Abstract
Vam2p/Vps41p is known to be required for transport vesicles with vacuolar cargo to bud from the Golgi. Like other VAM-encoded proteins, which are needed for homotypic vacuole fusion, we now report that Vam2p and its associated protein Vam6p/Vps39p are needed on each vacuole partner for homotypic fusion. In vitro vacuole fusion occurs in successive steps of priming, docking, and membrane fusion. While priming does not require Vam2p or Vam6p, the functions of these two proteins cannot be fulfilled until priming has occurred, and each is required for the docking reaction which culminates in trans-SNARE pairing. Consistent with their dual function in Golgi vesicle budding and homotypic fusion of vacuoles, approximately half of the Vam2p and Vam6p of the cell are recovered from cell lysates with purified vacuoles.
Keywords: Vps41/Vam2p, Vps39/Vam6p, priming, NSF/Sec18p, αSNAP-Sec17p
Introduction
The fusion of cellular membranes during secretion, endocytosis, and organelle inheritance is essential for cellular compartmentation. Highly conserved proteins are involved in fusion events throughout the cell and across species. These include soluble NSF attachment protein receptors (SNAREs), the ATP-driven chaperone NSF/Sec18p and its partner proteins αSNAP/Sec17p, and a large family of Rab GTPases. The pairing in trans of cognate v- (vesicle) and t- (target membrane) SNAREs is a central event in docking membranes before fusion (Rothman 1994; Hay and Scheller 1997). This pairing is regulated by the prior priming action of NSF/Sec18p and SNAP/Sec17p, which prepares the SNAREs for docking, and by Rab GTPases (Mayer and Wickner 1997; Novick and Zerial 1997). The relationship of the structurally diverse Rab effectors and tethering factors (Pfeffer 1996; TerBush et al. 1996; Cao et al. 1998; McBride et al. 1999) to docking is currently studied for several trafficking reactions.
We have studied the homotypic fusion of yeast vacuoles. It occurs in the ordered stages of priming, docking, and bilayer fusion. During priming, individual vacuoles are prepared for interaction with other vacuoles. The starting vacuoles contain a cis complex of SNAREs bound together on the same vacuole. This cis-SNARE complex includes a t-SNARE (Vam3p), v-SNAREs (Nyv1p, Vti1p, and Ykt6p), an s-SNARE (Vam7p, which is a homologue of the synaptic SNAP-23/25 protein), an α-SNAP (Sec17p; Haas et al. 1995; Haas and Wickner 1996; Nichols et al. 1997; Ungermann and Wickner 1998; Ungermann et al. 1999a), as well as a novel chaperone (LMA1; Barlowe 1997; Xu et al. 1997, Xu et al. 1998). In priming, the action of Sec17p, Sec18p, and LMA1 disassembles the cis-SNARE complex. Sec17p is released, the t-SNARE is activated, and LMA1 stabilizes the primed t-SNARE (Mayer et al. 1996; Ungermann et al. 1998; Xu et al. 1998). The primed vacuoles come into contact during docking. The initial tethering stage of docking is reversible and independent of SNAREs, but requires Ypt7p, a small GTP-binding protein of the Rab family (Ungermann et al. 1998). The interaction between vacuoles becomes irreversible through the association of SNARE proteins from apposed vacuoles, forming a trans-SNARE complex. trans-SNARE pairing triggers the release of lumenal calcium to interact with calmodulin and mediate downstream events that lead to fusion (Peters and Mayer 1998). These events include the release of LMA1 from the t-SNARE, which is regulated by a phosphatase-kinase pair (Xu et al. 1998).
Mutations in the genes encoding many of the above proteins, which were shown biochemically to catalyze each stage of the fusion reaction, cause severe vacuole fragmentation in the intact cell, presumably reflecting a failure of vacuole fusion. Type II vacuole morphology (vam) mutants were obtained in a nonselective screen for just such a highly fragmented vacuole morphology. Since Vam3p, Vam4/Ypt7p, and Vam7p are each involved in the reaction, we examined whether Vam2p and Vam6p are also required. These proteins are required for normal vacuole morphology (Wada et al. 1992; Nakamura et al. 1997; Zheng et al. 1998), for protein sorting to the vacuole (Raymond et al. 1992; Cowles et al. 1997; Rehling et al. 1999) and for cytosol-to-vacuole protein targeting (Harding et al. 1995). Though Vam2p and Vam6p are reported to be localized to the vacuole (Wada et al. 1992) and to be associated in a large complex (Nakamura et al. 1997), Vps41p/Vam2p also fulfills a specific role in transport vesicle budding from the Golgi (Rehling et al. 1999,), where it interacts with the AP3 coat. We now report that approximately half of the Vam2p and Vam6p of the cell copurifies with vacuoles, and that it is required for the docking stage of homotypic vacuole fusion and for the formation of stable trans-SNARE pairs.
Materials and Methods
Yeast Strains and Genetic Manipulations
Saccharomyces cerevisiae strains BJ3505 (MATa pep4::HIS3 prb1-Δ1.6R HIS3 lys2-208 trp1-D101 ura3-52 gal2 can) and DKY 6281 (MATa leu2-3 leu2-112 ura3-52 his3-D200 trp1-D901 lys2-801 suc2-D9 pho8::TRP1) were used. The VAM2 and VAM6 genes were disrupted in S. cerevisiae BJ3505 by recombination with PCR-generated cassettes containing 5′ and 3′ homologous regions of either VAM2 or VAM6 and the URA3 gene of S. cerevisiae. The PCR primers contained 40 bases of identity to the regions flanking the open reading frame (VAM6 5′ primer sequence: 5′ CAG CAA AAA CCC TTC AAA ATA TCA ATT TAT ACC AAA AAT TAA GAT TCC GGT TTC TTT GAA AT 3′; VAM6 3′ primer sequence 5′ ATA AGA AAT ACT AAC AAC AAT AAC AGC AGC TGT TAA GGG ATC TCT AAT TTG TGA GTT TAG TAT AC 3′; VAM2 5′ primer sequence: 5′ AAA GCA TTT TAA CGA AGA GTA TAT ACC TAC TAT TAG ACA TTA GAT TCC GGT TTC TTT GAA AT 3′; and VAM2 3′ primer sequence 5′ TGA AGT GTA CAC TTG CCT TGT GTA TTA AAT GAT GAT TCG ATA TCT AAT TTG TGA GTT TAG TAT AC 3′). Transformants were screened for the expected deletion by PCR using primers that anneal to the promoter region of the VAM2 or VAM6 genes (VAM2 primer sequence 5′ GGG CTA TTG AGA CTGV TTG 3′; and VAM6 primer sequence 5′ TAG GTT TAC GGC TGT TCA A 3′), the coding region of the URA3 gene (5′CCC AAT GCG TCT CCC TT 3′), and isolated genomic DNA from the selected clones by PCR (Ausubel et al. 1995). Deletions were confirmed by SDS-PAGE immunoblotting (Harlow and Lane 1988) with antibodies to Vam2p and Vam6p.
Biochemical Reagents
All inhibitors and antibodies were dissolved in or dialyzed into PS buffer (20 mM Pipes-KOH, pH 6.8, 200 mM sorbitol) unless stated otherwise.
Antibody Production and Purification
Antibodies against the Vam2 and Vam6 proteins were raised in rabbits using recombinant His6-tagged proteins purified from Escherichia coli. The coding sequences for S. cerevisiae VAM2 and VAM6 were PCR amplified (VAM2 5′ primer: 5′σ AGT ACC GGA TCC ATG ACT ACA GAT AAT CAT 3′; VAM2 3′ primer: 5′ AGT GTT GGT ACC TTA TAA ACA CCA TTT AA 3′; VAM6 5′ primer: 5′ AGT GTT GGT ACC TTA CTT ATT ATT TAG GTC 3′; and VAM6 3′ primer: 5′ AGT ACC GGA TCC ATG TTA AGA GCT CAA AAG 3′) and cloned into pQE30 (Qiagen). Antibodies were affinity-purified using columns of recombinant Vam2 or Vam6 proteins coupled to Affi-Gel (Bio-Rad Laboratories) and dialyzed into PS buffer (20 mM Pipes-KOH, pH 6.8, 200 mM sorbitol). Fab fragments of anti-Vam2 were prepared by papain digestion (ImmunoPure Fab kit; Pierce Chemical Co.), affinity-purified as described above, and dialyzed into PS buffer. Antibodies against Ypt7p (Haas et al. 1995), Sec18p (Haas and Wickner 1996), and Vam3p (Nichols et al. 1997) were purified as described previously and dialyzed into PS buffer.
Vacuole Fusion Assay
Vacuole isolation and fusion assays were performed as described previously (Haas 1995).
Results
Vam2p and Vam6p Are Required on both Partner Vacuoles for Fusion
Vam2/Vps41p and Vam6/Vps39p, fused to green fluorescent protein, are localized to the vacuole in vivo (Wada et al. 1992) and, yet, Vam2p is also found associated with AP-3 coat adapter subunits on the Golgi and is required there for vesicle budding (Rehling et al. 1999). To determine whether the proportion of Vam2p and Vam6p recovered with the vacuoles was consistent with vacuolar and/or nonvacuolar localizations, we purified vacuoles from cell lysates and compared the recovery of Vam2p and Vam6p with that of Pho8p, an established vacuolar marker protein. We find that 45% of the Vam2p and 54% of the Vam6p are recovered in our purified vacuoles, after correction for the loss of 23% of the vacuole marker Pho8p during isolation (Fig. 1). The presence of Vam2p and Vam6p in this fraction is not due to contaminating Golgi, as <5% of the late Golgi marker Kex2p is recovered with the vacuoles (Fig. 1). Thus, half of the Vam2p and Vam6p is localized to the vacuole while the rest is elsewhere in the cell, in accordance with both Wada et al. 1992 and Rehling et al. 1999.
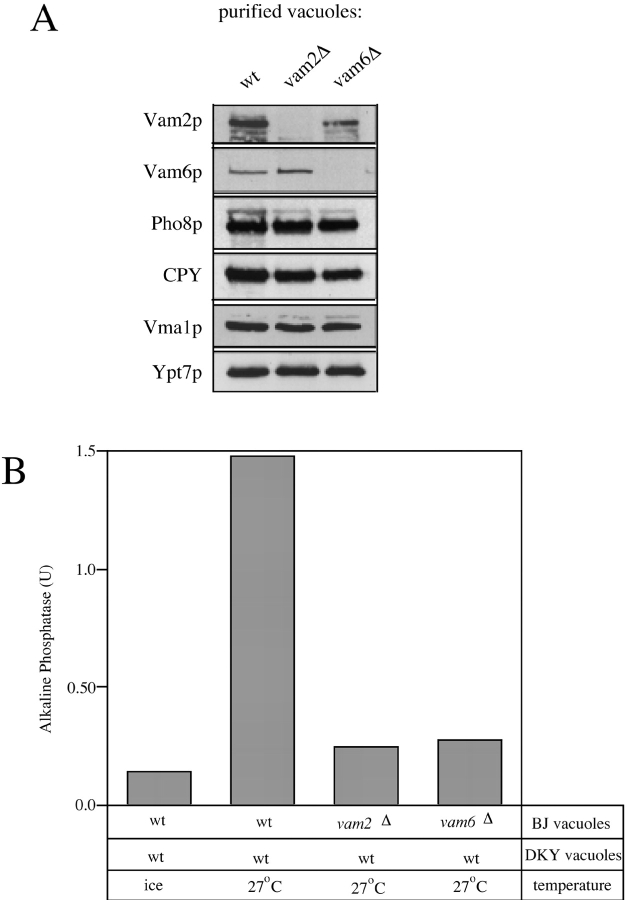
Figure 1.
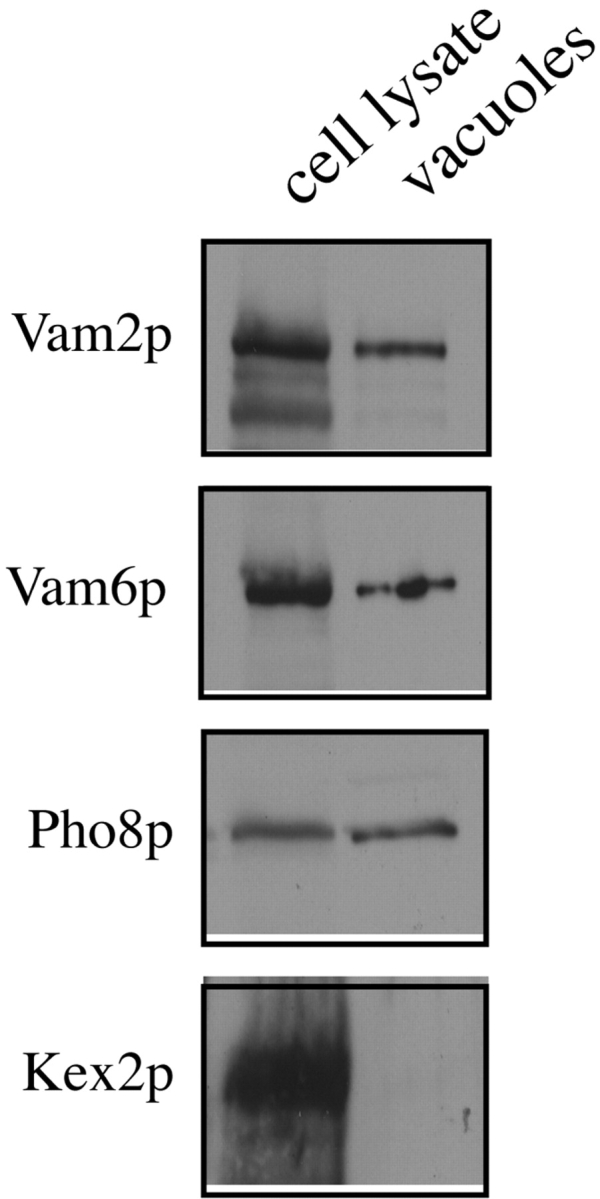
Vam2p and Vam6p are associated with purified vacuoles. A BJ3505 wild-type culture was processed for vacuole purification (Haas 1995). An aliquot of spheroplasted yeast cells was lysed by boiling in SDS-PAGE loading buffer, and the remaining spheroplasts were carried through the vacuole purification. Equivalent proportions of spheroplasts and purified vacuoles (0.1% of the total preparation) were analyzed by SDS-PAGE and immunoblotting with antisera against the indicated proteins. Recovery of proteins in the purified vacuoles was determined by scanning densitometry (see text).
To investigate the functions of Vam2p and Vam6p, we have employed an in vitro assay of vacuole fusion. Vacuoles are purified from two S. cerevisiae strains bearing deletions in either the PEP4 gene or the PHO8 gene. Fusion of these vacuoles exposes pro-alkaline phosphatase from one vacuole population to its activating protease from the other, resulting in the production of enzymatically active alkaline phosphatase, which is assayed spectrophotometrically. The VAM2 and VAM6 genes were deleted in one of our vacuole fusion tester strains, BJ3505. Although mutations in VAM2 and VAM6 affect the rate of trafficking of proteins to the vacuole in vivo (Nakamura et al. 1997; Radisky et al. 1997), the steady state levels of the vacuolar marker proteins Pho8p, CPY, and Vma1p are unaffected in our purified vacuole preparations (Fig. 2 A). Thus, whereas these vacuoles are smaller than wild type (Nakamura et al. 1997) and are often recovered with somewhat lower yield, they contain the normal complement of vacuolar proteins. However, when normal amounts (assayed by Bradford protein determination) of vacuoles derived from BJ3505 vam2Δ or vam6Δ strains are combined with wild-type DKY6281 partner vacuoles in standard fusion reactions, the resulting fusion is <10% of that seen with wild-type vacuoles (Fig. 2 B). Since these vacuoles have normal levels of vacuole marker proteins (Fig. 2 A), except for Vam2p or Vam6p, and since small vacuole size alone is not a hindrance to fusion (see Fig. 4 b in Nichols et al. 1997), the simplest inference is that Vam2p and Vam6p are directly involved in fusion.
Figure 2.
Vacuole fusion requires Vam2p and Vam6p. (A) Protein composition of vacuoles. Vacuolar protein (15 μg) from wild-type, vam2Δ, and vam6Δ strains was analyzed by SDS-PAGE and immunoblotting with antisera to the indicated vacuolar proteins. (B) In vitro fusion of vacuoles from wild-type, vam2Δ, and vam6Δ strains. Standard vacuole fusion reactions (see Materials and Methods) were performed with BJ3505 wild-type, vam2Δ, or vam6Δ vacuoles combined with wild-type DKY6281 vacuoles.
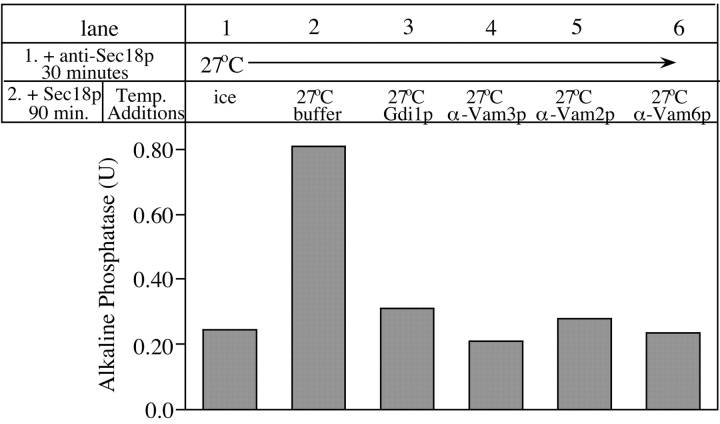
Figure 4.
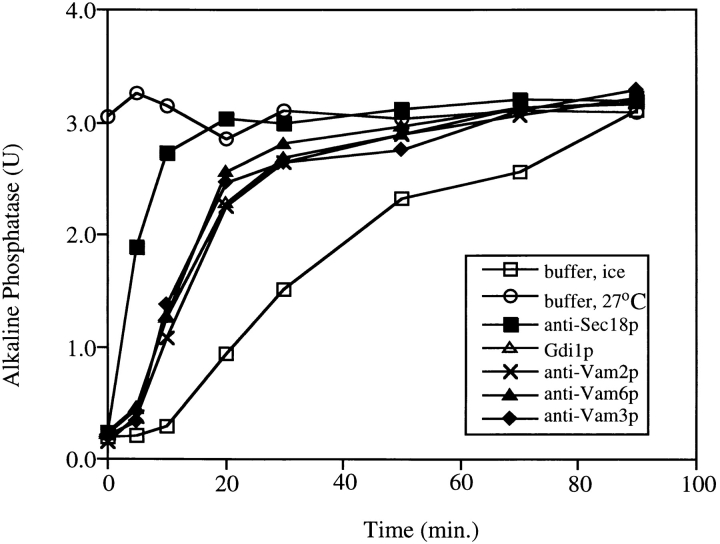
Kinetics of acquisition of resistance to Gdi1p and antibodies to Vam3p, Vam2p, and Vam6p. Aliquots (30 μl) were removed from 10× scale fusion reactions at the indicated times and added to control buffer on ice, buffer at 27°C, Gdi1p (64 μg/ml), or antibodies against Sec18p, Vam3p, Vam2p, or Vam6p at 27°C. Fusion reactions were incubated for a total of 90 min at 27°C before being assayed for alkaline phosphatase activity.
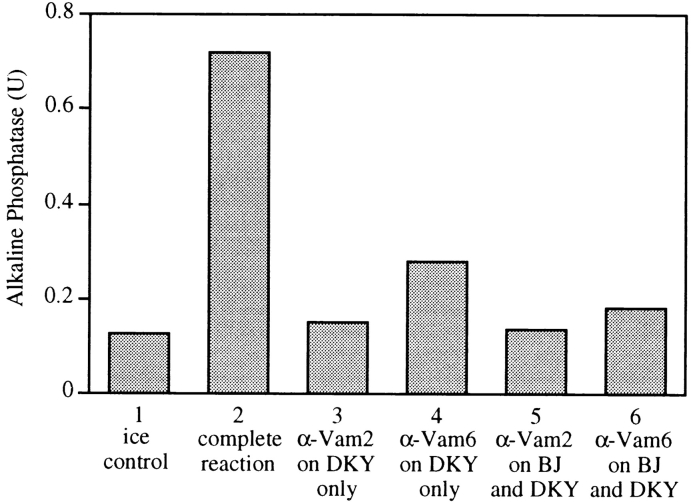
Affinity-purified antibodies to Vam2p or Vam6, or their respective Fab fragments, efficiently inhibited in vitro vacuole fusion, whereas preimmune IgG did not (data not shown). Since fusion is compromised when VAM2 or VAM6 are deleted on only one vacuole fusion partner (Fig. 2 B), we tested whether antibodies to Vam2p or Vam6p inhibit fusion if added to only one partner vacuole. Wild-type DKY6281 vacuoles were incubated on ice with antibody to either Vam2p or Vam6p and reisolated to remove unbound antibody. These vacuoles were combined with untreated or antibody-treated BJ3505 vacuole partners in standard fusion reactions. Treatment of only one vacuole type with antibody against Vam2p or Vam6p inhibited fusion (Fig. 3, lanes 3 and 4) to the same extent as seen when both fusion partners were treated (Fig. 3, lanes 5 and 6). Thus, homotypic vacuole fusion requires Vam2p and Vam6p on both fusion partners.
Figure 3.
Active Vam2p and Vam6p are required on both vacuole fusion partners. BJ3505 or DKY6281 vacuoles were mixed with buffer or antibodies against Vam2p or Vam6p. After 10 min, at 0°C, vacuoles were diluted 20-fold with PS buffer and reisolated to remove unbound antibody. Vacuole fusion partners were combined in standard fusion reactions supplemented with cytosol (30 μg/ml).
Vam2p and Vam6p Are Required for Docking Sec18-primed Vacuoles
Previous studies of vacuole fusion in vitro have defined three distinct reaction stages. First, priming requires the action of Sec17p and Sec18p (Mayer et al. 1996). Second, docking requires primed vacuoles, v- and t-SNAREs, and Ypt7p (Mayer and Wickner 1997; Ungermann and Wickner 1998). Third, fusion no longer requires trans-SNARE pairs (Ungermann et al. 1998) but needs calmodulin (Peters and Mayer 1998) and protein phosphatase 1 (Peters et al. 1999). To determine the stages where Vam2p and Vam6p act, aliquots were removed from vacuole fusion reactions at various times and mixed with buffer or inhibitors, or placed on ice, and the incubation was continued until all samples had been incubated for 90 min (Fig. 4). In contrast to the inhibition by anti-Sec18p (closed squares), which is only seen at early reaction times, resistance to anti-Vam2p (crosses) and anti-Vam6p (closed triangles) is achieved with intermediate kinetics that correspond to the docking stage inhibitors Gdi1p (open triangles) or the antibody to the t-SNARE Vam3p (diamonds; Mayer and Wickner 1997; Ungermann et al. 1998). Resistance to anti-Vam2p and anti-Vam6p is achieved before fusion itself (open squares), suggesting that these factors are not needed for the fusion stage of the reaction. These proteins are also not involved in priming, since antibodies to Vam2p and Vam6p have no effect on Sec17p release from the vacuoles (data not shown), indicating that these components are not involved in priming (Mayer et al. 1996).
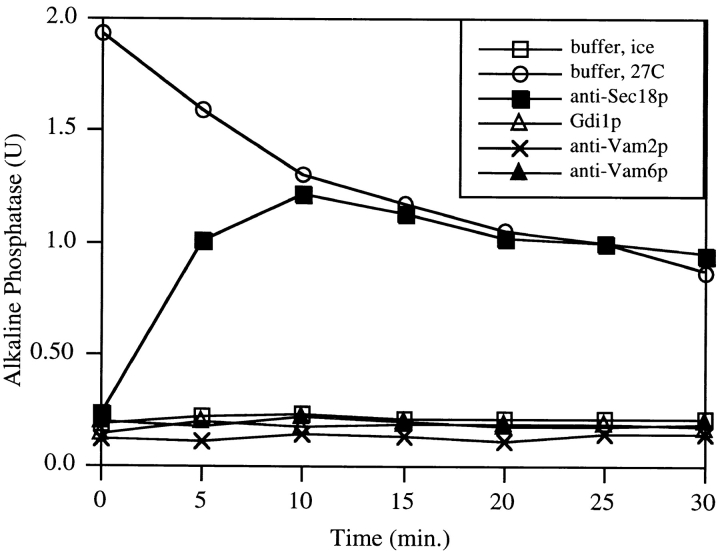
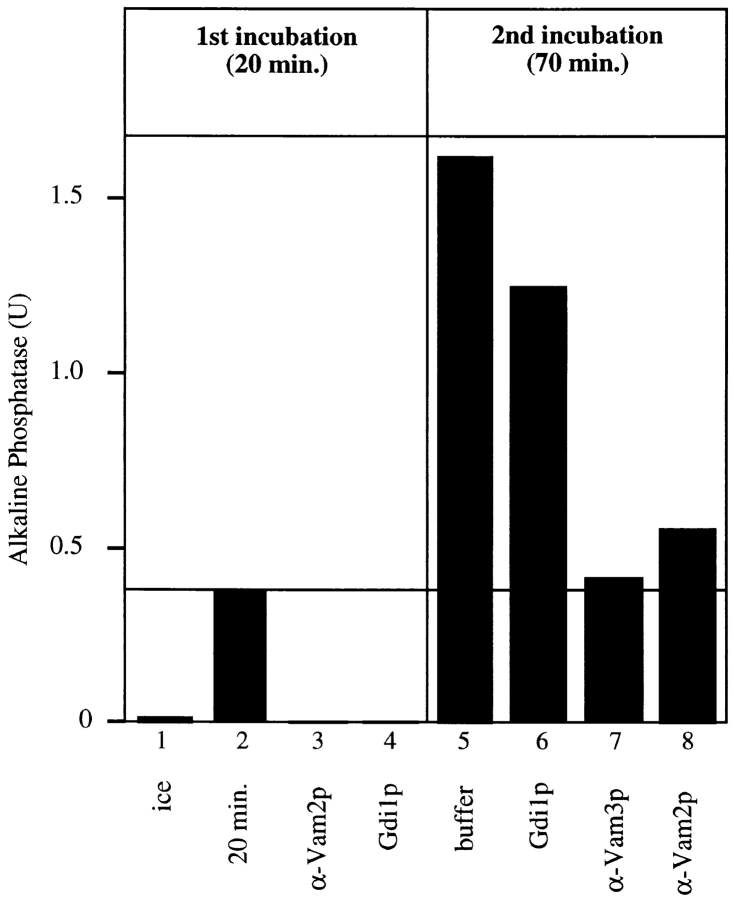
To further investigate the role of Vam2p and Vam6p in docking, fusion reactions without inhibitors were started with the partner vacuoles in separate tubes. At various times, aliquots of each preincubated vacuole population were combined with inhibitors and with each other, and the reaction was continued for 70 min (Fig. 5). Resistance to anti-Sec18p (closed squares) is achieved early, confirming that the priming subreaction can occur when vacuoles are separated (Mayer et al. 1996). However, as with an inhibitor of Ypt7p (open triangles), resistance to anti-Vam2p (crosses) and anti-Vam6p (closed triangles) is never achieved when the vacuole partners are in separate tubes. Therefore, the action of Vam2p and Vam6p requires contact between vacuole fusion partners and indicates a role in docking.
Figure 5.
Completion of Vam2p and Vam6p function requires vacuole docking. 10× scale reactions with vacuoles from either BJ3505 or DKY6281 were incubated at 27°C in the presence of ATP. At the indicated times, 15-μl aliquots were removed from each reaction and combined in the presence of control buffer on ice, control buffer at 27°C, Gdi1p, antibody to Vam3p, Vam2p, or Vam6p at 27°C, incubated for 70 min at 27°C, and analyzed for alkaline phosphatase activity.
Homotypic vacuole fusion requires the early action of Sec18p to prime the vacuoles for subsequent docking (Mayer et al. 1996; Mayer and Wickner 1997). To determine whether priming is a prerequisite for the action of Vam2p and Vam6p, a two-stage experiment was performed in which the activity of Sec18p was inhibited by anti-Sec18p antibody in the first incubation. After 30 min, a time which otherwise suffices for the acquisition of resistance to Gdi1p or to antibodies to Vam3p, Vam2p, or Vam6p (Fig. 4), Sec18p activity was restored by the addition of Sec18 protein, and the reaction was continued in the presence or absence of inhibitors. Fusion is restored by the addition of Sec18p in the second incubation (Fig. 6, lane 2). However, as with the docking inhibitors Gdi1p and anti-Vam3p, the reaction remains fully sensitive to anti-Vam2p and anti-Vam6p (Fig. 6, lanes 3–6). Thus, Sec18p must act before Vam2p and Vam6p can fulfill their functions.
Figure 6.
The action of Sec18p must precede that of Vam2p and Vam6p. A 10× scale fusion reaction with affinity-purified antibody against Sec18p was incubated at 27°C for 30 min. The vacuoles were reisolated and resuspended in the original volume of reaction buffer containing 25 μg/ml Sec18p and divided into 30-μl aliquots. Where indicated, Gdi1p or antibodies against Vam3p, Vam2p, or Vam6p were added. The incubations were continued at 27°C for 90 min and analyzed for alkaline phosphatase activity. The background activity of 0.22 U (a sample transferred to ice after the first 30-min incubation) was subtracted.
We have previously reported that docking can be divided into the stages of Ypt7p-dependent tethering, followed by trans-SNARE pairing. These stages are distinguished by the addition of an excess of Sec18p to vacuole fusion reactions, which allows Ypt7p to complete its function while preventing stable trans-SNARE pairing (Ungermann et al. 1998). To examine the role of Vam2/6p in docking, vacuoles were incubated under standard fusion conditions for 20 min, but with an excess of Sec18p, and an aliquot was assayed for the fusion that had occurred (Fig. 7, lane 2). Other portions were sedimented to remove the excess Sec18p and resuspended in buffer alone (lane 5) or with other fusion inhibitors (Fig. 7, lanes 6–8) for a second, 70-min incubation. Whereas the reaction had become resistant to Gdi1p (lane 6), it was still fully sensitive to antibody to Vam3p (lane 7) or to Vam2p (lane 8), suggesting that Vam2/6p may complete its function after Ypt7p but before SNAREs pair in trans.
Figure 7.
Vam2/6p continues to function after Ypt7p. Standard fusion reactions were supplemented with cytosol and excess Sec18p (3.8 μg) to prevent SNARE pairing and incubated on ice (lane 1) or at 27°C for 20 min. After 20 min, one reaction was placed on ice (lane 2), the others received inhibitors or antibodies and were placed on ice for 3 min. Reactions were diluted with 500 μl PS buffer. Vacuoles were sedimented (for 5 min, 10,000 g, at 4°C) and resuspended in the original volume of reaction buffer (without cytosol and Sec18p). The indicated inhibitors were added and the reaction continued for 70 min at 27°C. Fusion activity was measured and background activity (0.25 U) was subtracted.
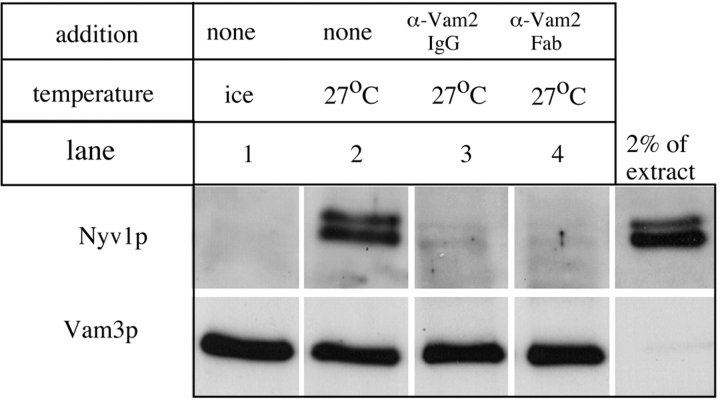
To directly assay whether Vam2/6p is needed, after priming and during docking, for the formation of trans-SNARE pairs between the t-SNARE Vam3p on one vacuole with the v-SNARE Nyv1p on another vacuole, we incubated vacuoles from a vam3Δ strain with vacuoles from a nyv1Δ strain, exploiting the fact that neither initial vacuole population can have Vam3p:Nyv1p complexes (Ungermann et al., 1998b). As previously shown (see Fig. 5 C in Ungermann et al., 1998b), the SNARE complexes that form at 27°C, and are assayed by the coprecipitation of Nyv1p by antibody to Vam3p, are truly in trans (i.e., between apposed vacuoles), as their formation is not blocked by Microcystin-LR, which is a potent inhibitor of fusion. We find that the formation of trans-SNARE pairs in such an assay (Fig. 8, lane 2) is blocked by antibody to Vam2p (lane 3) or by its monovalent Fab fragment (lane 4), confirming that Vam2/6p is required for the completion of docking.
Figure 8.
Vam2/6p is required for trans-SNARE pairing. The formation of trans-SNARE pairs between vacuoles was assayed as described in Ungermann et al. 1998. Vacuoles from strains BJ3505nyv1Δ and DKY6281vam3Δ (100 μg each) were incubated together at 27°C (lanes 2–4) or on ice (lane 1) with cytosol and ATP for 30 min. Reactions were supplemented with control buffer (lanes 1 and 2), affinity-purified anti-Vam2p IgG (lane 3), or affinity-purified anti-Vam2p Fab fragment (lane 4). Protein complexes were analyzed by coimmunoprecipitation with protein A–immobilized antibodies to Vam3p, SDS-PAGE, and immunoblotting with antibodies to Vam3p and Nyv1p.
Discussion
Vam2p and Vam6p are components of a multifunctional complex that is required both for protein trafficking to the vacuole (Raymond et al. 1992; Harding et al. 1995; Cowles et al. 1997; Nakamura et al. 1997) as well as for homotypic vacuole–vacuole fusion. Surprisingly, recent findings have suggested a novel function for Vam2/Vps41p. Emr and colleagues (Rehling et al. 1999) have shown that this protein is required for the formation of vesicular transport intermediates of the vacuole ALP (alkaline phosphatase) pathway at the trans-Golgi. They further demonstrated that Vam2/Vps41p interacts with components of a clathrin coat adapter complex by binding directly to the AP-3 subunit Apl5. On the other hand, we find that Vam2 and its partner protein Vam6 (Nakamura et al. 1997; Price et al., accompanying manuscript) are required for the docking stage of homotypic vacuole fusion. The fragmented vacuole phenotype seen in vam2 and vam6 mutants is identical to that seen in mutants of the small G protein Vam4/Ypt7p and the vacuolar t-SNARE (Wada et al. 1992; Haas et al. 1995; Nakamura et al. 1997; Nichols et al. 1997). Each of these proteins has been shown biochemically to act at the docking stage of the homotypic vacuole fusion reaction. Vam2p and Vam6p localize to the vacuole in vivo (Nakamura et al. 1997); additional localization to the Golgi or Golgi-derived vesicles might have been difficult to visualize. Vam2p and Vam6p presumably use different receptors at the Golgi and vacuole, which is consistent with the findings that vacuolar Ypt7p is needed for optimal steady state association of Vam2p and Vam6p at the vacuole (Price et al., 2000 [this issue]) and, yet, normal budding of trafficking vesicles from the Golgi (Rehling et al. 1999) may not require Ypt7p. We have shown that approximately half of the Vam2 and Vam6 copurify with vacuoles (when compared with the vacuole marker protein Pho8p), whereas Kex2p, a trans-Golgi marker protein, does not (Fig. 1). In the accompanying manuscript (Price et al., 2000 [this issue]), we demonstrate that both Vam2p and Vam6p are physically and functionally associated with factors specifically required for homotypic vacuole fusion. Except for the study of Springer and Schekman 1998, which showed that SNARE proteins nucleate COPII budding from the yeast ER, there is little precedent for such an unusual dual function in both vesicle budding and membrane docking. This raises the fascinating possibility that Vam2/6p may couple these processes in vivo. Further work will be required to test this idea.
Since many of the same proteins are needed for heterotypic trafficking to the vacuole and for homotypic vacuole fusion, the recovery of organelles with vacuole density from lysates of vam2Δ, vam6Δ, vam3Δ, and ypt7Δ strains, and the demonstration that these organelles contain vacuole marker proteins at approximately normal specific activities, raises a question of their identity and origins. They may be small, but rather normal, vacuoles that have obtained most of their proteins by slow, or even bypass, pathways and are small because of defective homotypic fusion. Alternatively, they may represent an earlier organelle on the Golgi to vacuole pathways that has expanded because of accumulated vacuole proteins until it acquires certain vacuole characteristics such as density. Further studies are needed to resolve this yet, in either case, assay of the capacity of this organelle to fuse with vacuoles from wild-type cells provides one measure of the role of the missing protein in fusion pathways. Other complementary assays are inhibition of the homotypic fusion of wild-type vacuoles by the relevant antibody and, as detailed for Vam2p and Vam6p in the accompanying manuscript (Price et al., 2000), direct demonstration of physical and functional associations with other catalysts of vacuole fusion such as Ypt7p and SNAREs.
How general is the yeast vacuole and the role of Vam2p and Vam6p in its fusion as a model for priming and docking in other vesicle trafficking pathways? Yeast vacuoles require priming before docking (Mayer and Wickner 1997) and, in this regard, might be thought to differ, at least superficially, from other trafficking reactions. For example, the priming of SNAREs by NSF and SNAP is not needed for docking at the neural synapse in Drosophila (Littleton et al. 1998) and Cao et al. 1998 have shown that the docking of ER-derived vesicles at the Golgi requires Uso1p but not the priming of SNAREs by Sec18p. However, we suggest that there is a fundamental unity in these three systems. Vacuole tethering is mediated by Ypt7p, but it is not mediated by the pairing of SNAREs in trans (Ungermann et al. 1998). Vacuoles from a vam3Δ strain, in which the SNAREs are not associated in cis with each other (Ungermann and Wickner 1998), can also tether without priming (Ungermann et al. 1998), as in the studies of Cao et al. 1998. Furthermore, since synaptic transmission is sensitive to some proteolytic toxins that only cleave unpaired SNAREs (Otto et al. 1997) and attachment of vesicles to the presynaptic membrane is unaffected by SNARE mutants (Broadie et al. 1995), the morphologically docked state at the synapse may correspond to tethering but not to complete trans-SNARE pairing. We propose that the disassembly of v- and t-SNAREs after heterotypic fusion and the sorting of the v- and t-SNAREs at the start of v-SNARE retrograde traffic may spatially disconnect the priming activity of Sec18p/NSF from docking, whereas these events are necessarily linked in homotypic fusion reactions.
The docking process is now known to require an unexpected variety of factors. These include t- and v-SNAREs on apposed vacuoles (Nichols et al. 1997), the Rab-like Ypt7p on both vacuoles (Haas et al. 1995), vacuolar phosphatidylinositol 4,5-bisphosphate (Mayer et al. 2000), vacuole acidification (Ungermann et al. 1999b), and now Vam2p and Vam6p. The relationships between these factors are not clear. However, in an accompanying paper, we show that a complex that contains Vam2p and Vam6p functions as an effector complex for Ypt7p, linking membrane priming to trans-SNARE pairing. Our finding (Fig. 7) that Vam2p is still needed even after Ypt7p has completed its function suggests that Vam2p might function between Ypt7p-mediated tethering and trans-SNARE pairing. This result is consistent with the finding that Vam2p is needed for trans-SNARE pairing (Fig. 8), but further studies will be needed to test this hypothesis.
Acknowledgments
We thank Dr. Yoh Wada (University of Tokyo, Tokyo, Japan) for providing the HA-Vam6 plasmid. We thank Drs. Charles Barlowe, Jerry Eichler, Gary Eitzen, Daniel Klionsky, Darren Seals, Zuoyu Xu, and especially Dr. Tom Rapoport for helpful discussions, and Dr. Scott Emr (University of California, San Diego, San Diego, CA) for communicating unpublished results. We thank Li Wang for sharing preliminary results.
This research was supported by a grant from the National Institute of General Medical Sciences. C. Ungermann was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
The website for this laboratory is located at http://www.dartmouth.edu/~wickner
The present address of A. Price is Department of Cell Biology, Sterling Hall of Medicine, C441, Yale University School of Medicine, 333 Cedar Street, PO Box 208002, New Haven, CT 06520-8002.
The present address of C. Ungermann is Biochemie-Zentrum Heidelberg, Im Neuenheimer Feld 328, 69120 Heidelberg, Germany.
Abbreviations used in this paper: SNARE, soluble NSF attachment protein receptor; v- or t-SNARE, vesicle or target SNARE.
References
- Ausubel, F., R. Brent, R.E. Kingston, D.D. Moore, and J.G. Seidman. 1995. Short Protocols in Molecular Biology. J.A. Smith and K. Struhl, editors. Third edition. John Wiley & Sons, Inc., New York.
- Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol. 1997;139:1097–1108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie K., Prokop A., Bellen H.J., O'Kane C.J., Schulze K.L., Sweeney S.T. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila . Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- Cao X., Ballew N., Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles C.R., Snyder W.B., Burd C.G., Emr S.D. Novel Golgi to vacuole delivery pathway in yeastidentification of a sorting determinant and required transport component. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. Methods Cell Sci. 1995;17:283–294. [Google Scholar]
- Haas A., Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A., Scheglmann D., Lazar T., Gallwitz D., Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding T.M., Moranno K.A., Scott S.V., Klionsky D.J. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Lane O. AntibodiesA Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1988. [Google Scholar]
- Hay J.C., Scheller R.H. SNAREs and NSF in targeted membrane fusion. Curr. Opin. Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Chapman E.R., Kreber R., Garment M.B., Carlson S.D., Ganetzky B. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J. Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner W., Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mayer A., Scheglmann D., Dove S., Glatz A., Wickner W., Haas A. Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol. Cell Biol. 2000;In press doi: 10.1091/mbc.11.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Rybin V., Murphy C., Giner A., Teasdale R., Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Hirata A., Ohsumi Y., Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae . J. Biol. Chem. 1997;272:11344–11349. doi: 10.1074/jbc.272.17.11344. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann C., Pelham H.R.B., Wickner W.T., Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Otto H., Hanson P.I., Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews P.D., Stark M.J.R., Cesaro-Tadic S., Glatz A., Podtelejnikov A., Mann M., Mayer A. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science. 1999;285:1084–1087. doi: 10.1126/science.285.5430.1084. [DOI] [PubMed] [Google Scholar]
- Pfeffer S.R. Transport vesicle dockingSNAREs and associates. Annu. Rev. Cell Dev. Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Radisky D.C., Snyder W.B., Emr S.D., Kaplan J. Characterization of VPS41, a gene required for vacuolar trafficking and high-affinity iron transport in yeast. Proc. Natl. Acad. Sci. USA. 1997;94:5662–5666. doi: 10.1073/pnas.94.11.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C.K, Roberts C.J., Moore K.E., Howald I., Stevens T.H. Biogenesis of the vacuole in Saccharomyces cerevisiae . Int. Rev. Cytol. 1992;139:59–120. doi: 10.1016/s0074-7696(08)61410-2. [DOI] [PubMed] [Google Scholar]
- Rehling P., Darsow T., Katzmann D.J., Emr S.D. Formation of AP-3 transport intermediates requires Vps41p function. Nat. Cell Biol. 1999;1:346–353. doi: 10.1038/14037. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Springer S., Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Wickner W. Vam7p, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato K., Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Fischer von Mollard G., Jensen O.N., Margolis N., Stevens T.H., Wickner W. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic vacuole fusion J. Cell Biol. 145 1999. 1435 1442a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C.U., Wickner W., Xu Z. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion Proc. Natl. Acad. Sci. USA. 96 1999. 11194 11199b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Ohsumi Y., Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- Xu Z., Mayer A., Muller E., Wickner W. A heterodimer of thioredoxin and IB 2 cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J. Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Sato K., Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]
- Zheng B., Wu J.N., Schober W., Lewis D.E., Vida T. Isolation of yeast mutants defective for localization of vacuolar vital dyes. Proc. Natl. Acad. Sci. USA. 1998;95:11721–11726. doi: 10.1073/pnas.95.20.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]