Abstract
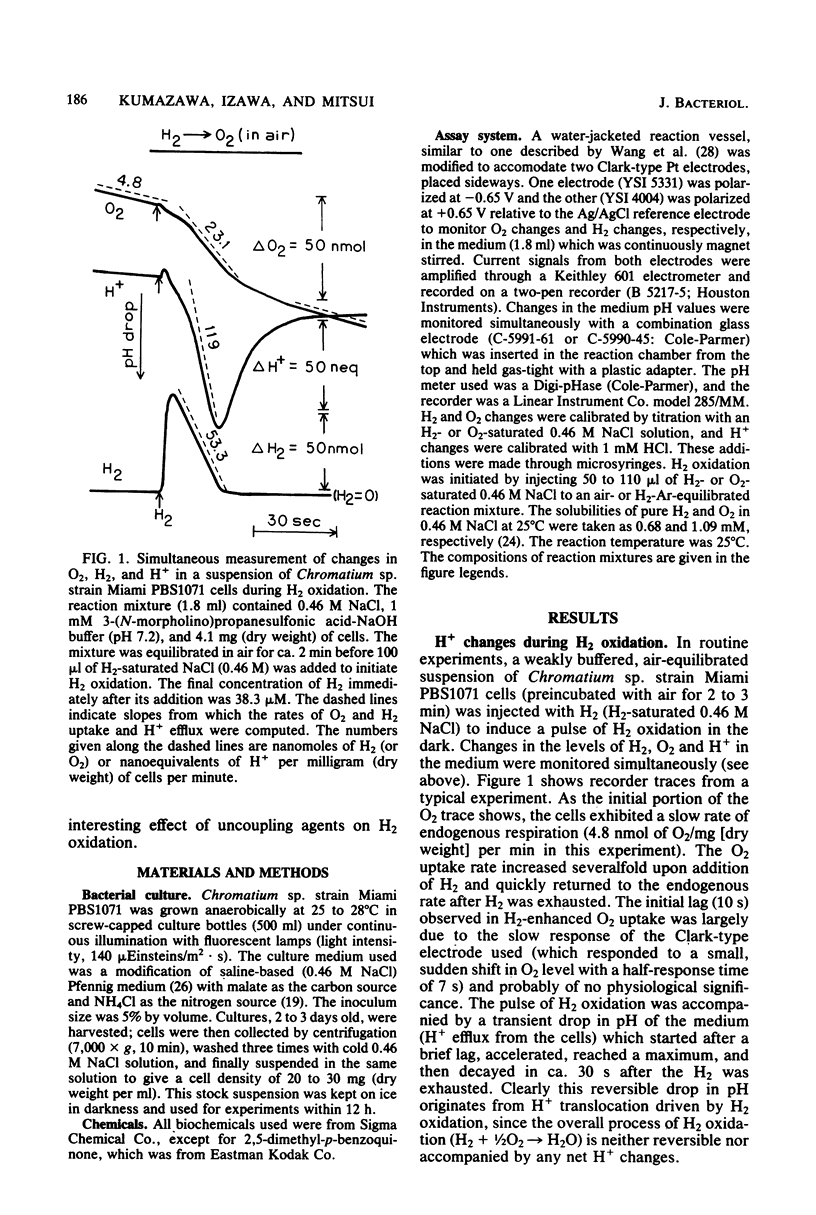
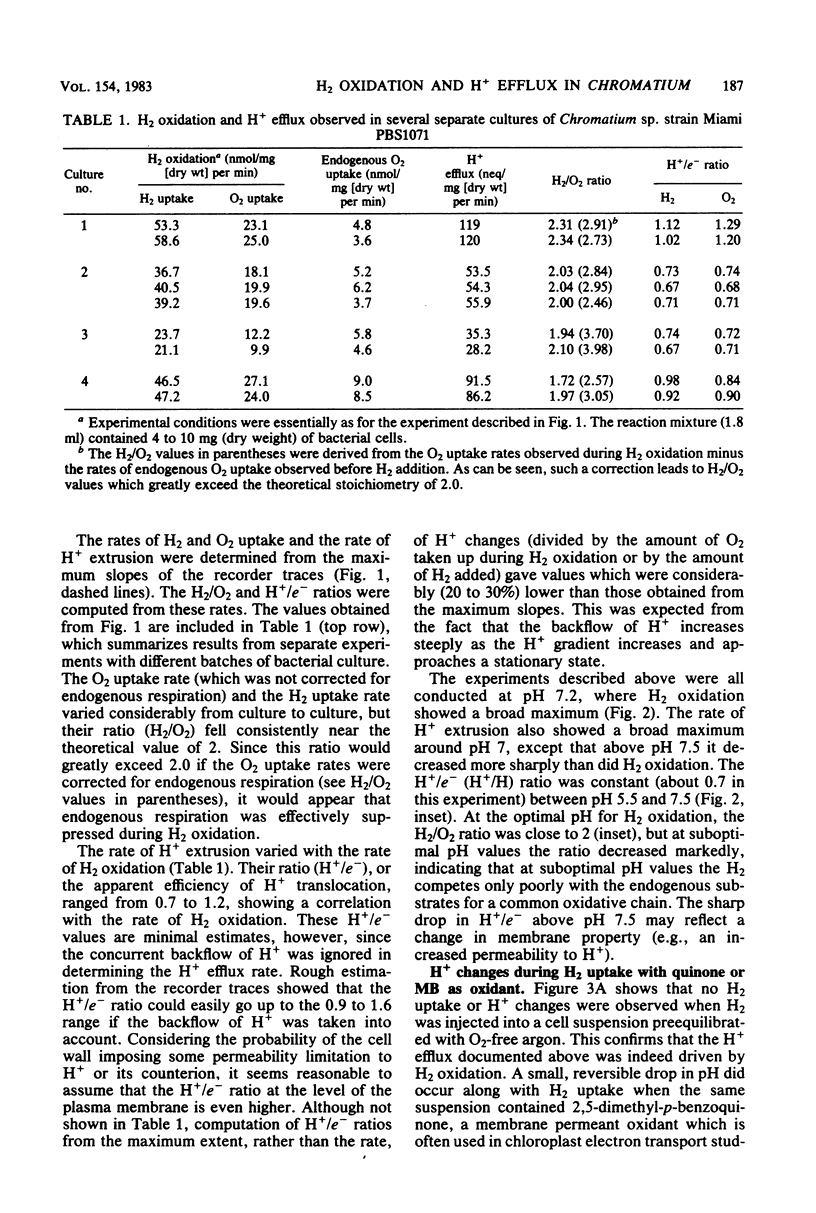
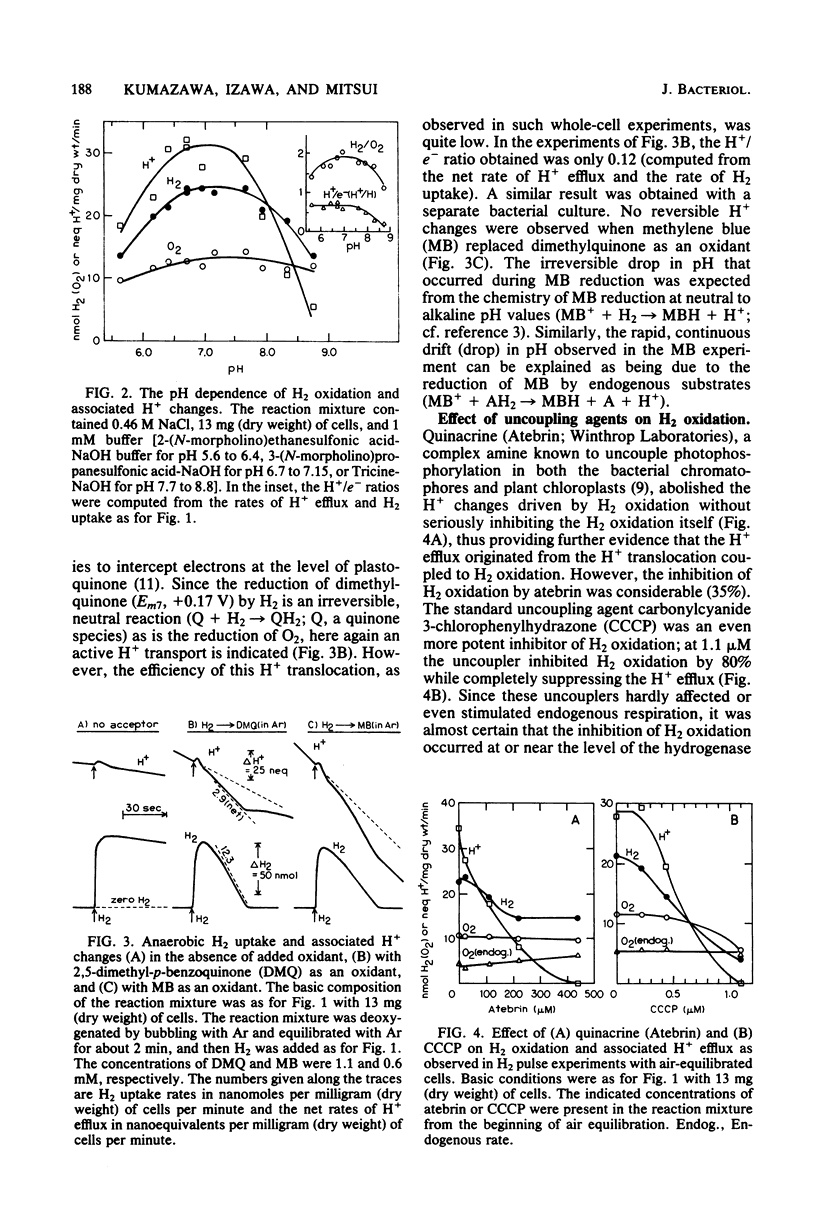
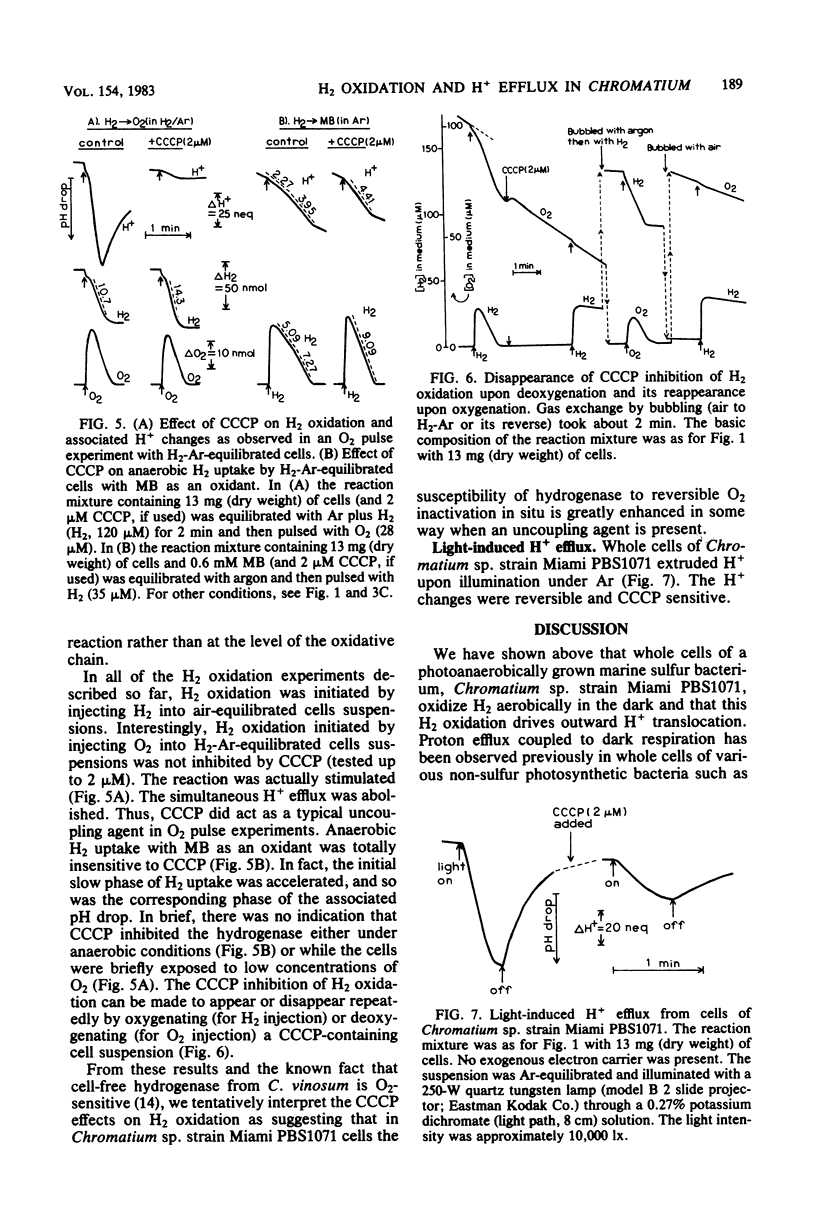
Whole cells of photoanaerobically grown Chromatium sp. strain Miami PBS1071, a marine sulfur purple bacterium, oxidized H2 in the dark through the oxyhydrogen reaction at rates of up to 59 nmol of H2 per mg (dry weight) per min. H2 oxidation was routinely measured in H2 pulse experiments with air-equilibrated cells. The reaction was accompanied by a reversible H+ efflux from the cells, suggesting an outward H+ translocation reaction coupled to H2 oxidation. The H+/e- ratio, calculated from simultaneous measurements of H2, O2, and H+ changes in the medium, varied with the cultures from 0.7 to 1.2. The ratio increased considerably when the backflow of H+ was taken into account. Anaerobic H2 uptake with 2,5-dimethyl-p-benzoguinone as an oxidant also showed a weak H+-translocating activity. No H+-translocating activity was detected with methylene blue as an oxidant. Carbonylcyanide 3-chlorophenylhydrazone (1 microM) stimulated H2 oxidation and abolished the associated H+ changes when H2 oxidation was observed in O2 pulse experiments with H2-Ar-equilibrated cells. However, the uncoupler inhibited both H2 oxidation and H+ changes when measurements were made in H2 pulse experiments with air-equilibrated cells. It is suggested that in this bacterium the susceptibility of hydrogenase to reversible O2 inactivation in situ is enhanced by the presence of uncoupling agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre A., Reynafarje B., Lehninger A. L. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5296–5300. doi: 10.1073/pnas.75.11.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Bachofen R. Ferredoxin-dependent reduction of nicotinamide-adenine dinucleotides with hydrogen gas by subcellular preparations from the photosynthetic bacterium, Chromatium. Biochim Biophys Acta. 1968 Nov 26;162(4):607–610. doi: 10.1016/0005-2728(68)90067-4. [DOI] [PubMed] [Google Scholar]
- Doussière J., Porte F., Vignais P. M. Orientation of hydrogenase in the plasma membrane of Paracoccus denitrificans. FEBS Lett. 1980 Jun 2;114(2):291–294. doi: 10.1016/0014-5793(80)81136-7. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Bovell C. R. Characteristics of a light-dependent proton transport in cells of Rhodospirillum rubrum. Biochim Biophys Acta. 1969 Jan 14;172(1):126–133. doi: 10.1016/0005-2728(69)90097-8. [DOI] [PubMed] [Google Scholar]
- Gibson J. Aerobic metabolism of Chromatium sp. strain D. Arch Mikrobiol. 1967;59(1):104–112. doi: 10.1007/BF00406321. [DOI] [PubMed] [Google Scholar]
- Gitlitz P. H., Krasna A. I. Structural and catalytic properties of hydrogenase from Chromatium. Biochemistry. 1975 Jun 17;14(12):2561–2568. doi: 10.1021/bi00683a001. [DOI] [PubMed] [Google Scholar]
- Kondratieva E. N., Zhukov V. G., Ivanovsky R. N., Petushkova U. P., Monosov E. Z. The capacity of phototrophic sulfur bacterium Thiocapsa roseopersicina for chemosynthesis. Arch Microbiol. 1976 Jul;108(3):287–292. doi: 10.1007/BF00454854. [DOI] [PubMed] [Google Scholar]
- Krasna A. I. Oxygen-stable hydrogenase and assay. Methods Enzymol. 1978;53:296–314. doi: 10.1016/s0076-6879(78)53036-x. [DOI] [PubMed] [Google Scholar]
- Lien S., Pietro A. S. Effect of uncouplers on anaerobic adaptation of hydrogenase activity in C reinhardtii. Biochem Biophys Res Commun. 1981 Nov 16;103(1):139–147. doi: 10.1016/0006-291x(81)91671-5. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1981 Jul;147(1):161–169. doi: 10.1128/jb.147.1.161-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Lascelles J. Thiosulphate metabolism and rhodanese in Chromatium sp. strain D. J Gen Microbiol. 1966 Mar;42(3):357–370. doi: 10.1099/00221287-42-3-357. [DOI] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


