Abstract
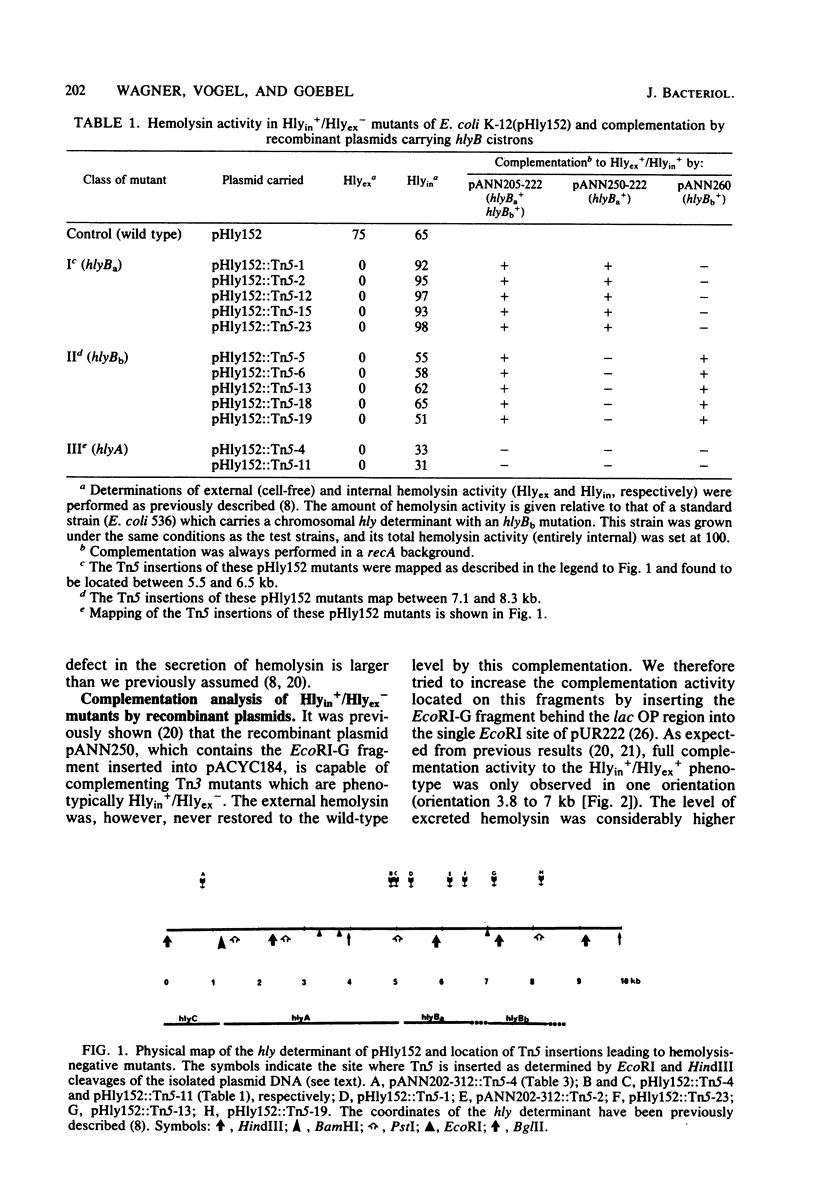
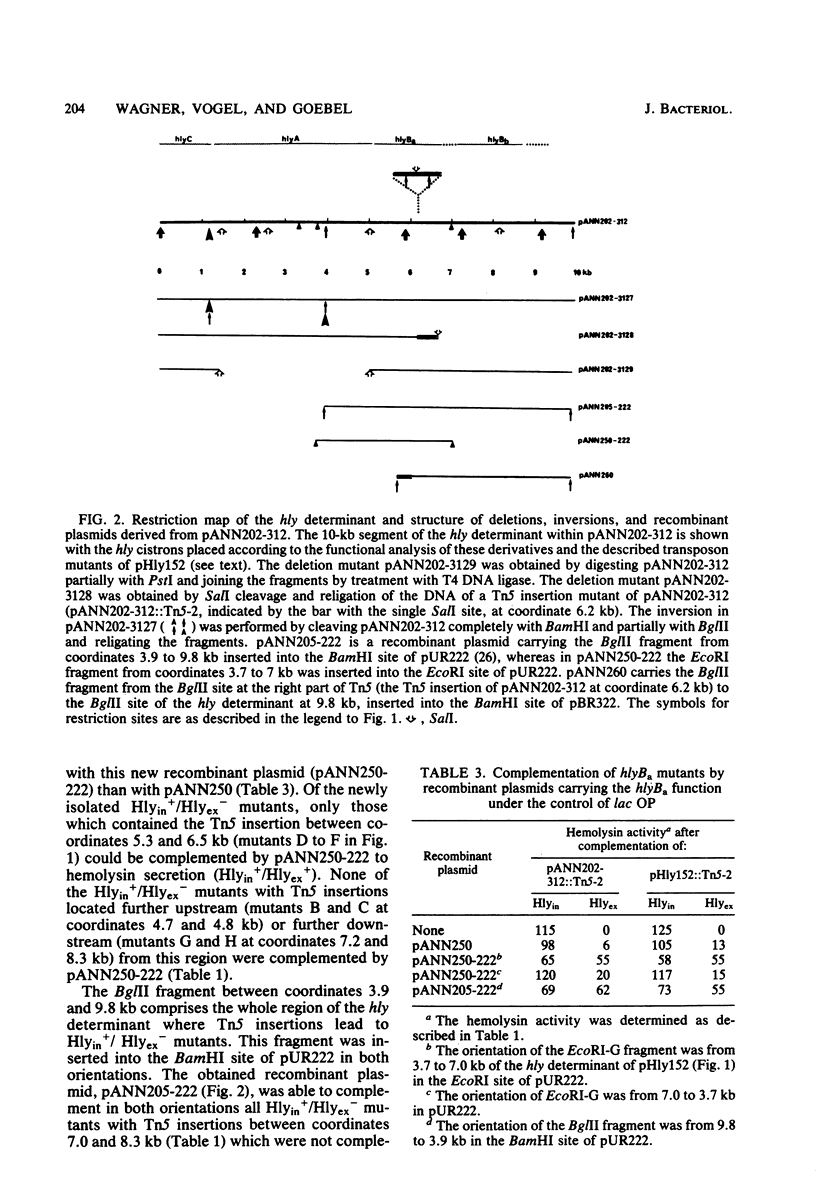
Among a large collection of hemolysis-negative mutants obtained by mutagenesis of the Hly plasmid pHly152 with Tn5, we have isolated two classes of mutants which are defective in the transport of hemolysin across the outer membrane. The two cistrons (hylBa and hlyBb) which are affected in these mutants are located adjacent to each other on the hly determinant but are transcribed from different promoters. Recombinant plasmids were constructed which carry the two functions as combined or separated cistrons. These were shown to complement the two types of transport mutants. Studies on the compartmentation of hemolysin in these two classes of mutants indicate that most hemolysin (greater than 70%) in hlyBa mutants is located in the periplasmic space, whereas in hlyBb mutants a larger portion of hemolysin is associated with the outer membrane fraction. The phenotypic appearance of colonies from hlyBb mutants is that of beta-hemolytic Escherichia coli strains, indicating that a substantial portion of hemolysin has already reached the outside of the outer membrane without being released into the medium. Release was achieved readily when hlyBb mutants were complemented with a recombinant plasmid carrying hlyBb.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Breton C., Hofnung M. In vivo and in vitro functional alterations of the bacteriophage lambda receptor in lamB missense mutants of Escherichia coli K-12. J Bacteriol. 1981 Dec;148(3):845–852. doi: 10.1128/jb.148.3.845-852.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J. M., Perrin D., Hedgpeth J. Analysis of lambda receptor and beta-lactamase synthesis and export using cloned genes in a minicell system. Mol Gen Genet. 1982;185(2):302–310. doi: 10.1007/BF00330802. [DOI] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Royer-Pokora B., Lindenmaier W., Bujard H. Plasmids controlling synthesis of hemolysin in Escherichia coli: molecular properties. J Bacteriol. 1974 Jun;118(3):964–973. doi: 10.1128/jb.118.3.964-973.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Clement J. M., Marchal C., Perrin D., Hofnung M. DNA sequence encoding the NH2-terminal peptide involved in transport of lambda receptor, an Escherichia coli secretory protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2621–2625. doi: 10.1073/pnas.77.5.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. C., Gho D., Müller-Hill B. Isolation, genetic analysis, and characterization of Escherichia coli mutants with defects in the lacY gene. J Bacteriol. 1977 Sep;131(3):830–838. doi: 10.1128/jb.131.3.830-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Phillips R., Roberts A. P. Serum resistance among Escherichia coli strains causing urinary tract infection in relation to O type and the carriage of hemolysin, colicin, and antibiotic resistance determinants. Infect Immun. 1982 Jan;35(1):270–275. doi: 10.1128/iai.35.1.270-275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K. S., Model P. Mechanism of export of colicin E1 and colicin E3. J Bacteriol. 1979 Jun;138(3):770–778. doi: 10.1128/jb.138.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D., Hughes C., Goebel W. Relationship between plasmid and chromosomal hemolysin determinants of Escherichia coli. J Bacteriol. 1983 Feb;153(2):846–851. doi: 10.1128/jb.153.2.846-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Noegel A., Rdest U., Goebel W. Determination of the functions of hemolytic plasmid pHly152 of Escherichia coli. J Bacteriol. 1981 Jan;145(1):233–247. doi: 10.1128/jb.145.1.233-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Springer W., Goebel W. Plasmid cistrons controlling synthesis and excretion of the exotoxin alpha-haemolysin of Escherichia coli. Mol Gen Genet. 1979 Oct 1;175(3):343–350. doi: 10.1007/BF00397234. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Oudega B., Stegehuis F., van Tiel-Menkveld G. J., de Graaf F. K. Protein H encoded by plasmid CloDF13 is involved in excretion of cloacin DF13. J Bacteriol. 1982 Jun;150(3):1115–1121. doi: 10.1128/jb.150.3.1115-1121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Rosenbusch J. P. Release of colicin E2 from Escherichia coli. J Bacteriol. 1981 Jul;147(1):186–192. doi: 10.1128/jb.147.1.186-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The transmissible nature of the genetic factor in Escherichia coli that controls haemolysin production. J Gen Microbiol. 1967 Apr;47(1):153–161. doi: 10.1099/00221287-47-1-153. [DOI] [PubMed] [Google Scholar]
- Springer W., Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. doi: 10.1128/jb.144.1.53-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenne S., Cavard D., Lazdunski C. Biosynthesis and export of colicin A in Citrobacter freundii CA31. Eur J Biochem. 1981 Jun 1;116(3):615–620. doi: 10.1111/j.1432-1033.1981.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Müller D., Ortiz J. M., Goebel W. Hemolysis determinant common to Escherichia coli hemolytic plasmids of different incompatibility groups. J Bacteriol. 1980 Aug;143(2):825–833. doi: 10.1128/jb.143.2.825-833.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F., Zabala J. C., Ortiz J. M. Incompatibility among alpha-hemolytic plasmids studied after inactivation of the alpha-hemolysin gene by transposition of Tn802. Plasmid. 1979 Oct;2(4):507–519. doi: 10.1016/0147-619x(79)90050-7. [DOI] [PubMed] [Google Scholar]


