Abstract
The homotypic fusion of yeast vacuoles requires Sec18p (NSF)-driven priming to allow vacuole docking, but the mechanism that links priming and docking is unknown. We find that a large multisubunit protein called the Vam2/6p complex is bound to cis-paired SNAP receptors (SNAREs) on isolated vacuoles. This association of the Vam2/6p complex with the cis-SNARE complex is disrupted during priming. The Vam2/6p complex then binds to Ypt7p, a guanosine triphosphate binding protein of the Rab family, to initiate productive contact between vacuoles. Thus, cis-SNARE complexes can contain Rab/Ypt effectors, and these effectors can be mobilized by NSF/Sec18p-driven priming, allowing their direct association with a Rab/Ypt protein to activate docking.
Keywords: Vps39/Vam6p, Vps41/Vam2p, Ypt7p, priming, Rab/Ypt effector
Introduction
Regulated fusion of organelle membranes (Rothman 1994) is essential for subcellular protein compartmentation. The protein and lipid factors that catalyze membrane fusion are being identified and their functional relationships are receiving intense study. Each fusion reaction seems to require integral membrane proteins termed SNAP receptors (SNAREs) (Pfeffer 1996), which function through the association of their cytoplasmic domains to form coiled-coils. Associated SNAREs, whether in cis (on the same membrane) or in trans (attached to apposed membranes), can be disassembled by the action of the ATP-driven chaperone, N-ethylmaleimide sensitive factor (NSF)/Sec18p, and its cochaperone αSNAP/Sec17p (Söllner et al. 1993; Hanson et al. 1995; Ungermann et al. 1998a). SNARE associations are regulated either directly or indirectly by members of the Rab/Ypt family of GTPases and their associated proteins, termed Rab/Ypt effectors (McBride et al. 1999; Carr et al. 1999; Ungermann et al. 1998a). In several systems, Rab/Ypt proteins and their effectors have been shown to mediate a SNARE-independent initial association of membranes, termed tethering, which is the first step of the docking stage (Cao et al. 1998; Ungermann et al. 1998b). Tethering is a necessary prelude to trans-SNARE pairing, which establishes a stably docked organelle pair. The recent finding (McBride et al. 1999) that Rab effectors can directly associate with SNAREs and that this association can be modulated by the chaperone NSF, suggested that these Rab effectors may directly mediate trans-SNARE pairing and the subsequent steps that lead to fusion.
We have studied the homotypic fusion of yeast vacuoles. This reaction occurs in obligately ordered subreactions of priming, docking, and fusion. Five SNAREs on the purified organelle are found in a cis complex with the chaperones Sec18p, Sec17p, and low molecular weight activity #1 (LMA1) (Ungermann et al. 1999). This complex has been viewed as simply the remains of SNARE associations from prior fusion events, though current studies reported below show that it actually has a greater complexity and distinct functions. The cis-SNARE complex is disassembled as Sec18p hydrolyzes ATP during priming (Barnard et al. 1997; Ungermann et al. 1998b). This priming event is necessary for docking (Mayer and Wickner 1997). Docking occurs in two stages, a reversible vacuole association termed tethering, which depends on the Rab-like GTPase Ypt7p, and the subsequent pairing of SNAREs in trans (Ungermann et al. 1998a). We report that Vam2/Vps41p and Vam6/Vps39p, identified in vacuole protein sorting (vps) screens for defective vacuole protein sorting (Stack et al. 1995) and in vacuole morphology (vam) screens for fragmented vacuole morphology (Wada et al. 1992), are also required for vacuole docking (Price et al. 2000). Docking triggers a brief release of calcium from the vacuole lumen (Peters and Mayer 1998) which leads to vacuole association of a complex of calmodulin and protein phosphatase 1 (Peters et al. 1999), a dephosphorylation of a vacuolar target protein(s), release of the LMA1 cochaperone (Xu et al. 1998), and membrane fusion.
Despite this progress in defining the components that catalyze each stage of the reaction, the mechanism whereby the different stages are coupled is unknown. One of the most intriguing questions is how the priming of vacuoles leads to their docking. Specifically, how does the disassembly of the cis-SNARE complex lead to the Ypt7p-dependent association of vacuoles, and what are the linking proteins? We now show that this function is fulfilled by a protein complex that contains Vam2/Vps41p and Vam6/Vps39p, termed Vam2/6p, working as a vacuolar Rab/Ypt effector. Vam2p and Vam6p are localized to the vacuole as part of a large complex (Nakamura et al. 1997) that is required for the docking stage of homotypic vacuole fusion (Price et al. 2000). However, unlike other known Rab/Ypt effectors, Vam2/6p is initially bound to the cis-SNARE complex and is released from the SNAREs upon Sec18p-dependent priming. Vam2/6p can only then bind to Ypt7p to initiate docking, providing an important physical and functional link between membrane priming and docking.
Materials and Methods
Biochemical Reagents
Gdi1p was purified according to Haas et al. 1995. All inhibitors and antibodies were dissolved or dialyzed in PS buffer (20 mM Pipes-KOH, pH 6.8, 200 mM sorbitol) unless stated otherwise.
Antibody Production and Purification
Antibodies against the Vam2 and Vam6 proteins were prepared as described in Price et al. 2000.
Assay for Release of Vam2/Vam6p
Vacuoles from S. cerevisiae BJ3505 (wild-type or with hemagglutinin [HA]-tagged Vam6p) were incubated under standard fusion reaction conditions for 0–90 min. The reactions were then diluted fivefold with PS buffer and the vacuoles sedimented by centrifugation (14,000 g, 5 min, 4°C). Proteins in the supernatant were precipitated by the addition of TCA to a final concentration of 15% (vol/vol), washed with ice-cold acetone, and analyzed by SDS-PAGE and immunoblotting.
For some experiments, the chromosomal VAM6 gene was replaced with a HA epitope–tagged version of the VAM6 gene by recombination with a plasmid cassette containing the HA-tagged VAM6 gene. A 1.2-Kb Kpn1-EcoRI fragment of the VAM6 gene containing six copies of the HA epitope at the 5′ end of the coding sequence (a gift from Dr. Yoh Wada, Osaka University, Osaka, Japan) was cloned into pRS306. This construct was then linearized at the MscI site in the VAM6 coding sequence and transformed into S. cerevisiae BJ3505. Transformants were analyzed for expression of HA-Vam6p by SDS-PAGE followed by immunoblotting with the anti-HA mAb 12CA5 (Boehringer) and antiserum against Vam6 protein. HA-tagged VAM6 rescues the fragmented vacuole phenotype of vam6Δ yeast (Nakamura et al. 1997), and fusion of HA-Vam6p vacuoles in vitro is indistinguishable from that of wild-type vacuoles (data not shown).
Immunoprecipitations
Anti-Vam2p, anti-Ypt7p, and anti-Vam3p were cross-linked to protein A–Sepharose CL-4B (Amersham Pharmacia Biotech) using dimethylpimelimidate (Harlowe and Lane 1988). Vacuoles (100 mg) were solubilized in 200 μl of solubilization buffer (20 mM Hepes-KOH, pH 7.4, 150 mM NaCl, 10% [wt/vol] glycerol, 1% [vol/vol] Triton X-100) with protease inhibitor cocktail (Haas 1995) for 10 min on ice. Insoluble material was removed by centrifugation (14,000 g, 10 min, 4°C). The supernatant was then incubated with protein A–Sepharose immobilized antibodies for 2 h at 4°C in a final volume of 500 μl of solubilization buffer. The beads were washed twice with solubilization buffer and bound proteins were eluted with 0.1 M glycine, pH 2.5. The eluates were then neutralized by the addition of 0.1 M Tris-HCl, pH 9.0, and analyzed by SDS-PAGE and immunoblotting.
Sucrose Velocity Gradients
Vacuoles were sedimented (14,000 g, 10 min, 4°C) and solubilized for 10 min on ice in PS solubilization buffer, which is PS buffer containing 1% (vol/vol) Triton X-100 and protease inhibitor cocktail (Haas 1995). Insoluble material was removed by sedimentation (14,000 g, 10 min, 4°C). The vacuole extract was layered onto a 12-ml gradient of 10–40% (wt/vol) sucrose in PS buffer with 1% (vol/vol) Triton X-100 and centrifuged (38,000 rpm, 6 h, 4°C; Beckman SW-41 rotor). Fractions were analyzed by SDS-PAGE followed by immunoblotting. BSA (4S), α2-macroglobulin (20S), and ferritin (65S) were centrifuged in a parallel gradient and analyzed by SDS-PAGE and staining with Coomassie brilliant blue.
Results
Association of Vam2/6p with SNAREs
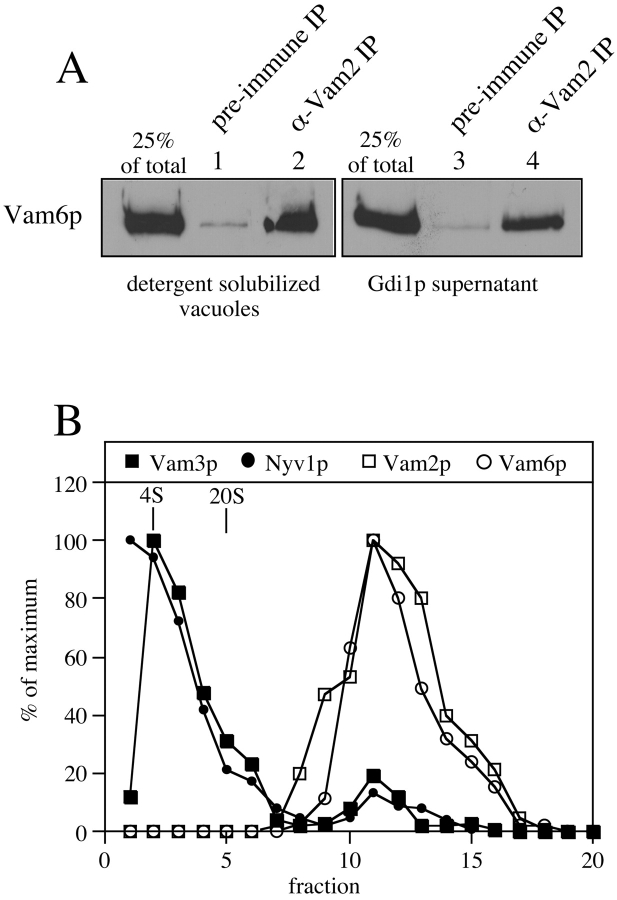
In accord with earlier studies (Nakamura et al. 1997), Vam2p and Vam6p are associated with each other as part of a large complex, as assayed in Triton X-100 extracts of vacuoles by coimmunoprecipitation (Fig. 1 A, lane 2) and cosedimentation at 65S in sucrose velocity gradients (Fig. 1 B). Gradient fractions were also assayed for the SNARE proteins Nyv1p and Vam3p. Surprisingly, although the cis-SNARE complex (Ungermann et al. 1999) was labile under these conditions, a small fraction of these SNAREs cosedimented with Vam2/6p (Fig. 1 B). This was confirmed directly by observing a coimmunoprecipitation of Vam2p by anti-Vam3p (Fig. 2 A, lane 1), but not by control IgG (Fig. 2 A, lane 4). This association was seen with other members of the SNARE complex, as antibody to the v-SNAREs Vti1p or Ykt6p immunoprecipitate Vam2p (Fig. 2 B, lanes 2 and 3) at least as efficiently as does antibody to Vam3p (Fig. 2 B, lane 1).
Figure 1.
Physical associations of Vam2p and Vam6p. (A) Vam2p and Vam6p form a stable complex. BJ3505 HA-Vam6p vacuoles were solubilized with buffer containing 1% Triton X-100 or incubated at 27°C in a complete fusion reaction containing Gdi1p (64 μg/ml) for 90 min. Detergent extracts and release reaction supernatants were immunoprecipitated with antibody against Vam2p or preimmune antibody as described in Materials and Methods. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-HA mAb 12CA5. (B) Sucrose velocity gradients. BJ3505 vacuoles (500 μg) were solubilized in 400 μl of PS solubilization buffer and analyzed on 10–40% sucrose gradients as described in Materials and Methods. Gradient fractions were analyzed by SDS-PAGE and immunoblotting with antisera against Vam2p, Vam6p, Nyv1p, and Vam3p. Immunoblots were then quantified by scanning densitometry and plotted as a percent of the maximum signal in the peak fraction.
Figure 2.
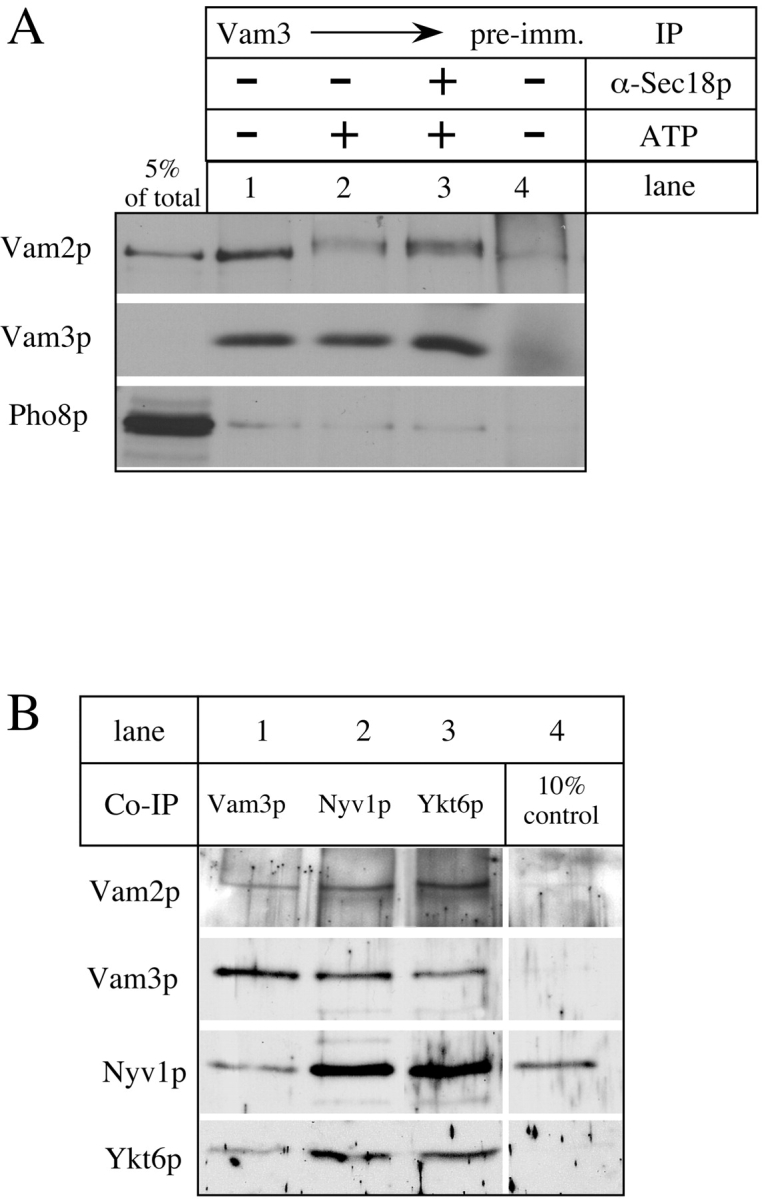
Vam2/6p is associated with the cis-SNARE complex. (A) Association of Vam2p with Vam3p. BJ3505 vacuoles were incubated in 10× scale fusion reactions at 27°C for 10 min with or without ATP. Lane 3 was supplemented with antibody to Sec18p. Vacuoles were solubilized in buffer containing 1% Triton X-100 as described in Materials and Methods, and proteins immunoprecipitated from the detergent extracts with antibody to Vam3p. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antisera to the indicated vacuolar proteins. (B) Vam2p is associated with Vti1p and Ytk6p. An immunoblot of vacuole protein immunoprecipitates from Ungermann et al. 1999 was probed with antibody to Vam2p.
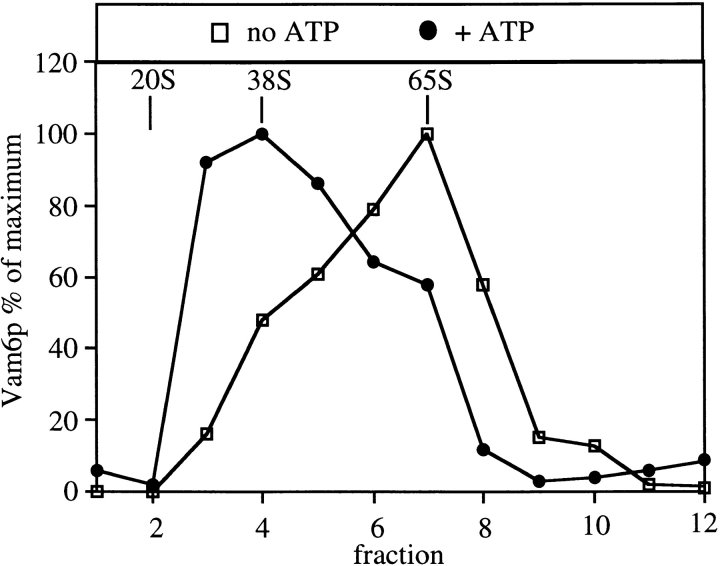
Vacuole cis-SNARE complexes are dissociated by the priming activity of Sec18p (Ungermann et al. 1999). We find that Vam2/6p is released from its association with the cis-SNARE complex during priming. Coimmunoprecipitation of Vam2p with Vam3p is diminished 77% upon incubation at 27°C with ATP (Fig. 2 A, lane 2), and this release is completely blocked by antibody to the priming catalyst Sec18p (Fig. 2 A, lane 3) but not by ligands that block steps other than priming (data not shown). Not only do SNAREs cosediment (Fig. 1 B) and coimmunoprecipitate (Fig. 2A and Fig. B) with Vam2/6p, but disruption of the cis-SNARE complex through priming the vacuoles (Fig. 3) causes a shift of the 65S Vam2/6p to a slower sedimenting species of 38S. As Vam2/6p is a peripheral membrane complex (Nakamura et al. 1997), this loss of its association with integral membrane SNAREs, as well as its involvement in the docking reaction (Price et al. 2000), raises the question of the identity of its subsequent physical and functional associations on the vacuole membrane.
Figure 3.
Vacuole priming dissociates the Vam2/6p complex. BJ3505 wild-type vacuoles (300 μg) were incubated at 27°C for 10 min with or without Mg2+/ATP in standard fusion reactions. Vacuoles were then sedimented, solubilized in PS buffer containing 1% Triton X-100, and analyzed on sucrose velocity gradients as in Fig. 1.
Vam2/6p Physically Associates with Ypt7p after Priming
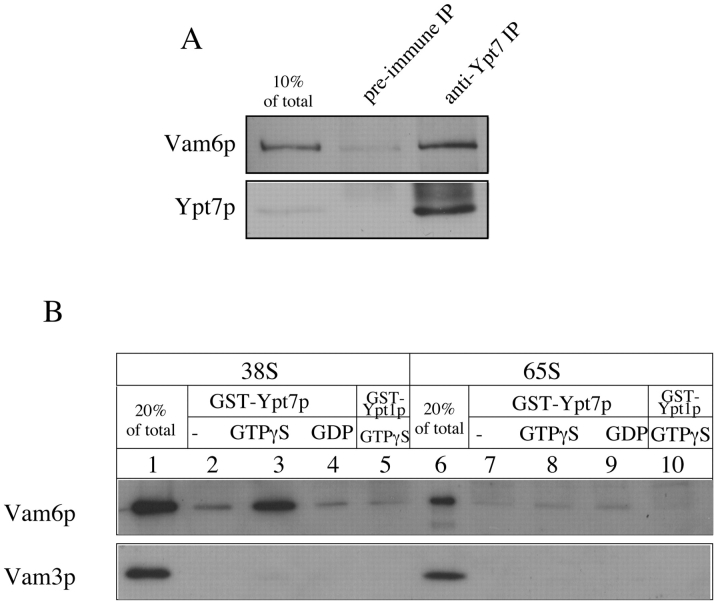
Since both Vam2/6p and Ypt7p are essential for docking (Mayer and Wickner 1997; Price et al. 2000), we asked whether Vam2/6p and Ypt7p are directly associated. We find that antibody to Ypt7p will cross-immunoprecipitate ∼13% of the Vam6p from vacuole detergent extracts (Fig. 4 A). Moreover, the GTP form of purified recombinant glutathione S-transferase (GST)–Ypt7p will bind the complex containing Vam6p (Fig. 4 B, lane 3) and Vam2p (data not shown) from the 38S gradient-purified fractions (Fig. 3) of Vam2/6p complex from primed vacuoles. This association is specific for the nucleotide (Fig. 4 B, lanes 2–4) and is not seen with the GTP form of the fusion protein of GST with Ypt1p (Fig. 4 B, lane 5), a closely related Rap/Ypt protein required for ER to Golgi traffic. Without priming, the 65S Vam2/6p complex (Fig. 4 B, lane 6) does not associate with GST–Ypt7p:GTPγS (Fig. 4 B, lane 8) above background levels (Fig. 4 B, lanes 7–10). These results indicate that the Vam2/6p complex acquires a direct affinity for Ypt7p after priming and establish that Vam2/6p is an important Ypt7p effector.
Figure 4.
Association of Vam2/6p with Ypt7p. A. Vam6p is associated with Ypt7p on isolated vacuoles. BJ3505 HA-Vam6p vacuoles were solubilized in buffer containing 1% Triton X-100 as described in Materials and Methods, and proteins were immunoprecipitated from the detergent extracts with preimmune serum or antiserum to Ypt7p. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-HA mAb 12CA5 and anti-Ypt7p. (B) The 38S Vam2/6p complex binds to GST–Ypt7p:GTPγS. BJ3505 vacuoles (650 μg) were sedimented (13,000 rpm, 10 min, 4°C; Microfuge, then incubated in a 100× scale fusion reaction (Haas 1995) with salt adjustment buffer for 10 min at 27°C. Primed vacuoles were then sedimented as above and resuspended twice in 1 ml PS buffer followed by sedimentation. Primed vacuoles, as well as a separate aliquot of unprimed vacuoles (650 μg), which had also been sedimented and resuspended, were solubilized in 650 μl PS solubilization buffer and fractionated on sucrose gradients as described in Materials and Methods. Vam2/6p peak fractions from unprimed (65S) and primed (38S) vacuoles (see Fig. 3) were brought to final concentration of 150 mM NaCl, 10% glycerol, and 1× protease inhibitor cocktail (Haas 1995), and incubated for 2 h at 4°C in the presence of 12 μg of glutathione–Sepharose-bound GST–Ypt7p or GST–Ypt1p. (Fusion protein preparation: The fusion proteins were preincubated in PS solubilization buffer containing 2 M NaCl, 10% glycerol, 1 mM DTT, 20 mM EDTA, and 5 mM GDP [PS elution buffer] for 20 min at room temperature with 40 μl (packed volume) glutathione–Sepharose 4B beads [Amersham Pharmacia Biotech]. Beads were sedimented [1 min, 13,000 rpm, 4°C] and resuspended four times as above in 1 ml of PS solubilization buffer containing 150 mM NaCl and 10% glycerol, then incubated in 25 μl of 1.2× PS buffer containing 36 mM TrisCl, pH 8.0, 180 mM KCl, 0.6 mM MnCl2, 6 mM MgCl2, 0.1× protease inhibitor cocktail, 1 mM DTT, and either 4 mM GTPγS, 4 mM GDP, or no nucleotide [−] for 30 min at 27°C). After the incubation of peak fractions and fusion protein beads, beads were collected and suspended twice in 1 ml PS solubilization buffer. Bound proteins were eluted with 500 μl PS elution buffer for 15 min at room temperature and separated from the beads by sedimentation as above. The elutate was TCA-precipitated, the precipitate suspended in 1 ml 80% acetone and collected by sedimentation, and analyzed by SDS-PAGE and immunoblotting with antisera to Vam6p and Vam3p.
Ypt7p Regulates the Localization of Vam2/6p to the Vacuole
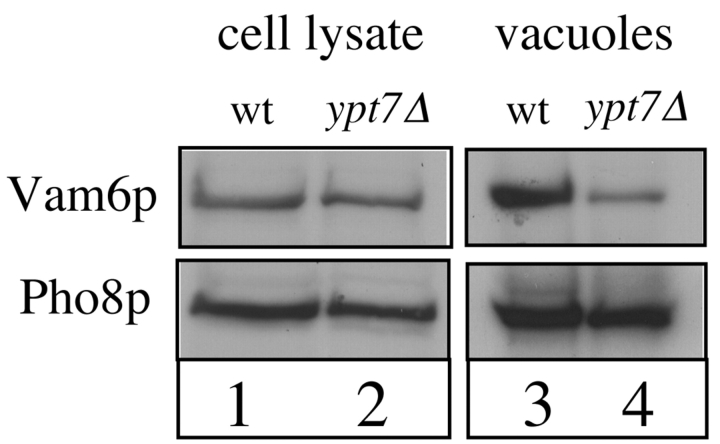
To investigate whether Vam2p and Vam6p also interact with Ypt7p in the cell, we examined the localization of Vam6p in a ypt7Δ strain. Purified vacuoles that lack Ypt7p had very little Vam6p (Fig. 5, lane 4) in comparison with wild-type vacuoles (Fig. 5, lane 3), whereas the amount of the vacuole marker Pho8p is unaffected by the ypt7Δ mutation. The amount of Vam6p is unchanged in whole cell lysates of ypt7Δ cells in comparison with wild-type (Fig. 5, lanes 1 and 2). Therefore, the ypt7Δ mutation affects the vacuolar localization of Vam6p but not its expression, indicating a functional relationship between these components. In contrast, the amount of Ypt7p on the vacuoles is unaffected by the vam2Δ and vam6Δ mutations (see Fig. 2 of Price et al. 2000, this issue), indicating that Ypt7p has a primary role in vacuolar localization and that Vam2p and Vam6p are dependent on Ypt7p.
Figure 5.
Vam6p is lost from the vacuole in ypt7Δ cells. BJ3505 wild-type and ypt7Δ cultures (1 l) were grown to mid-log phase. Portions (50 ml) of each were centrifuged and the cells were resuspended in buffer containing 2% (vol/vol) Triton X-100, 1% (wt/vol) SDS, 0.1 M NaCl, 1 mM EDTA, 1 mM PMSF, and 10 mM TrisCl, pH 8.0. Cells were broken by vortexing in the presence of acid-washed glass beads. Glass beads and cell debris were removed by centrifugation (4°C, 1 min, 14,000 g). The remainder of the culture was used for vacuole purification (see Materials and Methods). Lysed cell supernatant from equivalent amounts of cells or equivalent amounts of vacuolar protein (15 μg) were analyzed by SDS-PAGE and immunoblotting with antisera against Vam6p and Pho8p.
In accord with Ypt7p being required for the vacuolar localization of Vam6p in vivo, extraction of Ypt7p or inhibition of its function in vitro releases Vam2 and Vam6 proteins from the vacuole membrane. When vacuoles are incubated under standard fusion conditions, <1% of Vam2 or Vam6 protein is released into the reaction supernatant after sedimentation (Fig. 6 A, lane 2; supernatant of a standard fusion reaction). Vam2/6p release is strikingly enhanced by the addition of yeast Gdi1p (Sec19p), an inhibitor of Rab GTPases that binds and extracts the GDP-bound form of these proteins from membranes (Novick and Zerial 1997). As seen in Fig. 6 A, lane 4, the 22% of Vam2p and Vam6p that was released from the vacuoles upon Ypt7p release by Gdi1p is recovered in the reaction supernatant. The integral membrane protein Pho8p is not found in any of the reaction supernatants, indicating that the appearance of Vam2p and Vam6p in the supernatant is not due to fragmentation of the vacuoles. The Vam6p that is extracted by Gdi1p is still coimmunoprecipitated by anti-Vam2p (Fig. 1 A, lane 4), although the extracted complex sediments more slowly than Vam2/6p in association with the cis-SNAREs (data not shown). Thus, Vam2p and Vam6p remain together throughout priming and extraction by loss of their Ypt7p anchor. We also observed that some Vam2p is covalently modified upon release and migrates at a slightly lower mobility, though the nature of this modification and its significance have not yet been determined. The removal of Ypt7p from the vacuole by Gdi1p is dose-dependent and there is a corresponding release of Vam6p (data not shown). However, measurement of release as a function of reaction time shows that Ypt7p is removed very rapidly from the vacuoles by Gdi1p, whereas the loss of Vam6p occurs gradually (Fig. 6 B). Ypt7p is specifically required for the retention of Vam6p on the vacuole, since addition of inhibitory anti-Ypt7p IgG (Haas et al. 1995) causes 27% release of Vam6p (Fig. 6 A, lane 6), and is thus as effective as Gdi1p in releasing Vam6p, whereas preimmune IgG has no effect (Fig. 6 A, lane 8). However, unlike Gdi1p, anti-Ypt7p does not remove Ypt7p from the vacuole membrane (Fig. 6 A, lanes 5 and 6). Therefore, it is the loss of full Ypt7p function and not its removal from the vacuole membrane that causes release of the Vam2 and Vam6 proteins.
Figure 6.
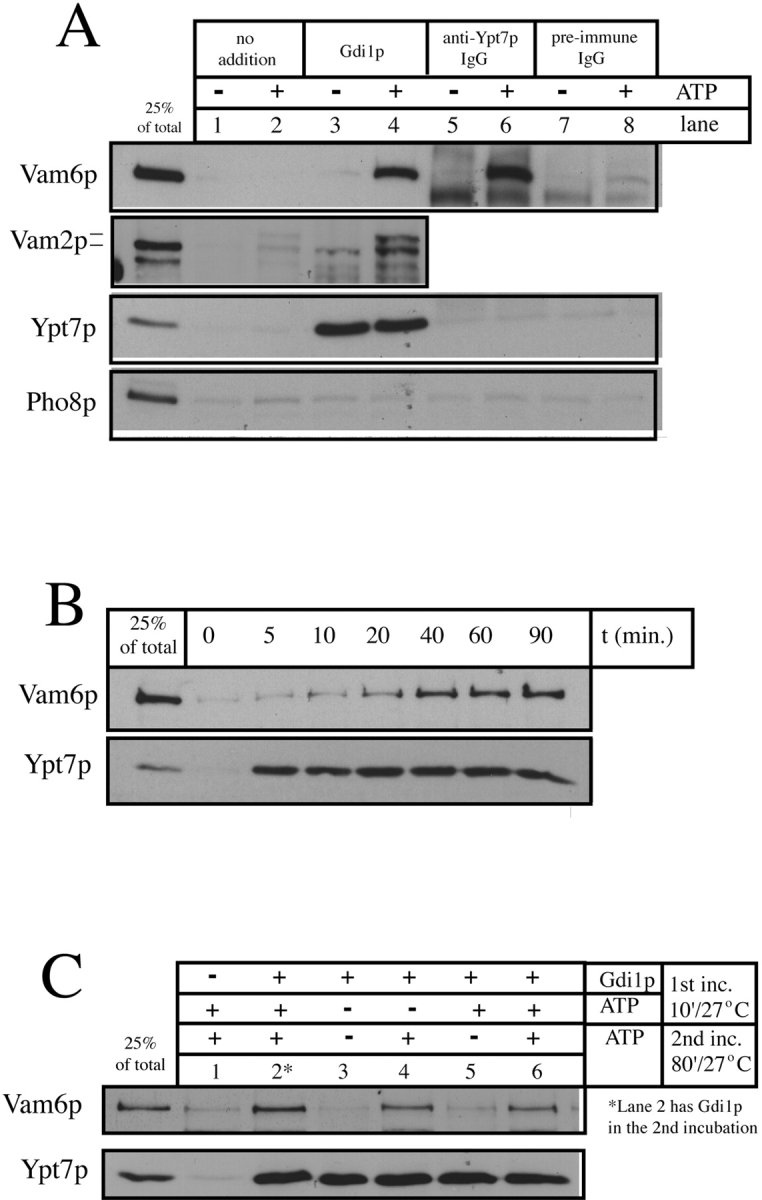
Release of Vam2/6p from the vacuole. (A) Vam2/6p release. BJ3505 HA-Vam6p vacuoles (see Materials and Methods) were incubated under standard fusion conditions with or without ATP at 27°C. Reactions were supplemented with control buffer, Gdi1p (64 μg/ml), IgG against Ypt7p, or preimmune IgG. After 90 min, the reactions were diluted fivefold with PS buffer and the vacuoles sedimented (5 min, 14,000 g, 4°C). The supernatants were then TCA-precipitated and analyzed by SDS-PAGE and immunoblotting with anti-HA mAb 12CA5 or antisera against Vam2p, Ypt7p, and Pho8p. Lanes 5–8 of the Vam2p panel were omitted because the immunoblot of Vam2p is obscured by the rabbit IgG added to the reactions. (B) Vam6p is released from the vacuole after the release of Ypt7p. BJ3505 vacuoles were incubated under standard fusion conditions with ATP at 27°C in the presence of Gdi1p (64 μg/ml). At the indicated times, reactions were placed on ice. After sedimenting as above, supernatants were analyzed by SDS-PAGE and immunoblotting with antibodies to Vam6p and Ypt7p. (C) The ATP requirement for Vam6p release is unrelated to the release of Ypt7p. BJ3505 vacuoles were incubated at 27°C for 10 min with ATP and/or Gdi1p (64 μg/ml) as indicated. The reactions were then diluted 10-fold with PS buffer and the vacuoles reisolated to remove ATP and Gdi1p. Vacuoles were then resuspended in reaction buffer with or without ATP as indicated and incubated at 27°C for an additional 80 min. Reactions were then analyzed for release of Vam6p and Ypt7p as above.
Although ATP is not required for the extraction of Ypt7p by Gdi1p, ATP is required for the release of Vam2p and Vam6p by either Gdi1p or anti-Ypt7 (Fig. 6 A, lanes 2–6). To determine whether ATP is needed at the same time as Gdi1p to promote Vam2/6p release, a two-stage reaction was performed. Vacuoles were first incubated with Gdi1p to remove Ypt7p, then reisolated to remove the Gdi1p. The vacuoles were then incubated with or without ATP and sedimented to allow assay of released Vam6p (Fig. 6 C). ATP alone in the second incubation is sufficient to extract Vam6p after Gdi1p treatment in the first incubation (Fig. 6 C, lane 4). However, whereas complete release of Ypt7p is achieved in the absence of ATP in the first 10-min incubation (Fig. 6 C, lane 3), little Vam6p is removed in this short incubation even when both Gdi1p and ATP are present (Fig. 6 C, lane 5). Therefore, the ATP requirement for the release of Vam6p cannot be simply due to effects it might have on Ypt7p, and may not simply reflect a need for priming, which is usually complete by 10 min (Mayer et al. 1996; Price et al., accompanying manuscript). The role of ATP in Gdi1p-mediated release of Vam2/6p remains to be determined.
Sec18-primed Vacuoles must Dock to Retain the Vam2/6 Complex
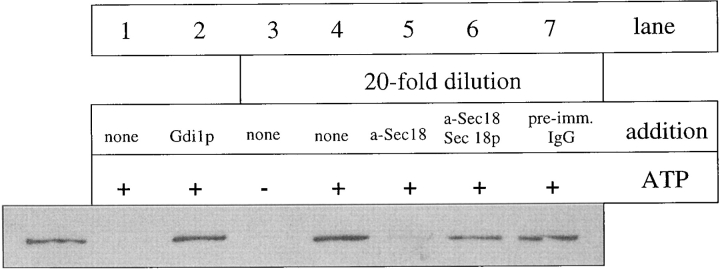
Since Ypt7p supports vacuole docking (Mayer and Wickner 1997; Ungermann et al. 1998b), we asked whether inhibition of docking itself would influence Vam2/6p localization. 20-fold dilution of the vacuoles, a nonpharmacological treatment that prevents vacuole docking (Mayer and Wickner 1997), promotes Vam6p release from the vacuoles (Fig. 7, lane 4). Like the Vam6p release promoted by Ypt7p inhibitors, ATP is required for Vam6p release upon dilution (Fig. 7, lane 3 vs. lane 4). Dilution does not cause release of Ypt7p (data not shown). This Vam6p release requires the normal priming action of Sec18p, as addition of anti-Sec18p blocks Vam6p release upon dilution of the fusion reaction (Fig. 7, lane 5), whereas preimmune IgG had no effect (Fig. 7, lane 7). The anti-Sec18p blockage of this reaction was relieved by the addition of excess Sec18 protein (Fig. 7, lane 6), demonstrating that the effect of the antibody and the promotion of Vam6p release is specifically due to the activity of Sec18p. Therefore, after priming releases Vam2/6p from its association with the cis-SNARE complex, vacuole docking must occur to avoid Vam2/6p release.
Figure 7.
Sec18-primed vacuoles must dock to retain Vam6p. BJ3505 HA-Vam6p vacuoles were incubated with or without ATP with control buffer, antibody against Sec18p, or preimmune IgG on ice for 10 min. Lane 6 was supplemented with Sec18 protein (100 μg/ml). Lane 2 received Gdi1p (64 μg/ml). Lanes 3–7 were then diluted 20-fold with reaction buffer with ATP where indicated. All reactions were then incubated at 27°C for 90 min and analyzed for HA-Vam6p release.
Discussion
Our current studies suggest a working model (Fig. 8) of how Vam2/6p links priming and docking. In this model, Sec18p-dependent priming releases Vam2/6p from its association with the cis-SNARE complex (Fig. 8 A), freeing it to interact with Ypt7p (Fig. 8 B) and other docking components. After priming, Vam2/6p is needed for docking (Fig. 8 C) and docking is needed for the continued vacuole association of Vam2/6p (Fig. 5 Fig. 6 Fig. 7), suggesting that Vam2/6p is at the docking site (Fig. 8 C). Since Vam2/6p is required on both partner vacuoles (Price et al. 2000), Vam2/6p complexes from apposing vacuoles may contact one another during docking, although their function may also require other associated proteins. Vam2/6p may promote trans-SNARE pairing (Fig. 8 D) largely through working together with Ypt7p to mediate tethering. In this model, Vam2/6p regulates docking through its cycle of associations. It remains on the vacuole in vivo and during standard fusion reactions, yet after priming, even dilution is sufficient to cause its release. The inability of Ypt7p, which is not released upon dilution, to anchor Vam2/6p to diluted primed vacuoles suggests that Vam2/6p is directly involved in trans interactions.
Figure 8.
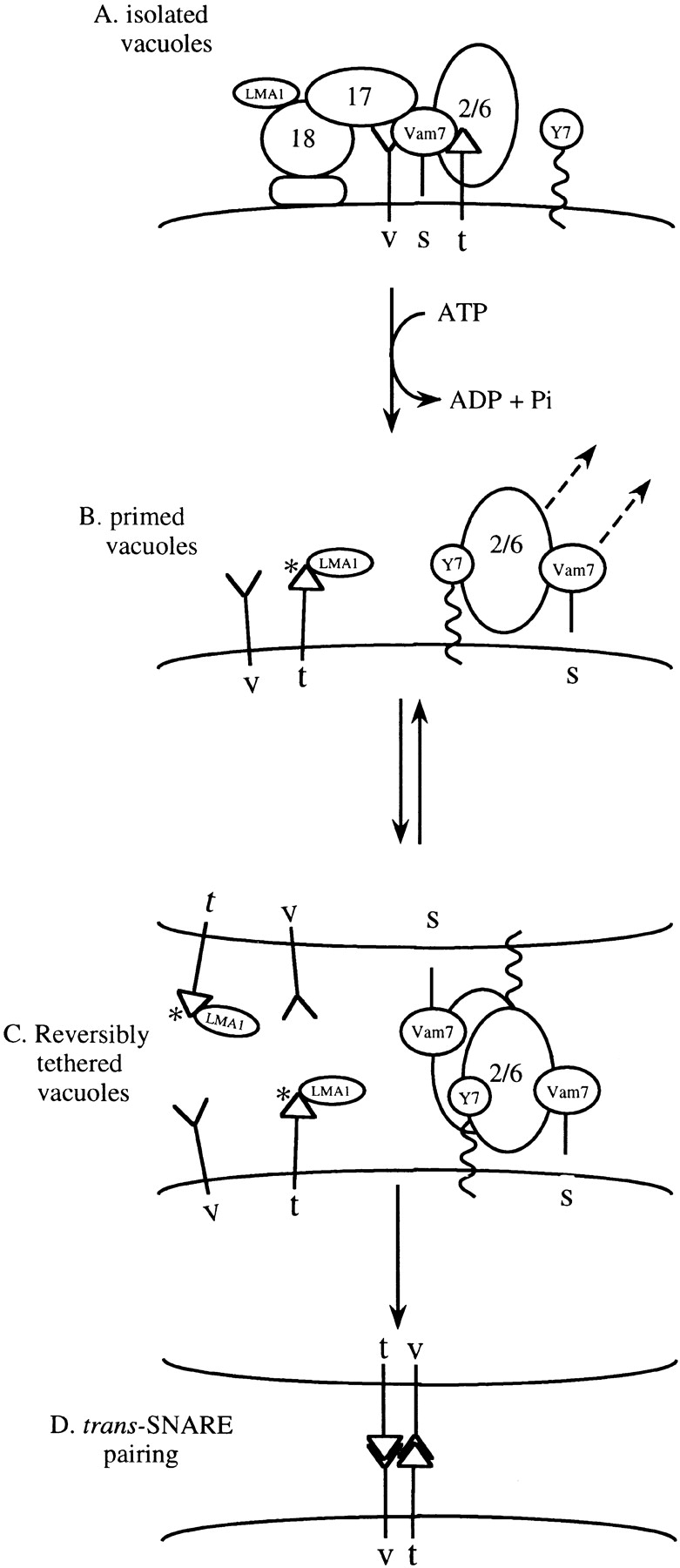
Working model for the coupling of vacuolar priming, docking, and trans-SNARE pairing. v, t, and s symbolize the v-SNAREs (Nyv1p, Vti1p, and Ykt6p), the t-SNARE Vam3p, and the SNAP-25 homologue Vam7p, respectively. Y7, Ypt7p; 2/6, Vam2/6 complex. See text for details.
Each aspect of this model (Fig. 8) is supported by our current studies. Vam2/6p is initially in a complex with SNAREs, as judged by cosedimentation (Fig. 1 B) and coimmunoprecipitation (Fig. 2). We find that priming, as it frees Vam2/6p from its association with SNAREs (Fig. 2 A), releases a 38S subcomplex (Fig. 3), which has gained an ability to associate with Ypt7p that was not seen before priming (Fig. 4 B). Functional in vitro studies are in complete accord with the transfer of Vam2/6p from association with SNAREs to Ypt7p, as Vam2/6p does not complete its function until docking (Price et al. 2000) and extraction of Ypt7p by Gdi1p, or its inhibition by anti-Ypt7p disrupts the vacuole association of Vam2/6p (Fig. 6). Finally, the absence of Ypt7p due to the deletion of its gene leads to a loss of Vam2/6p from the vacuole fraction (Fig. 5).
The 38S Vam2/6p complex from primed vacuoles will bind to GST–Ypt7p:GTPγS, as judged by recovery of Vam2p and Vam6p from the bound fraction (Fig. 4). Though little of the endogenous Ypt7p cosediments with Vam2/6p at 38S (data not shown), this may reflect a limited binding affinity and high dilution during purification that is overcome in experiments that employ glutathione–Sepharose beads with high levels of bound recombinant GST–Ypt7p. Recent studies (Seals, D., G. Eitzen, W. Wickner, and A. Price, manuscript in preparation) have shown that this bound complex, and the 65S and 38S complexes from which it derives, also include the four class C VPS proteins, Vps11p, Vps16p, Vps18p, and Vps33p. However, we cannot infer from these data which subunit or subunits of the 38S complex directly interact with Ypt7p, and which are only associated because they are part of the complex but perform Rab/Ypt effector functions. Further studies will be required to determine these functional distinctions.
Our current findings and the model that they suggest have several novel aspects. The cis-SNARE complex is seen to not just be a left-over from trans-SNARE complexes after membrane fusion is completed, but rather is embedded in a 65S complex, which has a vital signaling role, mediated by Vam2/6p, and triggered by the action of Sec18p and Sec17p. It will certainly be important to study the pathway and regulation of 65S complex assembly. Vam2/6p fulfills the definition of a Rab effector, yet it associates with a cis-SNARE complex upstream of its transfer to Ypt7p. The action of Sec18p and Sec17p is required for this transfer, providing the first functional evidence linking the priming factors with Rab/Ypt GTPases and their effectors. It will be important to determine whether the association of Rab effectors with their cognate SNAREs in other systems is with cis- or trans-SNARE complexes and whether it occurs before or after Rab association.
Vam2/6p functions on the vacuole with the Rab GTPase Ypt7p as a site-specific element for docking. It is, in this regard, functionally analogous to the plasma membrane–localized exocyst complex in yeast and mammals (Bowser et al. 1992; TerBush et al. 1996; Kee et al. 1997; Finger et al. 1998; Grindstaff et al. 1998), the cis-Golgi–localized transport protein particle (TRAPP) complex in yeast (Sacher et al. 1998), and early endosomal autoantigen 1 (EEA1) proteins in homotypic endosome–endosome fusion (Stenmark et al. 1995; Christoforidis et al. 1999; McBride et al. 1999). In addition, we propose that it functions as a link between priming and docking and suggest that other Rab/Ypt effectors may also fulfill these functions. Indeed, these complexes share a number of features. Like the TRAPP and exocyst complexes purified from cytosol (Bowser and Novick 1988; Sacher et al. 1998), the soluble Vam2/6p complex released by Gdi1p migrates at ∼20 S by gradient sedimentation (our unpublished data). We have shown biochemical interactions between the Vam2/6p complex, the SNAREs, and Ypt7p, whereas the TRAPP and exocyst complexes have been shown to interact genetically with homologous factors (Bowser et al. 1992; Sacher et al. 1998). The Rab5p effector EEA1 can also interact with NSF and with syntaxin 6, and with complexes containing syntaxin 13 (McBride et al. 1999; Simonsen et al. 1999), and thus there may be similarities to the functions of EEA1 and Vam2/6p. The yeast exocyst component Sec15p has also been shown to function with the plasma membrane Rab GTPase Sec4p (Guo et al. 1999), and Cao et al. 1998 have shown that the activity of Ypt1p is required for the proper localization of Uso1p to the cis-Golgi membrane in yeast. Like components of the TRAPP and exocyst complexes, Vam2p has homologues in other organisms, including worms, flies, plants, and mammals (Nakamura et al. 1997; Radisky et al. 1997; Warner et al. 1998). Significantly, the light gene product, the Drosophila VAM2 homologue, plays a role in protein trafficking to pigment granules, specialized lysosomes in the Drosophila eye, suggesting a conserved mode of action in lysosome biogenesis (Warner et al. 1998; Lloyd et al. 1998).
We are only beginning to learn the full structural and functional complexity of the cis-SNARE complex. The core of this and other SNARE complexes is undoubtedly composed of a four-helix bundle (Katz et al. 1998; Poirier et al. 1998; Sutton et al. 1998) with a t-SNARE, v-SNARE, and s-SNARE. However, these core elements may be insufficient for function. The vacuole 65S complex contains the cis-SNARE complex, consisting of Vam3p, Vam7p, Nyv1p, Vti1p, Ykt6p, Sec17p, Sec18p, and LMA1, and Vam2/6p itself contains additional subunits (Seals, D., G. Eitzen, W. Wickner, and A. Price, manuscript in preparation). Thus far, these multiple subunits do not appear to be redundant, as deletion or antibody inhibition of each of them (after priming, when the constituent subunits are separated from one another) has a major deleterious effect on the reaction (Nichols et al. 1997; Ungermann and Wickner 1998; Ungermann et al. 1999). We find a smaller percent of SNAREs in complex with Vam2/6p (Fig. 3 B) than the percent of Vam2/6p that is associated with SNAREs (Fig. 3 D), suggesting that there are extra cis-SNARE complexes without Vam2/6p. This fits with the observation that trans-SNARE complexes, which need Vam2/6p to form, only encompass a small percent of the SNAREs (Ungermann et al. 1998b), and with observations in other systems such as yeast plasma membrane t-SNAREs being all over the bud rather than exclusively at the fusion site at the bud tip (Brennwald et al. 1994), and in neurons where t-SNAREs are not exclusively at the synapse (Garcia et al. 1995). Vam2/6p is localized in vivo to one or two discrete spots per vacuole (Nakamura et al. 1997), suggesting that like the exocyst on the plasma membrane, the localization of this complex determines the site of membrane docking. Critical testing of these ideas must await the determination of whether the same SNAREs that are in complex with Vam2/6p are those that form the trans-SNARE pairs.
Acknowledgments
We thank Dr. Yoh Wada for providing the HA-VAM6 plasmid, Dr. Gary Eitzen for the pGST-Ypt7 construct, and Dr. Greg Payne for anti-Kex2p. We thank Drs. Charles Barlowe, Jerry Eichler, Gary Eitzen, Daniel Klionsky, Zuoyu Xu, and especially Dr. Tom Rapoport for helpful discussions, and Dr. Scott Emr for communicating unpublished results. We thank Li Wang for generously sharing preliminary results.
This research was supported by a grant from the National Institute of General Medical Sciences. C. Ungermann was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
A. Price's present address is Department of Cell Biology, Sterling Hall of Medicine, C441, Yale University School of Medicine, 333 Cedar Street, P.O. Box 208002, New Haven, CT 06520-8002.
C. Ungermann's present address is Biochemie-Zentrum Heidelberg, Im Neuenheimer Feld 328, 69120 Heidelberg, Germany.
The website for this laboratory is located at http://www.dartmouth.edu/~wickner
Abbreviations used in this paper: GST, glutathione S-transferase; HA, hemagglutinin; LMA1, low molecular weight activity #1; NSF, N-ethylmaleimide sensitive factor; SNARE, SNAP receptor; TRAPP, transport protein particle.
References
- Barnard R.J.O., Morgan A., Burgoyne R.D. Stimulation of NSF ATPase activity by α-SNAP is required for SNARE complex disassembly and exocytosis. J. Cell Biol. 1997;139:875–883. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser R., Novick P. Sec15 protein, an essential component of the exocytotic apparatus, is associated with the plasma membrane and with a soluble 19.5S particle. J. Cell Biol. 1988;112:1117–1131. doi: 10.1083/jcb.112.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser R., Muller H., Govindan B., Novick P. Sec8p and Sec15p are components of a plasma membrane-associated 19.5S particle that may function downstream of Sec4p to control exocytosis. J. Cell Biol. 1992;118:1041–1056. doi: 10.1083/jcb.118.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keranen S., Bankaitis V., Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Cao X., Ballew N., Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C.M., Grote E., Munson M., Hughson F., Novick P.J. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S., McBride H.M., Burgoyne R.D., Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Finger F., Hughes T.E., Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Garcia E.P., McPherson P.S., Chilcote T.J., Takei K., De Camilli P. rbSec1A and B colocalize with syntaxin and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J. Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff K.K., Yeaman C., Anandasabapathy N., Hsu S.-C., Rodriguez-Boulan E., Scheller R.H., Nelson W.J. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. Meth. Cell Sci. 1995;17:283–294. [Google Scholar]
- Haas A., Scheglemann D., Lazar T., Gallwitz D., Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.I., Otto H., Barton N., Jahn R. The N-ethylmaleimide-sensitive fusion protein and α-SNAP induce a conformational change in syntaxin. J. Biol. Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- Harlowe E., Lane D. AntibodiesA Laboratory Manual 1988. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: pp. 726 pp [Google Scholar]
- Katz L., Hanson P.I., Heuser J.E., Brennwald P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y., Yoo J.-S., Hazuka C.D., Peterson K.E., Hsu S.-C., Scheller R.H. Subunit structure of the mammalian exocyst complex. Proc. Natl. Acad. Sci. USA. 1997;94:14438–14443. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd V., Ramaswami M., Kramer H. Not just pretty eyesDrosophila eye-color mutations and lysosomal delivery. Trends Cell Biol. 1998;8:289–291. doi: 10.1016/s0962-8924(98)01270-7. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J. Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner W., Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McBride H.M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and Syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Hirata A., Ohsumi Y., Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae . J. Biol. Chem. 1997;272:11344–11349. doi: 10.1074/jbc.272.17.11344. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann C., Pelham H.R.B., Wickner W.T., Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Peters C., Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews P.D., Stark M.J.R., Cesaro-Tadic S., Glatz A., Podtelejnikov A., Mann M., Mayer A. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science. 1999;285:1084–1087. doi: 10.1126/science.285.5430.1084. [DOI] [PubMed] [Google Scholar]
- Pfeffer S.R. Transport vesicle dockingSNAREs and associates. Annu. Rev. Cell Dev. Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Poirier M.A., Xiao W., Macosko J.C., Chan C., Shin Y.K., Bennett M.K. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Price A., Wickner W., Ungermann C. Proteins needed for vesicle budding from the Golgi are also required for the docking step of homotypic vacuole fusion. J. Cell Biol. 2000;148:1223–1229. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky D.C., Snyder W.B., Emr S.D., Kaplan J. Characterization of VPS41, a gene required for vacuolar trafficking and high-affinity iron transport in yeast. Proc. Natl. Acad. Sci. USA. 1997;94:5662–5666. doi: 10.1073/pnas.94.11.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sacher M., Jiang Y., Barrowman J., Scarpa A., Burston J., Zhang L., Schieltz D., Yates J.R., Abeliovich H., Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Gaullier J.-M., D'Arrigo A., Stenmark H. The Rab5 effector EEA1 interacts directly with Syntaxin 6. J. Biol. Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M.K., Whiteheart S.W., Scheller R.H., Rothman J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Stack J.H., Horazdovsky B., Emr S.D. Receptor-mediated protein sorting to the vacuole in yeast. Annu. Rev. Cell Dev. Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer D., Jahn R., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Wickner W. Vam7p, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Nichols B.J., Pelham H.R.B., Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion J. Cell Biol. 140 1998. 61 69a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato K., Wickner W. Defining the functions of trans-SNARE pairs Nature. 396 1998. 543 548b [DOI] [PubMed] [Google Scholar]
- Ungermann C., Fischer von Mollard G., Jensen O.N., Margolis N., Stevens T.H., Wickner W. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic vacuole fusion. J. Cell Biol. 1999;145:1435–1442. doi: 10.1083/jcb.145.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Ohsumi Y., Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- Warner T.S., Sinclair D.A.R., Fitzpatrick K.A., Singh M., Devlin R.H., Honda B.M. The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome. 1998;41:236–243. [PubMed] [Google Scholar]
- Xu Z., Sato K., Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]