Abstract
Short seizure episodes are associated with remodeling of neuronal connections. One region where such reorganization occurs is the hippocampus, and in particular, the mossy fiber pathway. Using genetic and pharmacological approaches, we show here a critical role in vivo for tissue plasminogen activator (tPA), an extracellular protease that converts plasminogen to plasmin, to induce mossy fiber sprouting. We identify DSD-1-PG/phosphacan, an extracellular matrix component associated with neurite reorganization, as a physiological target of plasmin. Mice lacking tPA displayed decreased mossy fiber outgrowth and an aberrant band at the border of the supragranular region of the dentate gyrus that coincides with the deposition of unprocessed DSD-1-PG/phosphacan and excessive Timm-positive, mossy fiber termini. Plasminogen-deficient mice also exhibit the laminar band and DSD- 1-PG/phosphacan deposition, but mossy fiber outgrowth through the supragranular region is normal. These results demonstrate that tPA functions acutely, both through and independently of plasmin, to mediate mossy fiber reorganization.
Keywords: neurite, dentate, nonproteolytic, kainic acid, seizure
Introduction
Organization of neuronal pathways occurs during late prenatal and early postnatal development, and reorganization takes place continuously as neurons undergo stimulation. Short seizure episodes induce the development of new synapses and the reorganization of existing synapses along the mossy fiber pathway in the hippocampus. Such induced sprouting of mossy fibers (axons of dentate granule cells) can result in the formation of aberrant structures in the CA3 infrapyramidal layer and the dentate supragranular layer (Ben-Ari and Represa 1990). Synaptic organization and reorganization require remodeling of extracellular matrix (ECM) components through the action of extracellular proteases (Werb 1997). Previously, we described a role for the extracellular proteases, tissue plasminogen activator (tPA) and plasmin(ogen) in mediating neuronal cell death after excitotoxic injury to the hippocampus (Tsirka et al. 1995, Tsirka et al. 1996, Tsirka et al. 1997). tPA is made by neurons and microglia, whereas plasmin(ogen) is expressed by neurons (Tsirka et al. 1997). In addition to its role after excitotoxic events, tPA expression is upregulated in the cerebellum during normal neuronal stimulation and changes in plasticity (Seeds et al. 1995). Furthermore, in hippocampal slices from tPA-deficient (tPA−/−) mice (Carmeliet et al. 1994), or wild-type mice in which tPA activity is inhibited, deficits in the late phase of long-term potentiation (LTP) are observed (Frey et al. 1996; Huang et al. 1996; Baranes et al. 1998).
The mechanism(s) through which tPA mediates these changes is not understood, although the prevalent hypothesis has been that generation of plasmin by tPA leads to the degradation of ECM and cell surface components (Carroll et al. 1994; Werb 1997). Supporting this model, plasmin-mediated degradation of laminin has been shown to be an early step in the pathological neurodegeneration process triggered by excitotoxins (Chen and Strickland 1997).
tPA protein and activity in the mouse hippocampus localize along the CA3 pyramidal layer and the dentate gyrus (DG; Sappino et al. 1993). This expression pattern coincides with the mossy fiber pathway and the region in which sprouting and reorganization of mossy fibers occur (Ben-Ari and Represa 1990; Amaral and Witter 1995). There are several genes known to be involved in the formation and structural reorganization of mossy fibers. Neural cell adhesion molecule (NCAM), a membrane glycoprotein expressed by astrocytes (Jucker et al. 1995), mediates adhesion between neuronal elements, induces neurite outgrowth (Cremer et al. 1997), and may mediate the interaction of mossy fibers with other fibers and glial cells (Niquet et al. 1993). Among NCAM's ligands are chondroitin sulfate proteoglycans. These proteoglycans are present in the brain ECM and are involved in cell–cell and cell–substrate interactions (Levine and Nishiyama 1996). Two of these ligands are neurocan and DSD-1-PG/phosphacan. Neurocan is synthesized by neurons at high levels during late embryonic development (Margolis and Margolis 1997). DSD-1-PG/phosphacan is expressed primarily by astrocytes, but only at low levels prenatally. The levels increase steadily in late embryogenesis, reach a plateau two weeks postnatally, and then remain stable (Margolis and Margolis 1997). DSD-1-PG/phosphacan is the isolated, soluble extracellular domain of a receptor-type transmembrane protein tyrosine phosphatase (RPTPζ/β). RPTPζ/β induces cell adhesion and promotes neurite growth of primary tectal neurons (Peles et al. 1995; Sakurai et al. 1997), as well as neuronal migration (Maeda and Noda 1998). DSD-1-PG/phosphacan and RPTPζ/β both bind to NCAM and tenascin-C and -R. Accordingly, DSD-1-PG/phosphacan has been proposed to oppose RPTPζ/β by competing for its binding sites. DSD-1-PG/phosphacan can affect neuronal adhesion and neurite outgrowth (Margolis and Margolis 1997), although the precise effect may differ for hippocampal and spinal cord neurons (Garwood et al. 1999).
In this report, we demonstrate two roles for tPA in controlling mossy fiber sprouting, one of which involves regulated DSD-1-PG/phosphacan proteolysis.
Materials and Methods
Animals
We used wild-type C57Bl6, tPA−/−, and plasminogen-deficient (plg−/−) mice and their heterozygote littermates. The mice were genotyped as described previously (Bugge et al. 1996; Mecenas et al. 1997). The adult male mice, age-matched and ∼25 g, were injected intraperitoneally with atropine (0.6 mg/kg of body weight) and then deeply anesthetized with 2.5% avertin (0.02 ml/g of body weight). They were placed in a stereotaxic apparatus, and injected unilaterally with 1.0 nmol of kainic acid (KA) in 0.3 μl of PBS into the amygdala (Niquet et al. 1993). The coordinates of the injection were: bregma, −1.6 mm; medial-lateral, 3.3 mm; and dorsoventral, 4.5 mm (according to Franklin and Paxinos 1997). The excitotoxin was delivered over 30 s. After KA was delivered, the injection needle remained at the above coordinates for another 2 min to prevent reflux of fluid. Animals showing KA-induced seizures after recovery from anesthesia were used for subsequent experiments. Animals injected with PBS were used as controls. We have reported that tPA−/− mice have a higher threshold for seizures (after i.p. delivery of metrazol or KA) than wild-type mice (Tsirka et al. 1995, Tsirka et al. 1996, Tsirka et al. 1997). The current seizure promoting protocol is different than our published one (Tsirka et al. 1995), in that the injection is done locally into the amygdala. After variable lengths of time (12, 20, and 30 d; time indicated in each experiment), the animals were killed. The brains were removed and sectioned (30 μm). When plg activator inhibitor was used, tPA Stop (American Diagnostica, Inc.) was infused at a concentration of 0.12 mg/ml in PBS, approximately in the midline at the level of the hippocampus (stereotaxic coordinates: bregma, −2.5 mm; medial-lateral, 0.5 mm; and dorsoventral, 1.6 mm) using an Alza micro-osmotic pump (Model no. 2004) with a pumping rate of 0.25 μl/h. After the infusion, cannula was positioned and secured, and the mice were injected unilaterally with kainate into the amygdala at the coordinates cited above. 12 d after the infusion/injection, the mice were killed and their brains processed as above.
Histochemical Staining
Control and KA-treated animals were perfused with 0.2% sodium sulfide and then with 4% paraformaldehyde. Mossy fibers were stained according to a modified Timm silver staining method (Holm and Geneser 1991).
In vitro processing of purified 125I-neurocan and DSD-1-PG/phosphacan by tPA and plasmin purified 125I-neurocan (∼2.5 ng/μl, 0.06 μCi/μl), and 125I-DSD-1-PG/phosphacan (∼2.5 ng/μl, 0.09 μCi/μl), was pretreated with protease-free chondroitinase ABC (Retzler et al. 1996). 0.234 μCi of each proteoglycan was then incubated in 100 mM Tris-HCl, pH 7.5, 0.01% Triton X-100 in the absence or presence of various concentrations (1–100 ng) of recombinant mouse tPA or human plasmin for 30 min at 37°C. To test for specificity of the proteolytic reactions, PAI-1 (a tPA inhibitor) or α2-antiplasmin (a plasmin inhibitor) were included in the reaction. At the end of the incubation period, SDS sample buffer was added and the samples were analyzed by 8% (for neurocan) and 7% (for DSD-1-PG/phosphacan) SDS-PAGE. The gels were then processed for autoradiography.
Immunoblotting
Cerebral tissue at the level of the hippocampal formation (∼100 mg wet weight) was prepared for SDS-PAGE as described (Oohira et al. 1994). In brief, the tissue was homogenized in PBS containing protease inhibitors. An aliquot was centrifuged at 6,000 g for 20 min. The supernatant was mixed with 50 mM Tris-HCl, pH 7.5, containing protease inhibitors, and 2% SDS and was boiled for 5 min. 3 vol of 95% ethanol containing 1.3% potassium acetate was added to precipitate the proteoglycans. The pellet was washed with 70% ethanol/1% potassium acetate, and was then subjected to digestion with protease-free chondroitinase ABC at 37°C for 60 min before performing Western blot analysis. The amounts of protein loaded were controlled, both before loading (Bradford protein assay), as well as through assessment using postblot staining with Ponseau S.
Scoring Method for Timm Histochemistry
The extent of mossy fiber sprouting was analyzed independently by three examiners who were unaware of the genotype of each brain section, using a previously published, standardized scoring procedure (Cavazos et al. 1991). This method gives a scoring scale from 0 to 5 of the distribution of Timm granules in the supragranular region of the DG. This scoring method assesses only the presence or absence of granules in the DG supragranular region or in the inner molecular layer in a wild-type animal. It does not, however, evaluate the morphology of the extending neurites.
Immunohistochemistry
Brain sections or hippocampal neurons of the mice, manipulated as described above, were incubated with antisera against neurocan, DSD-1-PG/phosphacan (polyclonal provided by Dr. Margolis, and monoclonal purchased from Developmental Studies Hybridoma Bank, University of Iowa), PSA-NCAM (a gift of Dr. T. Seki, Department of Anatomy, Juntendo University, Japan), or dynorphin A (Peninsula Laboratories, Inc.), at the dilutions recommended by the suppliers. Biotinylated or rhodamine-conjugated (for dynorphin A) secondary antibodies were used (Vector Laboratories).
Substrate Preparation
To evaluate whether DSD-1-PG/phosphacan is inhibitory or repellent to elongating mossy fiber neurites, we used a previously described culture method (Dou and Levine 1994). In brief, 60-mm petri dishes were coated with 750 μl nitrocellulose dissolved in methanol (5 cm2 nitrocellulose in 12 ml of methanol) and air-dried in a tissue culture hood. To create a boundary between DSD-1-PG/phosphacan (proteolyzed or not) and laminin, a 2-μl drop of purified DSD-1-PG/phosphacan (20 μg/ml), or proteolyzed DSD-1-PG/phosphacan, was spotted onto the nitrocellulose; the drop was aspirated off after 10 min and then a larger droplet (8 μl) of laminin (0.7 μg/ml) was spotted over the DSD-1-PG/phosphacan spot, so that a circular border was formed with DSD-1-PG/phosphacan in the inner circle and laminin in the outer. When proteolyzed DSD-1-PG/phosphacan was used, the proteoglycan was incubated with plasmin (100 ng) for 4 h at 37°C. The reaction was terminated with the addition of 100 ng of α2-antiplasmin. The dish was washed with medium before adding the cells. Neurons from four tPA−/− hippocampi were plated on each 60-mm dish, as described (Rogove and Tsirka 1998). The cultures were photographed after 5 d of growth. The images of the cultures were captured, and the number of neurites (pyramidal vs. granule) that were in the proximity of the border crossing into the phosphacan substrate was quantitated. This quantitation was done in four individual experiments by two reviewers blinded to the experimental conditions.
Intrahippocampal Delivery of rtPA and S478A tPA and Injection of Kainate in the Amygdala
Adult tPA− / − male mice were subjected unilaterally into the hippocampus to infusion of either buffer (PBS; n = 4) or 200 μl of wild-type rtPA (n = 4), or S478A tPA (0.12 mg/ml; n = 8, a gift of Genentech, Inc.), as described (Rogove et al. 1999). Then, kainate (1.5 nmol of KA in 300 nl of PBS) was injected unilaterally into the amygdala, as above. The brains were processed and evaluated for mossy fiber sprouting and the presence of tPA protein (by activity and immunohistochemistry; Tsirka et al. 1995). The recombinant catalytically inactive tPA, S478A, has a serine to alanine mutation at the active site of tPA.
Results
tPA−/− and plg−/− Mice Display Differing Degrees of Aberrant Mossy Fiber Sprouting after Kainate Stimulation
Since tPA is active as an extracellular protease in the CA3 and DG, we sought to investigate whether it has a role along the mossy fiber pathway (Carroll et al. 1994; Werb 1997). We used a modification of a previously published protocol for stimulation-induced mossy fiber sprouting in the rat (Niquet et al. 1993). In brief, KA was injected into the amygdala of wild-type and tPA−/− mice, which were then monitored for development of seizures as evidence of adequate neural stimulation. Only mice that exhibited seizures soon after recovery from anesthesia were used subsequently to assess the pattern of sprouting using Timm staining. Marked differences were obvious in two respects between the injected and uninjected sides of the wild-type and tPA−/− mice (Fig. 1). First, extensive stimulation-dependent sprouting was evident in wild-type mice across the width of the supragranular region of the DG (Timm score 2.17 ± 0.39, see Table ), whereas only limited sprouting occurred in tPA−/− mice. The few sprouts observed in tPA−/− mice were not as well-defined or dense as the wild-type sprouts. This phenotype is comparable to the pattern of mossy fiber sprouting observed for mice treated with endo-Neuraminidase, which removes the polysialic acid associated with NCAM (Seki and Rutishauser 1998), in that after endo-Neuraminidase treatment, the bundles were less compact and the fascicles were much smaller and disorganized. Second, in tPA−/− mice, there was a marked stimulation-dependent accumulation of Timm-stained mossy fibers at the boundary between the supragranular region and the molecular layer, which resulted in the formation of a laminar band (Timm score 4.44 ± 0.51, see Table ). This band was not detectable (or, at best, was barely detectable) in wild-type mice with similar kainate stimulation. These findings suggest that tPA is required both to promote mossy fiber outgrowth in the supragranular region and to terminate mossy fiber outgrowth at the border of the supragranular region with the molecular layer.
Figure 1.
Neo-Timm staining (Holm and Geneser 1991) visualizing mossy fiber sprouting in coronal sections of the hippocampal formation of wild-type and tPA−/− mice 20 d after unilateral injection of KA in the amygdala. Note the strong silver deposits at the border of the molecular layer in tPA−/− mice (arrows). GCL, Supragranular cell layer; mol, molecular cell layer.
Table 1.
Kainate-induced Alterations in Timm Histochemistry
| Genotype | Average Timm score ± SD | Mice used |
|---|---|---|
| n | ||
| Wild-type | 2.17 ± 0.39 | 12 |
| tPA−/− | 4.44 ± 0.51 | 16 |
| plg−/− | 3.00 ± 0.00 | 6 |
| Wild-type/tPA Stop | 4.50 ± 0.71 | 6 |
Mossy fiber synaptic reorganization was evaluated using a previously established scoring scale, which used the following criteria: 0, no granules between the crest and tip of the DG; 1, sparse granules in the supragranular region in a patchy distribution between the tips and crest of the DG; 2, more numerous granules in the supragranular region in a continuous distribution between the tips and crest of the DG; 3, prominent granules in the supragranular region in a continuous pattern between tips and crest with occasional patches of confluent granules between the tips and crest of the DG; 4, prominent granules in the supragranular region that form a confluent dense laminar band between tips and crest; 5, confluent dense laminar band of granules in the supragranular region that extends into the inner molecular layer (Cavazos et al. 1991).
Since the primary function of tPA involves the catalytic conversion of plg to plasmin, we repeated the experiment using plg−/− mice (Fig. 2). Sprouting within the supragranular region (Fig. 2 A, plg−/−) was comparable (Timm score 3.00, see Table ) to that observed in wild-type mice (Fig. 2 A, wt). However, similar to tPA−/− mice (Fig. 2 A, tPA−/−), an abnormal laminar band still formed at the boundary between the supragranular region and the molecular layer (Fig. 2, plg−/−).
Figure 2.
A, High magnification of Timm-stained horizontal sections of the injected side of DG of wild-type, tPA−/−, and plg−/− mice near the tip of the injected DG. The arrows point to the newly formed mossy fiber sprouts, demonstrating that they accumulate and extend along the border of the granule cell and molecular layers. B, PSA-NCAM immunohistochemistry on horizontal sections of the injected side of DG of wild-type, tPA−/−, and plg−/− mice near the tip of the injected DG. The arrows point to the extending neurites through the granule cell layer (GCL), the arrowheads point to the accumulated PSA-NCAM expression on the border between GCL and molecular layer (mol).
In addition to the histological Timm stain, we evaluated neurite outgrowth along the mossy fiber pathway using immunohistochemistry for polysialylated NCAM (PSA-NCAM), which has been used extensively as a reliable marker for mossy fiber sprouting (Seki and Rutishauser 1998). PSA-NCAM is not normally expressed in the adult (Seki and Arai 1999), but its expression is required for activity-dependent morphological plasticity (Theodosis et al. 1999). As seen in Fig. 2 B, PSA-NCAM is expressed by the compact and dense extending neurites in wild-type DG (Fig. 2 B, wt). In tPA−/− mice, PSA-NCAM+ neurites are stubby and disorganized (Fig. 2 B, tPA−/−, arrows), and the laminar band is observed (Fig. 2 B, tPA−/−, arrowheads), in agreement with the Timm histological stain. plg−/− neurites are dense and compact through the granule cell layer (Fig. 2 B, plg−/−, arrows), but still form the laminar band (Fig. 2 B, plg−/−, arrowheads). Taken together, these results suggest that tPA, but not plasmin(ogen), promotes mossy fiber outgrowth across the supragranular layer, whereas formation of the aberrant laminar band occurs when plasmin (or tPA, which activates plg) is absent.
Identification of DSD-1-PG/Phosphacan as a Substrate for Plasmin in the Mossy Fiber Pathway
NCAM promotes mossy fiber sprouting by facilitating neuronal cell interactions (Cremer et al. 1997) and is essential for accurate neurite outgrowth, synaptogenesis, and long-term changes in synaptic strength (Cremer et al. 1998). In addition, it has been reported that tPA and plasmin can proteolyze the highly polysialylated NCAM that is associated with mossy fibers (Endo et al. 1998). Therefore, we investigated NCAM as a potential substrate for tPA/plasmin in the mossy fiber pathway. However, levels of NCAM in wild-type, tPA−/−, and plg−/− mice injected with buffer or kainate appeared similar, and no differences in degradation or cleavage of recombinant NCAM were observed after incubation with brain homogenates prepared from similarly treated animals (data not shown). Similarly, no changes were observed after immunostaining for laminin or GAP-43, which are targets for plasmin (Chen and Strickland 1997) or mediate mossy fiber reorganization (Cantallops and Routtenberg 1996), respectively (data not shown).
We next examined the NCAM ligands, DSD-1-PG/phosphacan and neurocan. Purified 125I-neurocan and DSD-1-PG/phosphacan were incubated with recombinant tPA or plasmin and analyzed by SDS-gel electrophoresis. tPA did not cleave neurocan or DSD-1-PG/phosphacan. However, both proteoglycans were cleaved by plasmin in vitro (Fig. 3). The plasmin inhibitor, α2-antiplasmin, blocked the proteolysis, demonstrating that the cleavage was mediated specifically by plasmin.
Figure 3.
In vitro cleavage of neurocan and DSD-1-PG/phosphacan by plasmin, but not tPA. Radiolabeled with 125I, purified neurocan (∼9.75 ng) and DSD-1-PG/phosphacan (∼6.5 ng) were incubated with recombinant mouse tPA or human plasmin in the absence or presence of PAI-1 or α2-antiplasmin. The inhibitors were preincubated with the recombinant enzymes for 5 min at 37°C. The samples were analyzed by SDS-PAGE and autoradiography. Lane 1, tPA 1 ng; lane 2, tPA 10 ng; lane 3, tPA 100 ng; lane 4, tPA 100 ng and PAI1 100 ng; lane 5, proteoglycan incubated with buffer alone; lane 6, proteoglycan by itself; lane 7, plasmin 1 ng; lane 8, plasmin 10 ng; lane 9, plasmin 100 ng; lane 10, plasmin 10 ng and α 2-antiplasmin 50 ng; lane 11, plasmin 10 ng and α2-antiplasmin 100 ng. The arrows point to bands that disappear after incubation with plasmin.
We then sought to determine whether processing of the proteoglycans might be abnormal in tPA−/− or plg−/− mice in vivo, using polyclonal antibodies specific for each proteoglycan. Neurocan was not observed using immunohistochemistry or Western blotting, consistent with its expression being primarily limited to development (Margolis and Margolis 1997). However, a stimulation-dependent DSD-1-PG/phosphacan accumulation was detected by immunohistochemistry in tPA−/− or plg−/− mice as a denser band starting at the boundary between the supragranular and molecular layer and covering the molecular layer (data not shown). Similarly, aberrant accumulation of DSD-1-PG/phosphacan has been reported before in lesioned rat hippocampi (Deller et al. 1997).
To determine whether the accumulated DSD-1-PG/phosphacan had in fact failed to be processed correctly, we prepared protein extracts from hippocampi of stimulated and control wild-type, tPA−/−, and plg−/− mice. The proteoglycans were partially purified (Retzler et al. 1996) and analyzed by gel electrophoresis. Extracts from all three genotypes contained similar basal levels of DSD-1-PG/phosphacan (Fig. 4, uninjected lanes). However, extensive DSD-1-PG/phosphacan proteolytic cleavage, primarily of the 180-kD band (the protein core), was observed in the extracts from stimulated wild-type mice, but not in the extracts from tPA−/− or plg−/− mice (Fig. 4, injected lanes). These data indicate that significant proteolytic processing of DSD-1-PG/phosphacan occurs via the tPA/plasmin system during mossy fiber sprouting in wild-type mice, that endogenous plasmin is produced in enough quantities locally to mediate phosphacan's cleavage, and suggest that this processing may be physiologically important.
Figure 4.
Proteolytic processing of DSD-1-PG/phosphacan in wild-type mice after KA injection. Partially purified protein extracts from KA-injected (inj) or PBS-injected (uninj) wild-type (wt), tPA−/− and plg−/− hippocampi, as described in Materials and Methods, were analyzed by SDS-PAGE and immunoblotting, using an anti-DSD-1-PG/phosphacan antibody. Note the processing that has occurred in the wild-type injected lane, and the absence of such processing in the corresponding tPA−/− and plg−/− lanes. The arrows point to the 400-kD DSD-1-PG/phosphacan protein band (upper arrow), and the DSD-1-PG/phosphacan protein core of 180 kD (lower arrow), which appears more susceptible to plasmin cleavage. Top, Coomassie-stained gel showing comparable loading among the different lanes; bottom, Western blot of the Coomassie-stained gel (top). This experiment has been repeated five times.
These results link tPA/plasmin activity to the regulation of a cell adhesion molecule known to inhibit neural adhesion and neurite outgrowth (in this case, mossy fiber sprouting) through interaction with NCAM.
Unprocessed DSD-1-PG/Phosphacan Directly Repels Mossy Fiber Outgrowth in Culture
To determine whether DSD-1-PG/phosphacan specifically inhibits mossy fiber sprouting, we plated hippocampal neurons onto nitrocellulose/laminin-coated plates that additionally contained a region in which DSD-1-PG/phosphacan had been applied before the laminin coating (Dou and Levine 1994). The pattern of neurite outgrowth was evaluated at the border between the laminin and the phosphacan/laminin regions. To differentiate between pyramidal neurites and genuine mossy fiber axons, immunohistochemistry was used (Fig. 5 E) to detect dynorphin A, which is a marker that visualizes mossy fiber axons from DG granule cells, but not those from CA1-CA3 cells (Baranes et al. 1996).
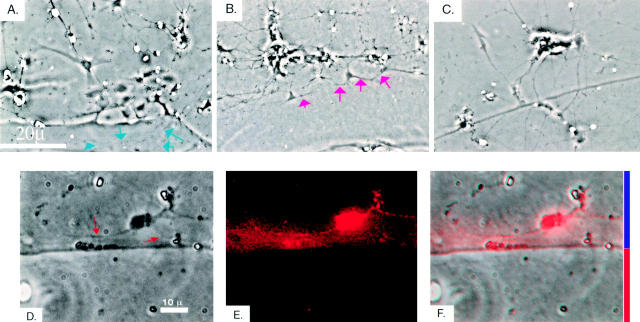
Figure 5.
DSD-1-PG/phosphacan repels mossy fiber outgrowth in culture. Hippocampal neurons were plated onto nitrocellulose-coated dishes containing: A, laminin in the center (bottom of A), and laminin in a surrounding annulus (top of A); B, laminin and DSD-1-PG/phosphacan in the center, and laminin alone in a surrounding annulus; C, laminin and plasmin-proteolyzed DSD-1/phosphacan in the center, and laminin alone in a surrounding annulus. The cultures were photographed after 5 d of growth. Phase-contrast image of hippocampal neuronal cells attached to the laminin region that are extending neurites (blue arrows denote neurites crossing through the layers, whereas red arrows indicate neurites that are being repelled). D, Phase-contrast image of neuronal cells attached to the laminin region and extending neurites through the laminin, but are being repelled and changing direction at the border of the DSD-1-PG/phosphacan/laminin region (red arrows). Immunohistochemistry with anti-DSD-1-PG/phosphacan antibody was used to confirm that the localization of DSD-1-PG/phosphacan coincided with the boundary at which neurite outgrowth terminated (data not shown). E, Immunostaining of mossy fiber-like axons with dynorphin A. F, Overlap of D and E, showing the avoidance of DSD-1-PG/phosphacan by the mossy fiber axons. Blue bar indicates the laminin region, and the red bar the DSD-1-PG/phosphacan/laminin region.
Neurons attached and extended neurites over the laminin-coated region. Pyramidal neurons could extend neurites into the phosphacan/laminin-coated region (98.71 ± 0.24% of all pyramidal neurites crossed the boundary between laminin and phosphacan). However, the phosphacan/laminin coated area was potently inhibitory to the axons of dentate granule cells (Fig. 5; only 7.55 ± 1.78% of granule cell neurites crossed the boundary). In fact, direct avoidance of DSD-1-PG/phosphacan by the outgrowing neurites was readily observed at the boundary (Fig. 5 B, red arrows, and D–F). This avoidance was not due to a border created by the two components, since when the border was generated by two different layers of laminin (Fig. 5 A, blue arrows), neurites could readily cross over. Furthermore, when phosphacan was incubated with plasmin before plating, no neurite outgrowth inhibition was observed (Fig. 5 C).
These results demonstrated that mossy fiber axons avoided regions that contained full-length DSD-1-PG/phosphacan, but otherwise continued to extend, supporting the in vivo data (Fig. 1 and Fig. 2).
tPA Promotes Mossy Fiber Outgrowth Acutely through a Proteolysis-independent Mechanism
To evaluate whether the involvement of tPA in neuritic pathfinding and outgrowth is due to its active participation in such processes (rather than to a developmental abnormality of the tPA−/− or plg−/− mice), we delivered back into tPA−/− mice recombinant active tPA or the catalytically inactive mutant, S478A tPA, and then stimulated amygdala with the injection of kainate. As seen in Fig. 6, the delivery of tPA into the tPA−/− animals just before kainate stimulation results in reversal (Fig. 6, tPA−/−/tPA) of the tPA−/− mossy fiber sprouting phenotype (Fig. 6, tPA−/−), making the animals comparable to wild-type animals (Fig. 6, wt). The effectiveness of the tPA infusion was assessed by visualizing tPA activity by in situ zymography (Sappino et al. 1993) or visualizing tPA protein by immunohistochemistry.
Figure 6.

The tPA−/− mossy fiber sprouting phenotype can be reversed to that of wild-type mice by infusion of recombinant tPA. Sections of KA-injected wild-type (wt) and tPA−/− mice are used as controls. When recombinant tPA (tPA−/−/tPA) is infused into the hippocampus and the intraamygdala KA injection follows, the Timm-positive mossy fiber sprouting pattern is comparable to that of wild-type mice. However, infusion of catalytically inactive S478A tPA (tPA−/−/S478A tPA) rescues only the neurite pathfinding through the supragranular region (arrowheads), but the aberrant laminar band (arrows) persists.
The more extensive phenotype observed for mossy fiber outgrowth in tPA−/− mice, as opposed to plg−/− mice, suggested either that tPA cleaves other effector proteins in addition to plg, or that it also acts through a proteolysis-independent pathway. To address this issue, we infused S478A tPA.
To investigate whether the above observation was reversible in wild-type mice, we employed tPA Stop, an inhibitor of tPA's proteolytic activity, to selectively block proteolysis-dependent pathways. tPA Stop (or buffer) was infused into the hippocampus of wild-type mice, which were subsequently injected unilaterally with kainate in the amygdala, and analyzed 12 d later. tPA activity was detected in the CA3/dentate hippocampal region of the buffer-infused mice, but not in the tPA Stop-infused mice (data not shown). Mossy fiber sprouting was evaluated by Timm staining. Extensive and dense sprouting was observed (Timm score 4.50 ± 0.71, Table ), in combination with a laminar band at the boundary between the supragranular region and the molecular layer, in the mice that received the tPA inhibitor (Fig. 7). In addition, accumulation of DSD-1-PG/phosphacan comparable to that seen in tPA−/− mice was observed (data not shown).
Figure 7.
Inhibition of tPA activity in wild-type mice generates only part of the aberrant sprouting phenotype observed in tPA−/− mice. A, Section of a KA-injected tPA−/− mouse as control. B, tPA Stop was infused into wild-type mice, which were then injected with KA into their amygdala. Strong, dense, and tight compacted Timm-positive sprouts are evident through the granular layer, as well as a laminar band at the border of granular and molecular layer of the injected side. The arrows point to the excessive sprouts present through the supragranular layer. The arrowheads point to the Timm-positive sprouts along the border of the molecular layer.
These results indicate that the tPA Stop infusion was effective, since DSD-1-PG/phosphacan processing, the step determined to be plasmin-dependent (and hence, tPA-dependent), was blocked and a laminar band was observed, yielding a phenotype similar to that observed for plg−/− mice (Fig. 2). However, since mossy fiber outgrowth was unperturbed, as opposed to the stunted outgrowth observed in tPA−/− mice (Fig. 1), mossy fiber outgrowth would appear to be mediated by a nonproteolytic tPA downstream effector pathway.
To demonstrate directly the physiological pathways mediated by tPA through this noncatalytic mechanism, we infused a recombinant, catalytically inactive tPA mutant, S478A, into tPA−/− mice. The inactivity of this enzyme was confirmed by zymography and its presence by tPA immunohistochemistry (Rogove et al. 1999; and data not shown). When S478A tPA was infused, the neurite outgrowth and pathfinding profile through the granule cell layer (Fig. 6, tPA/S478A tPA, arrowheads) was comparable to that of wild-type mice (Fig. 6, wt). An apparent laminar band, however, was present (Fig. 6, tPA−/−/S478A tPA, arrows), as had been observed in stimulated tPA−/− and plg−/− mice (Fig. 1, Fig. 2, and Fig. 6).
Discussion
NCAM promotes establishment of synaptic connections during development and synaptic plasticity in adults (Cremer et al. 1997) by regulating stimulation-dependent sprouting, growth, and synaptogenesis of mossy fibers (Niquet et al. 1993). NCAM-deficient mice exhibit learning and behavioral deficits ensuing from aberrant mossy fiber growth and pathfinding that leads to innervation of CA3 pyramidal cells at ectopic sites (Cremer et al. 1998). DSD-1-PG/phosphacan binds to many cell adhesion and ECM proteins, presumably to regulate cell–cell and cell–matrix interactions (Margolis and Margolis 1997). DSD-1-PG/phosphacan has been demonstrated to potently regulate neurite outgrowth in culture by opposing Ng-CAM and NCAM (Milev et al. 1994). Our results suggest that tPA/plasmin-mediated processing of DSD-1-PG/phosphacan is not necessary for mossy fiber outgrowth, but that it is critical for terminating appropriately the extension of mossy fibers at the supragranular/molecular boundary.
tPA has been reported to be secreted from neuronal growth cones, to promote the outgrowth of neurites (Krystosek and Seeds 1981a,Krystosek and Seeds 1981b), and to be required for the late phase of LTP (Frey et al. 1996; Huang et al. 1996). tPA is secreted from cultured dentate granule cells after stimulation with forskolin, and this release of tPA correlates with elongation of the dentate granule cell axons and the formation of active, presynaptic varicosities (Baranes et al. 1998).
tPA's involvement in L-LTP is likely to be dependent on N-methyl-d-aspartate (NMDA) receptors, since MK-801 blocks upregulation of tPA mRNA after LTP stimulation (Qian et al. 1993) and tPA−/− mice display a deficit in L-LTP that involves NMDA-mediated γ-aminobutyric acid (GABA) transmission (Frey et al. 1996; Huang et al. 1996; Baranes et al. 1998). It is also well established that a blockade of NMDA receptors inhibits neuronal stimulation-induced development of new attachment sites for mossy axons (McNamara and Routtenberg 1995), including at GABA+ interneurons in the DG (Acsády et al. 1998). It is intriguing to speculate that NMDA glutamate receptor stimulation results in upregulation and secretion of tPA, which would then regulate mossy fiber sprouting, and ultimately affect interneuron/GABA transmission.
Taken together, our findings suggest the following model for synaptic reorganization in the mossy fiber pathway. Upon stimulation, tPA released by the growth cones of granule cell axons promotes mossy fiber extension through a mechanism that does not involve the proteolytic conversion of plg to plasmin. At the supragranular/molecular border, tPA-mediated cleavage and activation of plg leads in turn to plasmin-mediated cleavage of DSD-1-PG/phosphacan and RPTPζ/β, which then counters NCAM's promotion of continued neurite extension. We do not presently know whether the termination of neurite extension is mediated directly by the processed DSD-1-PG/phosphocan or whether processing makes accessible a distinct termination signal otherwise hidden by the unprocessed DSD-1-PG/phosphocan. Our finding that unprocessed DSD-1-PG/phosphacan inhibits mossy fiber axon outgrowth (but not lateral growth) in culture is consistent with previous findings which demonstrated that both DSD-1-PG/phosphacan, as well as other brain chondroitin sulfate proteoglycans, can repel neurite outgrowth (Dou and Levine 1994; Margolis and Margolis 1997). Recently, it was reported that DSD-1-PG/phosphacan could promote or inhibit neurite outgrowth dependent on the lineage of neuronal cells (Garwood et al. 1999). In that context, pyramidal neurons transverse the phosphacan/laminin border (data not shown), indicating that the inhibitory effect is specific for the axons of dentate granule cells (Fig. 6 E).
The nature of the nonproteolytic mechanism exhibited by tPA remains to be defined. Previously, we reported that tPA-mediated activation of microglia does not require plasmin or catalytically active tPA (Tsirka et al. 1997). Accordingly, mossy fiber pathfinding and outgrowth might represent another such biological phenomenon. Alternatively, it is possible that microglia activated by secreted tPA may effect the mossy fiber pathfinding and outgrowth. In this context, other proteases released by macrophages or microglia have been shown to promote neurite growth (Petanceska et al. 1996; Bednarski et al. 1997).
Acknowledgments
We are grateful to Genentech for the kind gift of S478A tPA, Dr. T. Seki for the PSA-NCAM antibody, and to Drs. J. Benach, S. Strickland, K. Akassoglou, Z. Chen, and A.D. Rogove for assistance and critical reading of the manuscript, Dr. A. Pitkänen for thoughtful discussions, and Dave Colflesh for imaging expertise.
Epilepsy Foundation of America and the National Institutes of Health grants to S.E. Tsirka supported this work.
Footnotes
Abbreviations used in this paper: DG, dentate gyrus; ECM, extracellular matrix; GABA, γ-aminobutyric acid; KA, kainic acid; LTP, long-term potentiation; NCAM, neural cell adhesion molecule; NMDA, N-methyl-d-aspartate; plg, plasminogen; plg−/−, plg deficient; PSA-NCAM, polysialylated NCAM; RPTPζ/β, receptor-type transmembrane protein tyrosine phosphatase; tPA, tissue plasminogen activator; tPA−/−, tPA deficient.
References
- Acsády L., Kamodi A., Sik A., Freund T., Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D., Witter M. Hippocampal formation. In: Paxinos G., editor. The Rat Nervous System. Academic Press, Inc.; Orlando, FL: 1995. pp. 443–493. [Google Scholar]
- Baranes D., Lopez-Garcia J., Chen M., Bailey C., Kandel E. Reconstitution of the hippocampal mossy fiber and associational–commissural pathways in a novel dissociated cell culture system. Proc. Natl. Acad. Sci. USA. 1996;93:4706–4711. doi: 10.1073/pnas.93.10.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D., Lederfein D., Huang Y.-Y., Chen M., Bailey C., Kandel E. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Bednarski E., Ribak C., Lynch G. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J. Neurosci. 1997;17:4006–4021. doi: 10.1523/JNEUROSCI.17-11-04006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- Bugge T., Kombrinck K., Flick M., Daugherty C., Danton M., Degen J. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- Cantallops I., Routtenberg A. Rapid induction by kainic acid of both axonal growth and F1/GAP-43 protein in the adult rat hippocampal granule cells. J. Comp. Neurol. 1996;366:303–319. doi: 10.1002/(SICI)1096-9861(19960304)366:2<303::AID-CNE9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Schoonjans L., Kieckens L., Ream B., Degen J., Bronson R., De Vos R., van den Oord J., Collen D., Mulligan R. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Carroll P., Tsirka S., Richards W., Frohman M., Strickland S. The mouse tissue plasminogen activator gene 5′ flanking region directs appropriate expression in development and a seizure-enhanced response in the CNS. Development. 1994;120:3173–3183. doi: 10.1242/dev.120.11.3173. [DOI] [PubMed] [Google Scholar]
- Cavazos J., Golarai G., Sutula T. Mossy fiber synaptic reorganization induced by kindlingtime course of development, progression, and permanence. J. Neurosci. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-L., Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:1–20. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Cremer H., Chazal G., Goridis C., Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol. Cell. Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- Cremer H., Chazal G., Carleton A., Goridis C., Vincent J.-D., Lledo P.-M. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T., Haas C., Naumann T., Joester A., Faissner A., Frotscher M. Up-regulation of astrocyte-derived tenascin-C correlates with neurite outgrowth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neurosci. 1997;81:829–846. doi: 10.1016/s0306-4522(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Dou C.-L., Levine J. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Nagai N., Urano T., Ihara H., Takada Y., Hashimoto K., Takada A. Proteolysis of highly polysialylated NCAM by the tissue plasminogen activator-plasmin system in rats. Neurosci. Lett. 1998;246:37–40. doi: 10.1016/s0304-3940(98)00204-3. [DOI] [PubMed] [Google Scholar]
- Franklin K., Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press, Inc; Orlando, FL: 1997. [Google Scholar]
- Frey U., Müller M., Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J. Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood J., Schnadelbach O., Clement A., Schutte K., Bach A., Faissner A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J. Neurosci. 1999;19:3888–3899. doi: 10.1523/JNEUROSCI.19-10-03888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm I., Geneser F. Histochemical demonstration of zinc in the hippocampal region of the domestic pig. III. The dentate area. J. Comp. Neurol. 1991;308:409–417. doi: 10.1002/cne.903080308. [DOI] [PubMed] [Google Scholar]
- Huang Y., Bach M., Lipp H., Zhuo M., Wolfer D., Hawkins R., Schoonjans L., Kandel E., Godfraind J., Mulligan R. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc. Natl. Acad. Sci. USA. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M., Mondadori C., Mohajeri H., Bartsch U., Schachner M. Transient upregulation of NCAM mRNA in astrocytes in response to endorhinal cortex lesions and ischemia. Mol. Brain Res. 1995;28:149–156. doi: 10.1016/0169-328x(94)00206-t. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. Plasminogen activator release at the neuronal growth cone Science 213 1981. 1532 1534a [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. Plasminogen activator secretion by granule neurons of developing cerebellum Proc. Natl. Acad. Sci. USA 78 1981. 7810 7814b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Nishiyama A. The NG2 chondroitin sulfate proteoglycana multifunctional proteoglycan associated with immature cells. Perspect. Dev. Neurobiol. 1996;3:245–259. [PubMed] [Google Scholar]
- Maeda N., Noda M. Involvement of receptor-like protein tyrosine phosphatase ζ/RPTPβ and its ligand pleiotrophin/heparin binding growth associated molecule (HB-CAM) in neuronal migration. J. Cell Biol. 1998;142:203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R.U., Margolis R.K. Chondroitin sulfate proteoglycans as mediators of axon growth and pathfinding. Cell Tissue Res. 1997;290:343–348. doi: 10.1007/s004410050939. [DOI] [PubMed] [Google Scholar]
- McNamara R.K., Routtenberg A. NMDA receptor blockade prevents kainate induction of protein F1/GAP-43 mRNA in hippocampal granule cells and subsequent mossy fiber sprouting in the rat. Mol. Brain Res. 1995;33:22–28. doi: 10.1016/0169-328x(95)00083-5. [DOI] [PubMed] [Google Scholar]
- Mecenas P., Tsirka S., Sallés F., Strickland S. Lack of tissue plasminogen activator does not protect against neuronal degeneration in the cerebellum of weaver mice. Mol. Brain Res. 1997;772:233–238. doi: 10.1016/s0006-8993(97)00864-0. [DOI] [PubMed] [Google Scholar]
- Milev P., Friedlander D.R., Sakurai T., Karthikeyan L., Flad M., Margolis R.K., Grumet M., Margolis R.U. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J. Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquet J., Jorquera I., Ben-Ari Y., Represa A. NCAM immunoreactivity on mossy fibers and reactive astrocytes in the hippocampus of epileptic rats. Brain Res. 1993;626:106–116. doi: 10.1016/0006-8993(93)90569-9. [DOI] [PubMed] [Google Scholar]
- Oohira A., Matsui F., Watanabe E., Kushima Y., Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody 1G2 in the rat cerebrum. Neuroscience. 1994;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Peles E., Nativ M., Campbell P.L., Sakurai T., Martinez R., Lev S., Clary D.O., Schilling J., Barnea G., Plowman G.D. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Petanceska S., Canoll P., Devi L. Expression of rat cathepsin S in phagocytic cells. J. Biol. Chem. 1996;271:4403–4409. doi: 10.1074/jbc.271.8.4403. [DOI] [PubMed] [Google Scholar]
- Qian Z., Gilbert M., Colicos M., Kandel E., Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Retzler C., Göhring W., Rauch U. Analysis of neurocan structures interacting with the neural cell adhesion molecule NCAM. J. Biol. Chem. 1996;271:27304–27310. doi: 10.1074/jbc.271.44.27304. [DOI] [PubMed] [Google Scholar]
- Rogove A., Tsirka S. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr. Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Rogove A., Siao C.-J., Keyt B., Strickland S., Tsirka S. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J. Cell Sci. 1999;112:4007–4016. doi: 10.1242/jcs.112.22.4007. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Lustig M., Nativ M., Hemperly J.J., Schlessinger J., Peles E., Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta. J. Cell Biol. 1997;136:907–918. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A., Madani R., Huarte J., Belin D., Kiss J., Wohlwend A., Vassalli J. Extracellular proteolysis in the adult murine brain. J. Clin. Inves. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N., Williams B., Bickford P. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- Seki T., Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J. Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Arai Y. Temporal and spacial relationships between PSA–NCAM-expressing, newly generated granule cells, and radial glia-like cells in the adult dentate gyrus. J. Comp. Neurol. 1999;410:503–513. doi: 10.1002/(sici)1096-9861(19990802)410:3<503::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Theodosis D., Bonhomme R., Vitiello S., Rougon G., Poulain D. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J. Neurosci. 1999;19:10228–10236. doi: 10.1523/JNEUROSCI.19-23-10228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka S., Gualandris A., Amaral D., Strickland S. Excitotoxin induced neuronal degeneration and seizure are mediated by tissue-type plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tsirka S., Rogove A., Strickland S. tPA and neuronal death. Nature. 1996;384:123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- Tsirka S., Rogove A., Bugge T., Degen J., Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysisregulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]