Abstract
A mutant of Escherichia coli with a partially defective phosphoribosylpyrophosphate synthetase (ribosephosphate pyrophosphokinase) has been characterized genetically. The genetic lesion causing the altered phosphoribosylpyrophosphate synthetase, prs, was mapped at 26 min on the linkage map by conjugation. Transductional analysis of the prs region established the gene order as purB-fadR-dadR-tre-pth-prs-hemA-trp. Two additional mutations were identified in the mutant: one in gsk, the gene encoding guanosine kinase, and one in lon, conferring a mucoid colony morphology. The contribution of each mutation to the phenotype of the mutant has been evaluated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- Gibson K. J., Schubert K. R., Switzer R. L. Binding of the substrates and the allosteric inhibitor adenosine 5'-diphosphate to phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem. 1982 Mar 10;257(5):2391–2396. [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Jochimsen B., Koduri K. R. Microbial models and regulatory elements in the control of purine metabolism. Ciba Found Symp. 1977;(48):23–41. doi: 10.1002/9780470720301.ch3. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar R., Slonim A., Kuhn J. Role of D-tryptophan oxidase in D-tryptophan utilization by Escherichia coli. J Bacteriol. 1976 Mar;125(3):1096–1104. doi: 10.1128/jb.125.3.1096-1104.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
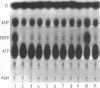
- Hove-Jensen B., Nygaard P. Phosphoribosylpyrophosphate synthetase of Escherichia coli, Identification of a mutant enzyme. Eur J Biochem. 1982 Aug;126(2):327–332. doi: 10.1111/j.1432-1033.1982.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Houlberg U., Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979 Oct 1;98(2):254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Jochimsen B., Garber B., Gots J. S. Phosphoribosylpyrophosphate (PRPP) synthetase mutant in Salmonella typhimurium. Adv Exp Med Biol. 1979;122B:131–136. doi: 10.1007/978-1-4684-8559-2_23. [DOI] [PubMed] [Google Scholar]
- Jochimsen B., Nygaard P., Vestergaard T. Location on the chromosome of Escherichia coli of genes governing purine metabolism. Adenosine deaminase (add), guanosine kinase (gsk) and hypoxanthine phosphoribosyltransferase (hpt). Mol Gen Genet. 1975 Dec 30;143(1):85–91. doi: 10.1007/BF00269424. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R., Walker C., Tan P. F. Studies on the metabolic role of peptidyl-tRNA hydrolase. I. Properties of a mutant E. coli with temperature-sensitive peptidyl-tRNA hydrolase. Mol Gen Genet. 1973 Mar 19;121(4):307–324. doi: 10.1007/BF00433230. [DOI] [PubMed] [Google Scholar]
- Olszowy J., Switzer R. L. Specific repression of phosphoribosylpyrophosphate synthetase by uridine compounds in Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):450–451. doi: 10.1128/jb.110.1.450-451.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey N. K., Switzer R. L. Mutant strains of Salmonella typhimurium with defective phosphoribosylpyrophosphate synthetase activity. J Gen Microbiol. 1982 Aug;128(8):1863–1871. doi: 10.1099/00221287-128-8-1863. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler W. C., Switzer R. L. Regulation of Salmonella phosphoribosylpyrophosphate synthetase activity in vivo. Deductions from pool measurements. J Biol Chem. 1977 Dec 10;252(23):8504–8511. [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Switzer R. L., Shelton E. Studies of the quaternary structure and the chemical properties of phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem. 1975 Sep 25;250(18):7492–7500. [PubMed] [Google Scholar]
- Simons R. W., Egan P. A., Chute H. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. J Bacteriol. 1980 May;142(2):621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Sadler J. R. The nature of lactose operator constitive mutations. J Mol Biol. 1971 Jul 28;59(2):273–305. doi: 10.1016/0022-2836(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Switzer R. L., Sogin D. C. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. V. Inhibition by end products and regulation by adenosine diphosphate. J Biol Chem. 1973 Feb 10;248(3):1063–1073. [PubMed] [Google Scholar]
- Tyrsted G., Sartorelli A. C. Inhibition of the synthesis of 5-phosphoribosyl-1-pyrophosphate by 3'-deoxy-adenosine and structurally related nucleoside analogs. Biochim Biophys Acta. 1968 Feb 26;155(2):619–622. doi: 10.1016/0005-2787(68)90209-8. [DOI] [PubMed] [Google Scholar]
- White M. N., Olszowy J., Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase: repression by end products. J Bacteriol. 1971 Oct;108(1):122–131. doi: 10.1128/jb.108.1.122-131.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J., Klopotowski T. D-Amino acid dehydrogenase of Escherichia coli K12: positive selection of mutants defective in enzyme activity and localization of the structural gene. Mol Gen Genet. 1981;181(3):373–378. doi: 10.1007/BF00425614. [DOI] [PubMed] [Google Scholar]
- Wild J., Walczak W., Krajewska-Grynkiewicz K., Klopotowski T. D-amino acid dehydrogenase: the enzyme of the first step of D-histidine and D-methionine racemization in Salmonella typhimurium. Mol Gen Genet. 1974;128(2):131–146. doi: 10.1007/BF02654486. [DOI] [PubMed] [Google Scholar]