplitudes by diet for the T17M mice. The two values in the control diet data designated by arrows were borderline outliers (see text).
There was no significant benefit of vitamin A supplementation on the rate of ERG amplitude decline for the P347S mice at the 1-mo follow-up (not shown) or at the 2-mo followup, after controlling for baseline amplitude (Table 2 and Fig. 2).
Figure 2.
Scatter diagrams and mean ± 1 standard error values for the daily percent change in a-wave and b-wave amplitudes by diet for the P347S mice.
Retinal Morphology.
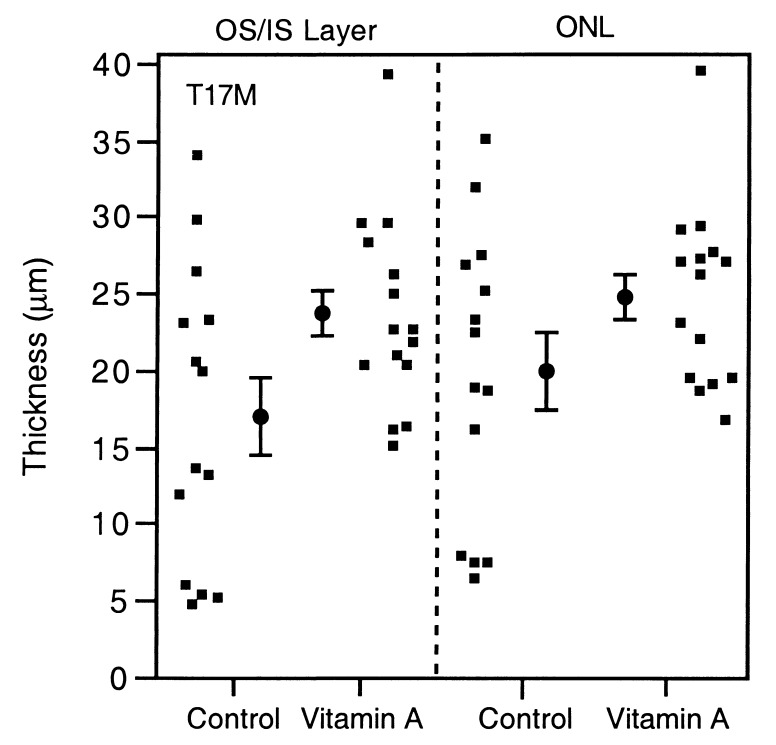
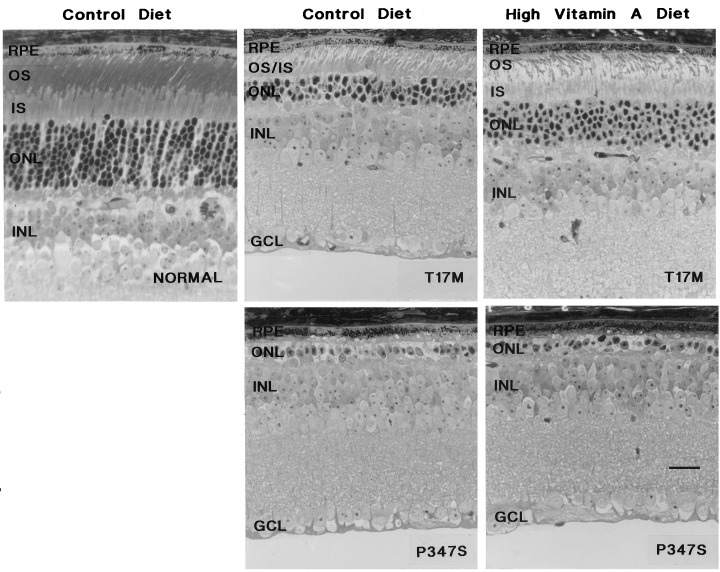
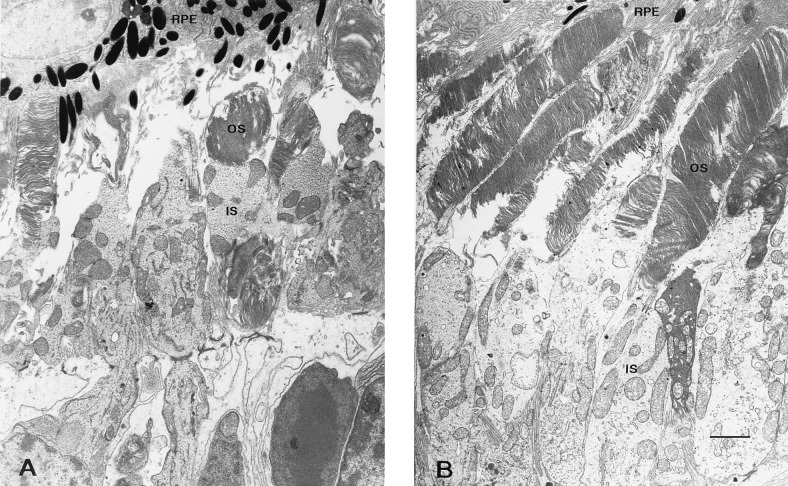
Fig. 3 and Table 3 show that the T17M mice on the high vitamin A diet had a 41% thicker layer of inner and outer segments and a 24% thicker outer nuclear layer than those on the control diet (P = 0.009 and P = 0.050, respectively). Light microscopy (Fig. 4) shows that the outer segments in mice fed the control diet were not only shorter but also appeared less well organized. In contrast, the P347S mice, whether fed the high vitamin A or control diet, had predominantly one row of photoreceptor nuclei without recognizable outer segments. The findings by electron microscopy (Fig. 5) were consistent with those by light microscopy. The T17M mice fed the high vitamin A diet had more well-packed discs and longer outer segments than did mice fed the control diet.
Figure 3.
Scatter diagrams and mean ± 1 standard error values for the thickness of the layer of inner/outer segments and the outer nuclear layer by diet in the T17M mice.
Table 3.
Retinal histology of T17M mice by diet
| Retinal layer | Thickness,
μm ± SEM
|
P value | |
|---|---|---|---|
| Control (n = 14) | Vitamin A (n = 15) | ||
| Inner/outer segments | 17.0 ± 1.6 | 23.9 ± 1.6 | 0.009* |
| Outer nuclear | 20.3 ± 1.6 | 25.1 ± 1.5 | 0.050 |
Controlling for baseline weight and litter.
Figure 4.
Light micrographs of retinas from treated and control mice carrying either transgene. The genotypes and diets are labeled. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer containing photoreceptor cell nuclei; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium. (Bar = 20 μm.)
Figure 5.
Electron micrographs of retinas from T17M mice fed either the control (A) or the high vitamin A diet (B). For labeling, see Fig. 4 legend. (Bar = 2 μm.)
Correlation of Outer Nuclear Layer Thickness and ERG Amplitude.
The amplitudes of the a-wave and b-wave were good indicators of the thickness of the outer nuclear layer (r = 0.85, P < 0.001 and r = 0.81, P < 0.001, respectively) based on data from both murine models, irrespective of diet. The thickness increased by about 1 μm for each 4 μV increase in amplitude.
Expression of Mutant Opsins in Vitro in the Presence of 11-cis-Retinal.
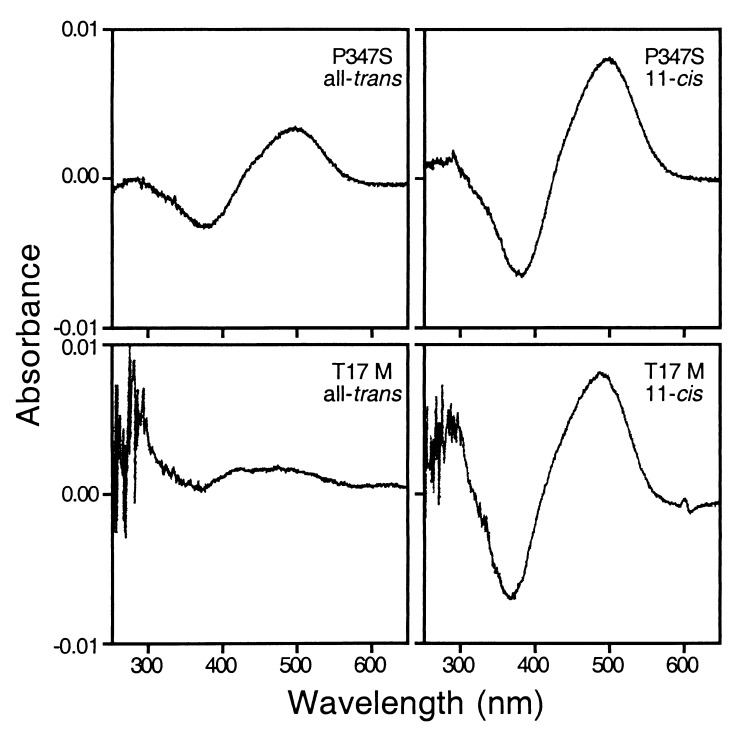
When transiently transfected 293 cells were used as host and in the presence of all-trans-retinal during culture, about 2 μg of P347S rhodopsin could be produced after regeneration from one 150-mm dish of confluent cells (containing about 2 × 107 cells); approximately twice the amount of rhodopsin was produced when 11-cis-retinal was present during the culture (Fig. 6), presumably because of the stabilizing effect of chromophore binding. Under identical culture conditions and in the presence of all-trans-retinal or without any retinal, little if any T17M rhodopsin could be detected; however, in the presence of 11-cis-retinal there was at least a 10-fold increase in the yield of T17M rhodopsin, representing a much greater increase in yield than for the P347S rhodopsin (Fig. 6). The increased yield of T17M rhodopsin could not be attributed to an increase in the mRNA level, as indicated by Northern blotting (not shown). On immunoblots, the T17M mutant expressed in the presence of 11-cis-retinal had a much more prominent band, corresponding to a mature opsin that had exited the endoplasmic reticulum (4) but had a similar amount of immature opsin and aggregates (not shown).
Figure 6.
Photobleaching difference spectra of mutant opsins expressed in 293 cells. Genotypes of each mutant and the retinals present during cell culture are indicated. Cell culture was scaled up 3-fold for the T17M mutant (using three 150-mm dishes) relative to the P347S mutant because the T17M rhodopsin was produced at a lower level.
Under either culture condition, the P347S mutant opsin exhibited a plasma membrane localization (Fig. 7), as has been described previously (5). The T17M mutant, however, displayed a pattern consistent with endoplasmic reticulum accumulation in the absence of 11-cis-retinal (4) (Fig. 7). Addition of 11-cis-retinal to the T17M culture changed the localization pattern so that many cells exhibited a pattern of plasma membrane localization resembling that of the P347S mutant (Fig. 7). 9-cis-Retinal was similarly effective in causing this change of staining pattern (not shown).
Figure 7.
Localization of P347S (stained with rho-4D2) and T17M (stained with rho-1D4) mutants in 293 cells by immunofluorescence. The genotypes and the retinals present during cell culture are indicated. Under phase contrast, cell morphology in all panels was indistinguishable.
DISCUSSION
This study shows that vitamin A supplementation slowed the course of photoreceptor degeneration in one of two murine models of retinitis pigmentosa. The beneficial effect of vitamin A was observed with respect to preserving both the ERG amplitude and retinal morphology. Furthermore, the amplitude of the ERG correlated with histologic evidence of photoreceptor survival. These results are consistent with an earlier finding that vitamin A supplementation slows, on average, the course of photoreceptor degeneration in patients with retinitis pigmentosa, as monitored by the ERG (2).
The beneficial effect of vitamin A supplementation on the T17M mice was anticipated from the nature of its biochemical defect and from our assumption that increased vitamin A intake ultimately leads to increased supply of 11-cis-retinal in the retina. The mechanism by which class II rhodopsin mutants cause photoreceptor degeneration is not yet fully understood but is presumably related to the instability defect seen in vitro. If binding to the chromophore confers a stabilizing effect similar to that seen in wt opsin, then increased availability of the chromophore may drive the balance toward a higher proportion of the chromophore-bound form, thus stabilizing the mutant opsins. The present study shows that the instability defect of the T17M mutant opsin can indeed be alleviated to a large extent through chromophore binding.
The lack of effect on the P347S mice is also consistent with the known biochemical defect in this mutant. Like all members of class I, the P347S mutant opsin does not exhibit decreased thermal stability. Therefore, there is a priori no theoretical basis to anticipate that an increase in chromophore supply might alleviate the pathogenic effect caused by the P347S mutant. We note here that the P347S mice had more advanced disease at baseline (when they were randomized to high vitamin A) and a faster rate of degeneration thereafter than the T17M mice. The possibility cannot be excluded at this time that the rate of degeneration impacts responsiveness to vitamin A supplementation and thus masks a treatment effect on the P347S mutation. Future experiments using a line of P347S mice or other class I mutant mice with a rate of degeneration more comparable to that of the T17M mice may help to address this possibility.
In the retina, a portion of all-trans-retinal released from light-activated rhodopsin is oxidized to retinoic acid by retinaldehyde dehydrogenase (18). Thus, increased availability of the chromophore may cause a corresponding increase in retinoic acid during light exposure. Retinoic acid plays a critical role during photoreceptor development (19) and regulates the expression of certain phototransduction-related genes in adult retinas (20). Interestingly, the growth factor midkine, encoded by a retinoic acid responsive gene, has been shown to promote photoreceptor survival (21). These data suggest a potential role for retinoic acid as a mediator for the preservation of photoreceptor cells in T17M rhodopsin transgenic mice observed in this study. However, the fact that P347S rhodopsin transgenic mice did not respond to vitamin A treatment and a report by others that administration of retinoic acid in vivo accelerated photoreceptor degeneration in proline-23→histidine rhodopsin transgenic mice (22) are not consistent with retinoic acid acting as the mediator for survival.
The present study raises the question whether vitamin A supplementation will help preserve photoreceptor function in all patients with retinitis pigmentosa. The conclusions of the treatment study in patients with retinitis pigmentosa were based on group averages irrespective of genotypes; the sample size with known mutations was too small to assess the benefit of vitamin A on subgroups of patients with different genotypes (2). Our animal study raises the possibility that vitamin A helps to preserve rod photoreceptors in the majority of patients with rhodopsin mutations (i.e., class II) but may confer little such benefit to patients with class I mutations. Study of additional murine models with class I and class II rhodopsin mutations as well as non-rhodopsin mutations will help establish to what extent the observations in the T17M and P347S mice are representative of rhodopsin mutations and whether vitamin A supplementation confers comparable benefit to individuals with rhodopsin and non-rhodopsin mutations.
Acknowledgments
We thank Dr. Ching-Hwa Sung for the P347S and T17M expression plasmids, Dr. Rosalie Crouch for 11-cis-retinal, and Dr. Robert Molday for the anti-rhodopsin antibodies. This work was supported by grants from the National Eye Institute (EY10309 and EY00169) and the Foundation Fighting Blindness, and by a career development award from Research to Prevent Blindness (T.L.).
ABBREVIATIONS
- ERG
electroretinogram
- T17M
threonine-17 → methionine
- P347S
proline-347 → serine
- wt
wild-type
References
- 1. Berson E L. Invest Ophthalmol Visual Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 2.Berson E L, Rosner B, Sandberg M A, Hayes K C, Nicholson B W, Weigel-DiFranco C, Willett W. Arch Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 3.Daiger S, Sullivan L, Rodriguez J. Behav Brain Sci. 1995;18:452–467. [Google Scholar]
- 4.Sung C H, Schneider B G, Agarwal N, Papermaster D S, Nathans J. Proc Natl Acad Sci USA. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung C H, Davenport C M, Nathans J. J Biol Chem. 1993;268:26645–26649. [PubMed] [Google Scholar]
- 6.Kaushal S, Khorana H G. Biochemistry. 1994;33:6121–6128. doi: 10.1021/bi00186a011. [DOI] [PubMed] [Google Scholar]
- 7.Sung C H, Makino C, Baylor D, Nathans J. J Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Snyder W K, Olsson J E, Dryja T P. Proc Natl Acad Sci USA. 1996;93:14176–14181. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deretic D, Puleo-Scheppke B, Trippe C. J Biol Chem. 1996;271:2279–2286. doi: 10.1074/jbc.271.4.2279. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard R. J Gen Physiol. 1958;42:259–280. doi: 10.1085/jgp.42.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieri J G, Tolliver T J, Catignani G L. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 12.Broich C R, Gerber L E, Erdman J W., Jr Lipids. 1983;18:253–258. doi: 10.1007/BF02534557. [DOI] [PubMed] [Google Scholar]
- 13.Rosner B. Fundamentals of Biostatistics. Boston: Duxbury Press; 1994. [Google Scholar]
- 14.Jones G J, Crouch R K, Wiggert B, Cornwall M C, Chader G J. Proc Natl Acad Sci USA. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlman J I, Nodes B R, Pepperberg D R. J Gen Physiol. 1982;80:885–913. doi: 10.1085/jgp.80.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathans J. Biochemistry. 1990;29:937–942. doi: 10.1021/bi00456a013. [DOI] [PubMed] [Google Scholar]
- 17.Hodges R, Heaton R, Parker J, Molday L, Molday R. J Biol Chem. 1988;263:11768–11775. [PubMed] [Google Scholar]
- 18.McCaffery P, Mey J, Drager U C. Proc Natl Acad Sci USA. 1996;93:12570–12574. doi: 10.1073/pnas.93.22.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyatt G A, Dowling J E. Invest Ophthalmol Visual Sci. 1997;38:1471–1475. [PubMed] [Google Scholar]
- 20.Wagner E, McCaffery P, Mey J, Farhangfar F, Applebury M L, Drager U C. FASEB J. 1997;11:271–275. doi: 10.1096/fasebj.11.4.9068616. [DOI] [PubMed] [Google Scholar]
- 21.Unoki K, Ohba N, Arimura H, Muramatsu H, Muramatsu T. Invest Ophthalmol Visual Sci. 1994;35:4063–4068. [PubMed] [Google Scholar]
- 22.Birnbach C D, Reh T A. Invest Ophthalmol Visual Sci. 1997;38:S311. [Google Scholar]