Abstract
The muscle-specific receptor tyrosine kinase (MuSK) forms part of a receptor complex, activated by nerve-derived agrin, that orchestrates the differentiation of the neuromuscular junction (NMJ). The molecular events linking MuSK activation with postsynaptic differentiation are not fully understood. In an attempt to identify partners and/or effectors of MuSK, cross-linking and immunopurification experiments were performed in purified postsynaptic membranes from the Torpedo electrocyte, a model system for the NMJ. Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analysis was conducted on both cross-link products, and on the major peptide coimmunopurified with MuSK; this analysis identified a polypeptide corresponding to the COOH-terminal fragment of membrane-associated guanylate kinase (MAGUK) with inverted domain organization (MAGI)-1c. A bona fide MAGI-1c (150 kD) was detected by Western blotting in the postsynaptic membrane of Torpedo electrocytes, and in a high molecular mass cross-link product of MuSK. Immunofluorescence experiments showed that MAGI-1c is localized specifically at the adult rat NMJ, but is absent from agrin-induced acetylcholine receptor clusters in myotubes in vitro. In the central nervous system, MAGUKs play a primary role as scaffolding proteins that organize cytoskeletal signaling complexes at excitatory synapses. Our data suggest that a protein from the MAGUK family is involved in the MuSK signaling pathway at the vertebrate NMJ.
Keywords: MAGUKs, MAGI-1c, muscle-specific receptor tyrosine kinase, neuromuscular junction, acetylcholine receptor
Introduction
The aggregation of acetylcholine receptors (AChRs), as well as a host of molecules at postsynaptic membrane sites, is the hallmark of synaptic differentiation of the neuromuscular junction (NMJ). This process is triggered by agrin, a heparansulfate proteoglycan synthetized by the motor axon and released into the synaptic basal lamina. An unusual receptor tyrosine kinase (Jennings et al. 1993; Valenzuela et al. 1995), the muscle-specific receptor tyrosine kinase (MuSK), is a critical component for agrin-mediated signaling and synapse formation. Mice lacking MuSK do not form functional NMJs, similar to agrin mutants (DeChiara et al. 1996; Gautam et al. 1996). MuSK is part of an agrin–receptor complex, and differs from other members of the receptor tyrosine kinase family in that agrin-dependent activation of MuSK is insufficient to mediate its effects (Hoch 1999). Distinct domains in the extracellular and cytoplasmic regions of MuSK are required for inducing AChR clustering. In the cytoplasmic moiety in particular, binding site NPXY for phosphotyrosine-binding (PTB) domains in adaptor proteins potentially confers to MuSK its ability to induce postsynaptic specializations (Zhou et al. 1999; Herbst and Burden 2000). However, the PTB-containing signaling proteins Shc, IRS-1/2, and FRS-2, as well as components of mitogen-activated protein kinase or phosphatidylinositol 3-kinase signaling pathways, failed to be tyrosine phosphorylated by agrin (Herbst and Burden 2000). Meanwhile, two small guanosine triphosphatases, Rac and Cdc-42, have been shown to be involved in agrin-induced AChR clustering in myotubes in culture (Weston et al. 2000). However, little is known about the signal transduction cascade that follows MuSK activation, and so far no MuSK effectors have been identified. To understand how MuSK leads to the formation of synaptic specialization, we sought to identify its partners and/or effectors in the postsynaptic membrane. To this end, we carried out chemical cross-linking and immunopurification experiments in a model system for the NMJ, the Torpedo electrocyte. Chemical cross-linking was previously used to identify the molecules closely associated with rapsyn, the 43-kD receptor-associated peripheral component, in the postsynaptic membrane: the β subunit of AChR (Burden et al. 1983) and β-dystroglycan (Cartaud et al. 1998). The postsynaptic membrane from Torpedo electrocyte comprised molecules important for cholinergic synaptic transmission including AChR, and components of the machinery triggering postsynaptic differentiation such as rapsyn and an orthologue of MuSK, referred to as MuSK in this paper (Jennings et al. 1993). Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry analysis of the MuSK partners allowed us to identify membrane-associated guanylate kinase (MAGUK) with inverted domain organization (MAGI-1c), a member of the MAGUK family, as a candidate for MuSK downstream signaling at the NMJ.
Materials and Methods
Antibodies
Polyclonal 2847 anti-Torpedo MuSK antibodies directed against the COOH-terminal 20 amino acids (Watty et al. 2000) were a gift from Dr. S. Burden (Skirball Institute, New York University Medical School, New York, NY). Polyclonal antibodies raised against the cytoplasmic domain of rat MuSK (cyt-MuSK) have been previously characterized (Hopf and Hoch 1998). Expression of the fusion protein in Escherichia coli and injections into rabbits were performed as described (Hopf and Hoch 1998). IgGs were purified from the serum on a protein A Sepharose Hi–Trap column (Amersham Pharmacia Biotech). Polyclonal antibodies R 499 (Dobrosotskaya et al. 1997) and R 497 (Dobrosotskaya and James 2000) anti–MAGI-1 were a gift from Drs. I. Dobrosotskaya and G. James (University of Texas, Southwestern Medical Center, Dallas, TX) Polyclonal antibodies directed against two peptides from the COOH-terminal domain of mouse MAGI-1c, CPRNPPEQRRRPYKE (R 84) and CRPKDRPPDAWREAQP (R 85), have been raised in rabbits after coupling to the carrier protein KLH using standard protocol.
Purification of AChR-rich Membranes
AChR-rich membranes were purified from fresh Torpedo electric tissue as described previously (Cartaud et al. 1998).
Cross-linking Experiments
Cross-linking experiments were performed as described in Burden et al. 1983, using succinimidyl 4(p-maleimidophenyl)-butyrate (SMPB) that contains N-ethylmaleimide and N-hydroxysuccinimide as reactive groups, which reacted with free sulfhydryls and primary amines, respectively. In brief, AChR-rich membranes were washed with 10 mM sodium phosphate buffer, 1 mM EDTA, 1 mM EGTA, 0.3 mM PMSF, 0.02% sodium azide, pH 7.4 (buffer A), pelleted by centrifugation, and resuspended in 10 mM sodium phosphate buffer, 1 mM EDTA, and pH 8.0 at a final concentration of 4 mg proteins/ml. SMPB in DMSO (2% vol/vol stock solution) was added to the membranes at concentrations ranging from 10−6 to 10−4 M, and incubated in the dark for 30 min at room temperature. Membranes were then pelleted and washed in 10 mM sodium phosphate buffer, 1 mM EDTA, pH 8.0, before solubilization in SDS-PAGE sample buffer. The detection of MuSK in the cross-linked products was subsequently achieved by Western blotting with anti–cyt-MuSK or 2847 anti-MuSK antibodies (1:2,000).
Immunoaffinity Purification of the MuSK–MAGI-1c Complexes
Native or cross-linked AChR-rich membranes were resuspended in buffer A (2 mg/ml), and the nonionic detergent Triton X-100 was added to a final concentration of 1%. After 1 h on ice and centrifugation at 100,000 g for 40 min, the supernatant was diluted 10 times and incubated on a rocking platform overnight at 4°C with hydrazide gel (Affigel 10; Bio-Rad Laboratories) coupled to anti–cyt-MuSK or R 84/R 85 anti–MAGI-1c antibodies. After washings of the columns, the complexes were eluted with 0.1 M glycine-HCl, pH 2.5, containing 0.02% Triton X-100.
SDS-PAGE and Western Blotting
Samples were run on 8 or 10% SDS-PAGE, using a Bio-Rad Laboratories Mini Protean II slab cell. Proteins separated by gel electrophoresis were then electrotransferred to nitrocellulose membranes (Schleicher & Schuell). Western blots were performed as described elsewhere (Cartaud et al. 1998), revealed using enhanced chemiluminescent detection (ECL; Amersham Pharmacia Biotech), and exposed to Fuji x-ray films. Quantification of MuSK cross-link products was achieved by analysis of immunoblots using the NEN Life Science Products/Eastman Kodak Co. Image Station 440 CF.
MALDI-TOF Mass Spectrometry
Protein bands were cut off and digested in gel slices with trypsin (EC 3.421.4; Roche Diagnostics Corp.) as described by Shevchenko et al. 1996. Digests were resuspended in 20 μl 1% formic acid, desalted using Zip Tips C 18 (Millipore), and eluted with 50 and 80% acetonitrile. The desalted peptide mixture was dried and dissolved in 3 μl 1% formic acid. The matrix used was a saturated solution of 2,5-dihydroxybenzoic acid in 0.1% trifluoroacetic acid. The sample and the matrix (1:1, vol/vol) were loaded on the target using the dried droplet method. MALDI-TOF spectra of the peptides were obtained with a Voyager-DE STR Biospectrometry Workstation mass spectrometer (PE Biosystems). The analyses were performed in positive ion reflector mode with an accelerating voltage of 20 kV and a delayed extraction of 200 ns; ∼250 scans were averaged. For subsequent data processing, the Data Explorer software (PE Biosystems) was used. Spectra obtained for the whole protein were calibrated externally using the [M+H]+ ion from Des-Arg Bradykinin peptide (mol wt 904.47) and ACTH 18–39 fragment peptide (MW 2465.20). The trypsin autoproteolysis products (132–142 fragment [MW 1153.57] and 56–75 fragment [MW 2163.06]) were used as the second caliber. Data mining was performed using the ProFound™ (The Dialog Corporation) and MS-Fit softwares. A mass deviation of 100 ppm was usually allowed in the database searches.
Immunofluorescence
Indirect immunofluorescence experiments were performed on 4-μm-thick cryostat sections of unfixed pieces of Torpedo electric tissue or sternomastoid muscles from adult rat. After preincubation in PBS containing 1% BSA and 5% goat serum, sections were incubated overnight with primary antibodies (R 499, 1:200, R 497, 1:200, or R-84, 1:200 anti–MAGI-1 antibodies) at 4°C in PBS containing 0.1% BSA and 0.5% goat serum; sections were washed four times for 5 min in PBS, and incubated with Cy3-conjugated secondary antibodies (GAR Cy3, 1:400; Jackson ImmunoResearch Laboratories) for 1 h at 25°C. Fluorescein isothiocyanate–conjugated α-bungarotoxin (1 μg/ml; Molecular Probes) was added with the secondary antibodies to label AChRs in the postsynaptic membranes. Sections were mounted in Citifluor (UKC; Chemlab). Micrographs were taken with a Leica DMR microscope equipped with a Micro Max cooled CCD camera (Princeton Instruments, Inc.). For double-fluorescence pictures, controls confirm that no bleedthrough was detectable under the conditions used (filters L5 for fluorescein, filters TX for Cy3). Digital images were captured using MetaView Imaging System (Universal Imaging Corp.) and arranged using Adobe Photoshop® v5.0.
Assay of Agrin-induced AChR Clustering
C2C12 mouse muscle cells (American Type Culture Collection) were cultured on Matrigel-coated (Collaborative Biomedical Products) glass coverslips according to the manufacturer's instructions. 3–4-d-old myotubes were then incubated with agrin purified from Torpedo electric organ according to Nitkin et al. 1987 (Cibacron pool, 10 ng/ml) for 16–24 h at 37°C. AChR clusters were detected on live cells with fluorescein isothiocyanate–conjugated α-bungarotoxin (1 μg/ml). MAGI-1 was subsequently detected with antibody R 499 after permeabilization with 95% ethanol at −20°C.
Results and Discussion
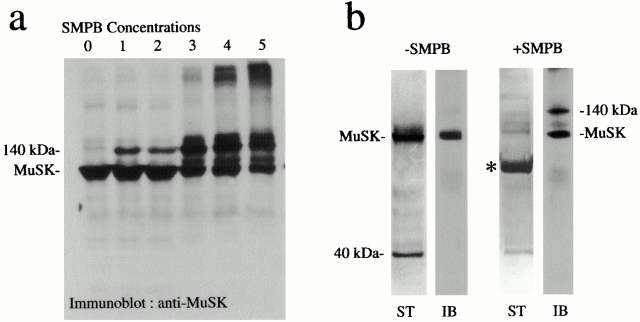
To identify the potential partners of MuSK in the postsynaptic membrane, we performed cross-linking experiments according to Burden et al. 1983, using a noncleaveable, heterobifunctional reagent SMPB in purified AChR-rich membranes from Torpedo electric tissue, as well as immunopurification of the MuSK complex. The cross-linked products containing MuSK were identified by Western blotting using anti-MuSK antibodies (Fig. 1 a). At SMPB concentrations of 10−5 M, a cross-linked product of 140 kD containing MuSK (97 kD) was detected. Quantitation of immunoblots (Fig. 1 a, lane 3) revealed that up to 30% of total MuSK was present in the 140-kD cross-link product. At higher SMPB concentrations, several high molecular mass cross-linked products (≥250 kD) appeared at the top of the gels. An alternative approach to the identification of MuSK partners in the membrane was achieved by affinity chromatography of MuSK complexes after Triton X-100 extraction (Fig. 1 b). We observed that in addition to MuSK, a 40-kD polypeptide was consistently present in immunoextracts after separation by SDS-PAGE. Taken together, these experiments point to the existence of a complex containing MuSK and a 40-kD polypeptide in the postsynaptic membrane in situ. This protein was not recognized by antibodies directed against rapsyn, a central component of the AChR clustering process, critically placed downstream of MuSK (Apel et al. 1997). Thus, the 40-kD protein was not identical to 43-kD rapsyn or to Rac or Cdc-42, two signaling molecules recently reported to mediate agrin-induced AChR clustering (Weston et al. 2000), as indicated by Western blotting (data not shown).
Figure 1.
MuSK cross-link products and immunopurification of MuSK complexes. (a) Proteins from Torpedo AChR-rich membranes were cross-linked with various concentrations of SMPB (lane 0, control; lane 1, 7 × 10−6 M; lane 2, 2 × 10−5 M; lane 3, 4 × 10−5 M; lane 4, 10−4 M; lane 5, 2.5 × 10−4 M) and separated by SDS-PAGE. Western blots revealed that in addition to MuSK (97 kD), a 140-kD cross-link product was detected at low SMPB concentrations. Other cross-link products were observed at higher concentrations. (b) Purification of uncross-linked (−SMPB) and cross-linked (+SMPB, 4 × 10−5 M) MuSK complexes was achieved by immunoaffinity chromatography. The immunopurified polypeptides or the MuSK cross-link products were detected by silver staining (ST) or identified by immunoblotting (IB), respectively. In both experiments, a 40-kD polypeptide was detected in addition to MuSK. After SMPB treatment, a major cross-link product of 140 kD was detected. The asterisk indicates residual IgGs.
The polypeptide associated with MuSK in the 140-kD cross-link product, and the 40-kD polypeptide copurifying with MuSK, were identified by MALDI-TOF mass spectrometry after tryptic digestion of the polypeptides separated by SDS-PAGE. To achieve this, the tryptic peptides characterized by their mass-to-charge ratios (m/z) were compared with masses of theoretical tryptic peptides of proteins listed in SwissProt database using ProFound™ or MS-Fit softwares (a 100-ppm mass deviation was allowed). To validate our approach, a search for MuSK peptide sequences in the 140-kD cross-link product was first undertaken. A sequence coverage of 6% was obtained, compared with rat MuSK sequence contained in databases with a probability score of 10−4 (Fig. 2 a). When a manual search was done with the sequence of Torpedo MuSK, which displays an overall amino acid identity of 70% with mammalian MuSK, a higher coverage was obtained (13%, data not shown). In addition to MuSK, MALDI-TOF mass spectrometry analysis of the 140-kD band highlighted the COOH-terminal domain of mouse MAGI-1c, the third isoform of MAGI-1, with a coverage of 16% (probability score 6.5 × 10−3, Fig. 2 b). The analysis of the tryptic peptides from the 40-kD band revealed a striking coverage of 35% for 100-ppm mass deviation (probability score 1; estimated Z score = 2.30) with the same COOH-terminal domain of mouse MAGI-1c (Fig. 2 c). For a mass deviation of 50 ppm, a coverage of 26% was obtained. This indicates an extensive sequence homology between Torpedo and mouse MAGI-1c. The difference between the coverage maps of the 140-kD cross-link product and the 40-kD immunopurified peptide results from the cross-link treatment which likely modifies some peptide masses and perturbs the accessibility to trypsyn, therefore reducing the number of tryptic peptides.
Figure 2.
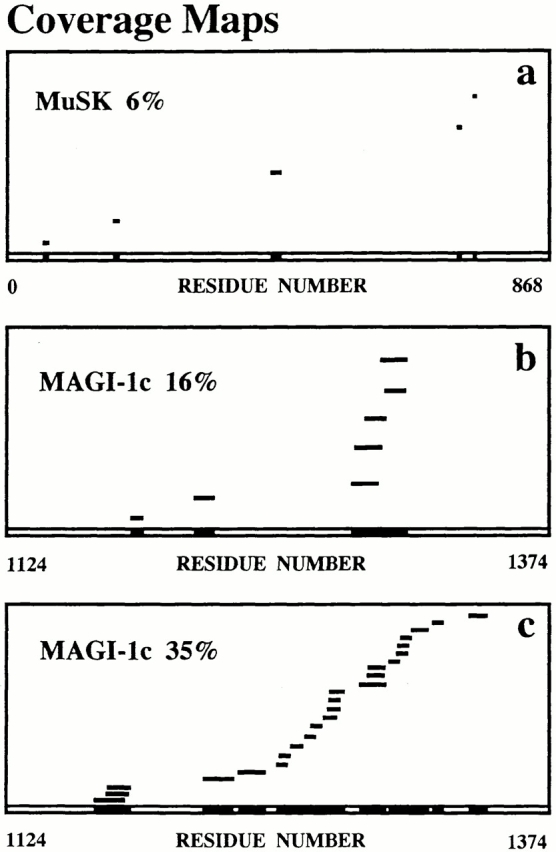
MALDI-TOF mass spectometry analysis of Torpedo MuSK binding partners. (a–c) Coverage maps of experimental tryptic peptides compared with theoretical tryptic peptides from databases obtained with ProFound™ software were shown. Coverages of 6% with rat MuSK (a), and of 16% with the COOH-terminal peptide (residues 1124–1374) of mouse MAGI-1c (b) were obtained from the 140-kD cross-link product. A coverage of 35% with the COOH-terminal peptide of MAGI-1c was obtained from the 40-kD polypeptide coimmunopurified with MuSK (c).
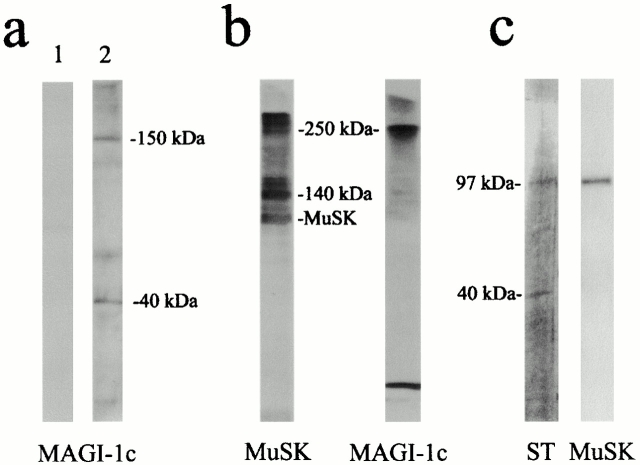
To ascertain MAGI-1c as a candidate protein associated with MuSK complex, immunoblots were performed with AChR-rich membranes from Torpedo electric tissue using anti–MAGI-1 antibodies; a 150-kD polypeptide that corresponded to a bona fide MAGI-1c protein, the larger MAGI-1 isoform (148 kD; Dobrosotskaya et al. 1997), was detected (Fig. 3 a). Interestingly, antibody R 85 revealed a 40-kD polypeptide in addition to the 150-kD protein (Fig. 3 a). This 40-kD polypeptide most likely represents a COOH-terminal proteolytic fragment of MAGI-1c, and corresponds to the 40-kD polypeptide cross-linked with MuSK. Moreover, the detection of MAGI-1c in a high molecular mass (250 kD) MuSK cross-link product (Fig. 3 b) argues in favor of a direct interaction between full-length MAGI-1c and MuSK. In addition, MuSK coprecipitated with MAGI-1c COOH-terminal fragment after immunopurification with anti–MAGI-1c antibodies from a Triton X-100 extract of purified AChR-rich membranes (Fig. 3 c). Taken together, our data highlight MAGI-1c as a possible component of the MuSK signaling complex.
Figure 3.
Characterization of the MuSK–MAGI-1c interaction in Torpedo postsynaptic membranes. (a) Western blots of AChR-rich Torpedo membranes were probed with preimmune serum (lane 1), and anti–MAGI-1c (R 85) (lane 2). A bona fide MAGI-1c (150 kD) and a 40-kD putative proteolytic fragment were detected (lane 2). (b) Western blots probed with R 497 and mab 2847 antibodies show that full-length MAGI-1 and MuSK were present together in a 250-kD cross-link product. (c) MuSK was coimmunopurified with the COOH-terminal fragment of MAGI-1c upon immunoaffinity chromatography with R 84 or R 85 antibodies. The immunopurified polypeptides were detected by silver staining (ST), and MuSK was detected by immunoblotting.
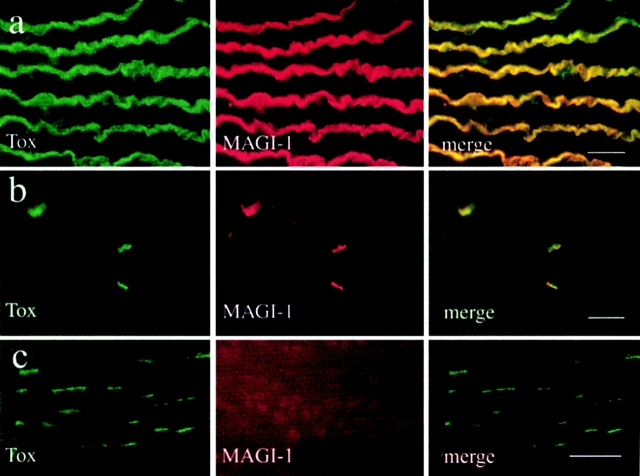
To evaluate the relevance of these in vitro data, immunofluorescence experiments were performed on Torpedo electric tissue and on rat sternomastoid muscle. Several antibodies were directed against the NH2-terminal domain (R 499; Dobrosotskaya et al. 1997), the first postsynaptic density (PSD)-95/synapse-associated protein (SAP)-90, Discs-large, ZO-1 homologous (PDZ) domain (R 497; Dobrosotskaya and James 2000) of MAGI-1, or the MAGI-1c–specific COOH-terminal domain (R84; see Materials and Methods); the antibodies cross-reacted specifically with the innervated membrane of the electrocytes (Fig. 4 a) and the postsynaptic membrane of adult rat NMJ (Fig. 4 b), identified by means of α-bungarotoxin staining.
Figure 4.
MAGI-1c localizes at the Torpedo and rat cholinergic synapses. (a) Double-fluorescence experiment showing the colocalisation of MAGI-1 (antibody R 499) and AChRs (α-bungarotoxin staining) at the innervated faces of Torpedo electrocytes. (b) In rat skeletal muscle fibers, MAGI-1 (antibody R 499) also strictly colocalized with AChRs at the NMJs. (c) At variance, MAGI-1 was not detected in agrin-induced AChR clusters in C2C12 myotubes. Bars, 30 μm.
Finally, we used an in vitro asay in order to gain insight into the function of MAGI-1 in AChR aggregation. When added to myotubes in culture, agrin induces the formation of AChR clusters, thus mimicking the first steps of postsynaptic specialization at the developing NMJ. We observed that upon agrin incubation, MAGI-1 was not concentrated at AChR clusters (Fig. 4 c).
MAGI-1 and its two isoforms, WWP3 (Pirozzi et al. 1997) and synaptic scaffolding molecule (S-SCAM) (Hirao et al. 1998), are unique MAGUKs with three features that distinguish them from all other known members of the family (including DLG, ZO-1, and PSD-95/SAP-90, among others): (a) the guanylate kinase domain is at the NH2 terminus rather than at the COOH terminus, (b) the SH3 domain is replaced by two WW domains, and (c) they contain five PDZ domains rather than the usual one or three. MAGI-1 mRNAs are widely expressed in several tissues including skeletal muscle, where they are present at low levels (Dobrosotskaya et al. 1997). A low level of expression is typical for proteins highly concentrated at the NMJ, as reported for MAGI-1 in this work. Among the three splice variants of MAGI-1, MAGI-1c contains three bipartite nuclear localization signals in its unique COOH-terminal sequence, and this protein was found preferentially in the nucleus of transfected MDCK cells (Dobrosotskaya et al. 1997). The COOH-terminal part of MAGI-1c contains the fifth PDZ domain, and consists of 37% Lys and Arg. It is reasonable to assume that this part of the molecule was associated with the cytoplasmic domain of MuSK which contains a COOH-terminal VXV motif that resembles the consensus binding site for PDZ domains and was efficiently cross-linked after SMPB treatment. However, Zhou et al. 1999 reported that the deletion of the COOH-terminal three residues VGV of rat MuSK is dispensable for agrin-induced AChR clustering in vitro. Because we observed that MAGI-1c is absent from agrin-induced AChR clusters, but is concentrated at the adult NMJ, we favor a role of MAGI-1 in late steps of synapse formation, rather than in initial steps of AChR clustering. This is in agreement with the conclusion of Zhou et al. 1999 which favors the possibility that PDZ domain proteins do play roles in MuSK signaling, but that these roles are not readily detected in in vitro assays.
The function of this new member of the MAGUK family being still unknown, the association of MAGI-1c with MuSK in the postsynaptic membrane at the NMJ represents the first indication that MAGI-1c could organize postsynaptic domains, like other synaptically localized MAGUKs. MAGUKs are multidomain scaffolding proteins involved in the clustering of ion channels, receptors, adhesion molecules, and cytosolic signaling proteins at several types of cellular junctions including epithelial tight and septate junctions, excitatory central synapses, and the NMJ in Drosophila (Ponting et al. 1997; Dimitratos et al. 1999; Fanning and Anderson 1999; Sheng and Pak 2000). At excitatory synapses in particular, members of the MAGUK protein family such as the PSD-95/SAP-90 proteins, the scaffolding proteins GRIP (Dong et al. 1997) and HOMER (Brakeman et al. 1997), and the Shank proteins (Sheng and Kim 2000), use multiple domains to cluster ion channels and cytosolic signaling proteins at postsynaptic sites. Interestingly, members of the PSD-95 family have recently been found to interact with receptor tyrosine kinases, such as the neuregulin receptor ErbB4, at neuronal synapses (Garcia et al. 2000).
S-SCAM has a cellular distribution reminiscent of that of PSD-95/SAP-90, and has been shown to bind both NMDA receptors and a neuroligin, a neuronal cell adhesion molecule, in vitro (Hirao et al. 1998). Therefore, the inverted MAGUKs of the MAGI-1/S-SCAM/WWP3 family may function as novel scaffolding molecules to assemble various components at synaptic junctions. Our data being inconsistent with a role of MAGI-1 in AChR aggregation in vitro, we propose that MAGI-1c could trigger postsynaptic and/or presynaptic differentiation by coordinating downstream MuSK signaling at the vertebrate NMJ. Agrin activates MuSK-inducing clustering of AChRs via intracellular pathways likely involving PTB domain–containing proteins required for activation of MuSK's kinase activity. On the other hand, MAGI-1c would recruit several effectors involved in downstream signaling to muscle-triggering and/or nerve-triggering synapse formation. This latter hypothesis is in agreement with the function of PSD-95 family proteins, which are not believed to be the primary organizers of the excitatory synapses (Lee and Sheng 2000). Owing to its multiple PDZ domains, MAGI-1c might interact through PDZ–PDZ interactions with a variety of effectors, including postsynaptic α-syntrophin and neuronal NOS, two PDZ-containing proteins present at the NMJ (Colledge and Froehner 1998), and K-RasB, because the first PDZ domain of MAGI-1 was isolated on the basis of its interaction with the COOH terminus of K-RasB in the yeast two-hybrid system (Dobrosotskaya et al. 1997). Also, the neuregulin receptors ErbB2 and ErbB4, which possess the consensus COOH-terminal VXV motif in their cytoplasmic domain, represent potential partners of MAGI-1c at the synapse. In addition, MAGI-1c, having the possibility to shuttle between the nucleus and the plasma membrane (Dobrosotskaya et al. 1997), an additional function for MAGI-1c at the NMJ would be its participation in the transfer of information from the postsynaptic membrane to subneural nuclei similar to the potential MuSK binding protein Syne-1 (Apel et al. 2000).
Acknowledgments
W. Hoch thanks Uli Schwarz for his continuous support.
This work was supported by the Centre National de la recherche Scientifique, Universités Paris 6 and Paris 7, and by grants to J. Cartaud from the Association Française Contre les Myopathies.
Footnotes
Part of this work was presented at the 40th Annual Meeting of the American Society for Cell Biology, San Francisco, CA, 9–13 December 2000 (Strochlic, L., A. Cartaud, V. Labas, W. Hoch, J. Rossier, and J. Cartaud. 2000. Mol. Biol. Cell. 11[Suppl.]:475a).
Abbreviations used in this paper: AChR, acetylcholine receptor; MAGI, MAGUK with inverted domain organization; MAGUK, membrane-associated guanylate kinase; MALDI-TOF, matrix-assisted laser desorption ionization–time of flight; MuSK, muscle-specific receptor tyrosine kinase; NMJ, neuromuscular junction; PDZ, PSD-95/SAP-90, Discs-large, ZO-1 homologous domain; PSD, postsynaptic density; PTB, phosphotyrosine-binding; SAP, synapse-associated protein; S-SCAM, synaptic scaffolding molecule; SMPB, succinimidyl 4(p-maleimidophenyl)-butyrate; ZO, zona occludens.
References
- Apel E.D., Glass D.J., Moscoso L.M., Yancopoulos G.D., Sanes J.R. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;4:623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Apel E.D., Lewis R.M., Grady R.M., Sanes J.R. Syne-1a dystrophin- and klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- Brakeman P.R., Lanahan A.A., O'Brien R., Roche K., Barnes C.A., Huganir R.L., Worley P.F. Homera protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Burden S.J., DePalma R.L., Gottesman G.S. Crosslinking of proteins in acetylcholine receptor-rich membranesassociation between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35:687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Cartaud A., Coutant S., Petrucci T.C., Cartaud J. Evidence for in situ and in vitro association between beta-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J. Biol. Chem. 1998;273:11321–11326. doi: 10.1074/jbc.273.18.11321. [DOI] [PubMed] [Google Scholar]
- Colledge M., Froehner S.C. Signals mediating ion channel clustering at the neuromuscular junction. Curr. Opin. Neurobiol. 1998;8:357–363. doi: 10.1016/s0959-4388(98)80061-5. [DOI] [PubMed] [Google Scholar]
- DeChiara T.M., Bowen D.C., Valenzuela D.M., Simmons M.V., Poueymirou W.T., Thomas S., Kinetz E., Compton D.L., Park J.S., Smith C. The receptor tyrosine kinase, MuSK, is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Dimitratos S.D., Woods D.F., Stathakis D.G., Briant P.J. Signaling pathways are focussed at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays. 1999;21:912–921. doi: 10.1002/(SICI)1521-1878(199911)21:11<912::AID-BIES3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya I., Guy R.K., James G.L. MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J. Biol. Chem. 1997;272:31589–31597. doi: 10.1074/jbc.272.50.31589. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya I.Y., James G.L. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem. Biophys. Res. Commun. 2000;270:903–909. doi: 10.1006/bbrc.2000.2471. [DOI] [PubMed] [Google Scholar]
- Dong H., O'Brien R.J., Fung E.T., Lanahan A.A., Worley P.F., Huganir R.L. GRIPa synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Fanning A.S., Anderson J.M. Protein modules as organizers of membrane structure. Curr. Opin. Cell Biol. 1999;4:432–439. doi: 10.1016/S0955-0674(99)80062-3. [DOI] [PubMed] [Google Scholar]
- Garcia R.A.G., Vasudevan K., Buonanno A. The neuregulin receptor Erb B-4 interacts with PDZ-containing proteins at neuronal synapses. Proc. Natl. Acad. Sci. USA. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M., Noakes P.G., Moscoso L., Rupp F., Scheller R.H., Merlie J.P., Sanes J.R. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Herbst R., Burden S.J. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 2000;19:67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao K., Hata Y., Ide N., Takeuchi M., Irie M., Yao I., Deguchi M., Toyoda A., Sudhof T.C., Takai Y. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J. Biol. Chem. 1998;273:21105–21110. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- Hoch W. Formation of the neuromuscular junction. Agrin and its unusual receptors. Eur. J. Biochem. 1999;265:1–10. doi: 10.1046/j.1432-1327.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- Hopf C., Hoch W. Dimerization of the muscle-specific kinase induces tyrosine phosphorylation of acetylcholine receptors and their aggregation on the surface of myotubes. J. Biol. Chem. 1998;273:6467–6473. doi: 10.1074/jbc.273.11.6467. [DOI] [PubMed] [Google Scholar]
- Jennings C.G., Dyer S.M., Burden S.J. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc. Natl. Acad. Sci. USA. 1993;90:2895–2899. doi: 10.1073/pnas.90.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Sheng M. Development of neuron-neuron synapses. Curr. Opin. Neurobiol. 2000;10:125–131. doi: 10.1016/s0959-4388(99)00046-x. [DOI] [PubMed] [Google Scholar]
- Nitkin R.M., Smith M.A., Magill C., Fallon J.R., Yao Y.M.M., Wallace B.G., McMahan U.J. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol. 1987;105:2471–2478. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzi G., McConnell S.J., Uveges A.J., Carter J.M., Sparks A.B., Kay B.K., Fowlkes D.M. Identification of novel human WW domain-containing proteins by cloning of ligand targets. J. Biol. Chem. 1997;272:14611–14616. doi: 10.1074/jbc.272.23.14611. [DOI] [PubMed] [Google Scholar]
- Ponting C.P., Phillips C., Davies K.E., Blake D.J. PDZ domainstargeting signalling molecules to sub-membranous sites. Bioessays. 1997;19:469–479. doi: 10.1002/bies.950190606. [DOI] [PubMed] [Google Scholar]
- Sheng M., Kim E. The Shank family of scaffold proteins. J. Cell Sci. 2000;113:1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- Sheng M., Pak D.T. Ligand-gated ion channel interactions with cytoskeletal and signalling proteins. Annu. Rev. Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Jensen O.N., Podtelejnikov A.V., Sagliocco F., Wilm M., Vorm O., Montensen P., Shevchenko A., Boucherie H., Mann M. Linking genome and proteome by mass spectrometrylarge-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D.M., Stitt T.N., DiStefano P.S., Rojas E., Mattsson K., Compton D.L., Nunez L., Park J.S., Stark J.L., Gies D.R. Receptor tyrosine kinase specific for the skeletal muscle lineageexpression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15:573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Watty A., Neubauer G., Dreger M., Zimmer M., Wilm M., Burden S.J. The in vitro and in vivo phosphotyrosine map of activated MuSK. Proc. Natl. Acad. Sci. USA. 2000;97:4585–4590. doi: 10.1073/pnas.080061997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston C., Yee B., Hod E., Prives J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatase Rac and Cdc 42. J. Cell Biol. 2000;150:205–212. doi: 10.1083/jcb.150.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Glass D.J., Yancopoulos G.D., Sanes J.R. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J. Cell Biol. 1999;146:1133–1146. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]