Abstract
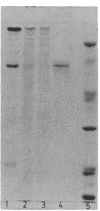
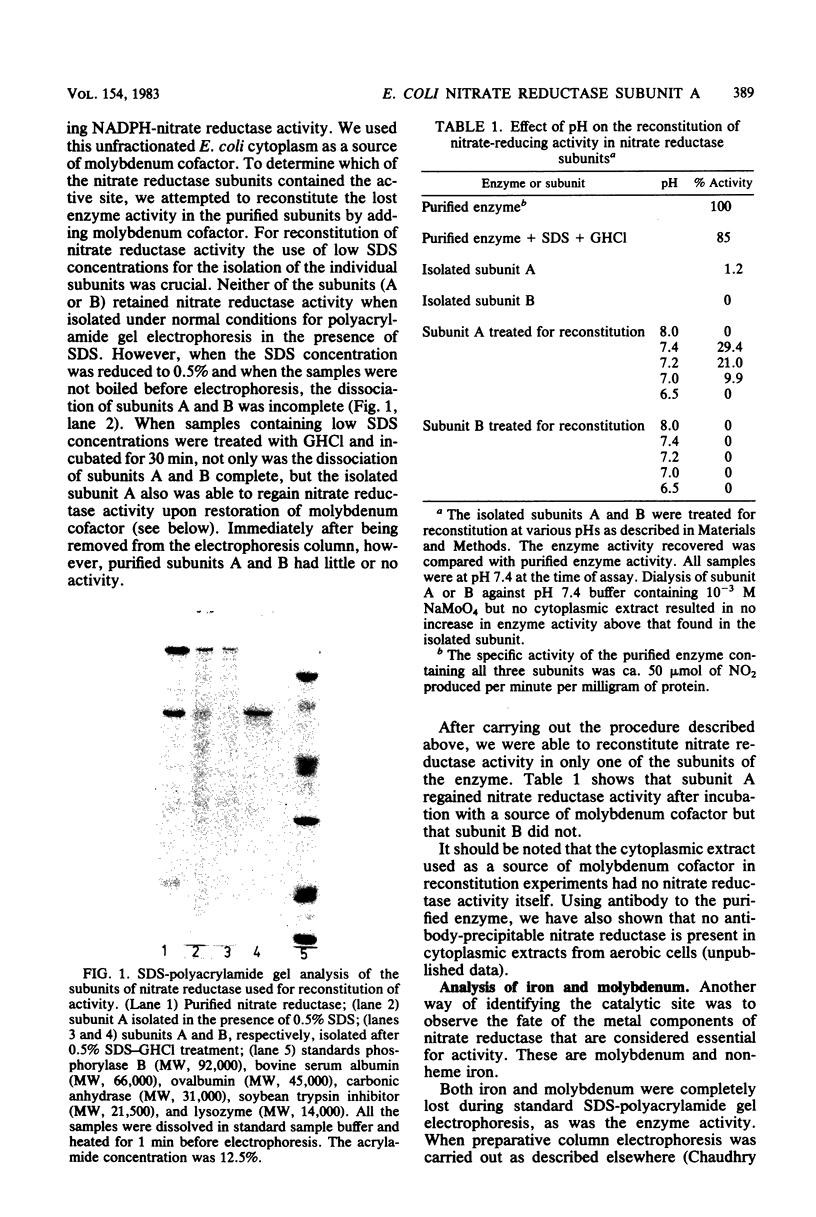
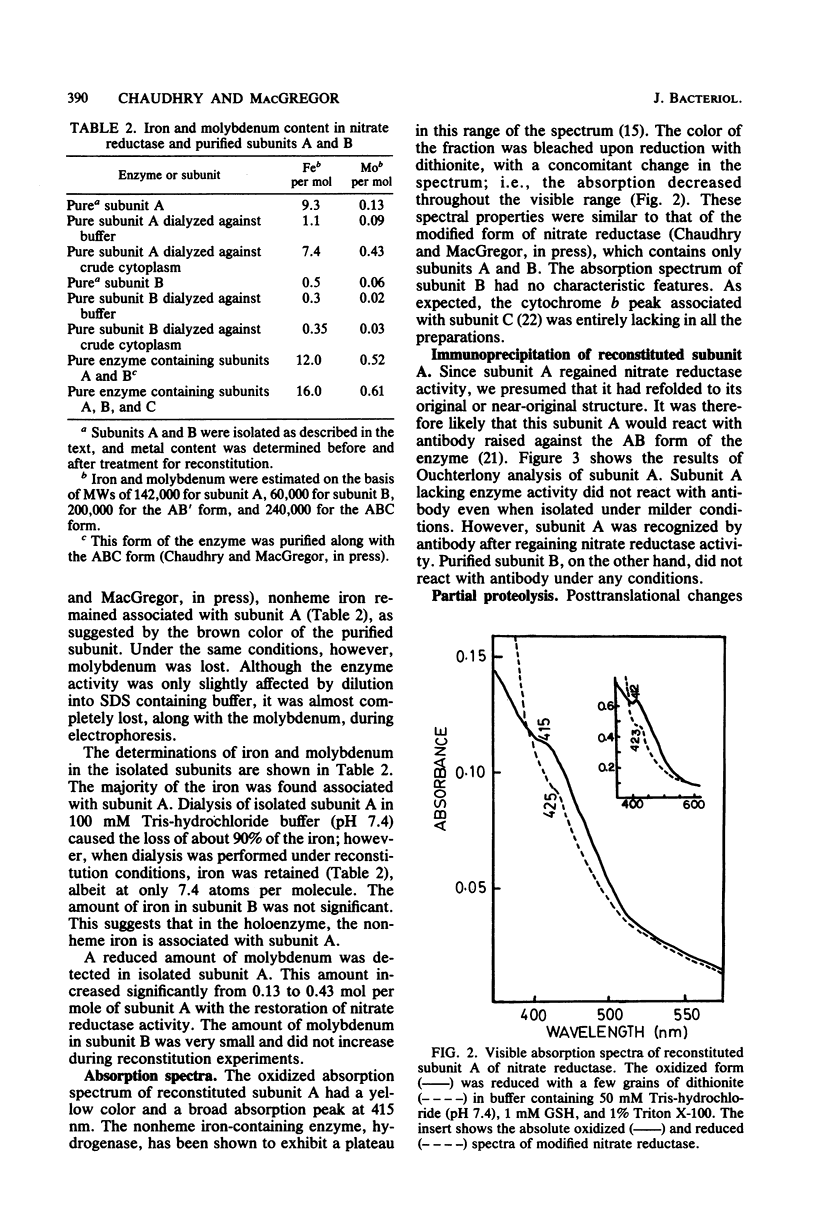
Subunits A and B were isolated from purified nitrate reductase by preparative electrophoresis in low levels of sodium dodecyl sulfate. Nonheme iron and low levels of molybdenum were associated with isolated subunit A but not with isolated subunit B. After dialysis against a source of molybdenum cofactor, subunit A regained tightly bound molybdenum and concomitantly regained enzyme activity and reactivity with anti-nitrate reductase antiserum. Subunit B neither bound cofactor nor regained activity or reactivity with antiserum. These data indicate that subunit A contains the active site of the enzyme. Subunit A was also found to be modified posttranslationally in a similar fashion as is subunit B. This was determined by comparison of partial proteolytic digests and amino acid analyses of A subunits from precursor and membrane-bound forms of nitrate reductase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy N. K., Rajagopalan K. V. Characterization of molybdenum cofactor from Escherichia coli. J Bacteriol. 1979 Oct;140(1):114–124. doi: 10.1128/jb.140.1.114-124.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- DeMoss J. A. Limited proteolysis of nitrate reductase purified from membranes of Escherichia coli. J Biol Chem. 1977 Mar 10;252(5):1696–1701. [PubMed] [Google Scholar]
- Demoss J. A., Fan T. Y., Scott R. H. Characterization of subunit structural alterations which occur during purification of nitrate reductase from Escherichia coli. Arch Biochem Biophys. 1981 Jan;206(1):54–64. doi: 10.1016/0003-9861(81)90065-5. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. H. The induction of nitrite reductase in Neurospora crassa. Biochim Biophys Acta. 1972 May 16;264(3):481–489. doi: 10.1016/0304-4165(72)90011-6. [DOI] [PubMed] [Google Scholar]
- Gewitz H. S., Piefke J., Vennesland B. Purification and characterization of demolybdo nitrate reductase (NADH-cytochrome c oxidoreductase) of Chlorella vulgaris. J Biol Chem. 1981 Nov 25;256(22):11527–11531. [PubMed] [Google Scholar]
- Giordano G., Grillet L., Pommier J., Terriere C., Haddock B. A., Azoulay E. Precursor forms of the subunits of nitrate reductase in chlA and chlB mutants of Escherichia coli K12. Eur J Biochem. 1980 Apr;105(2):297–306. doi: 10.1111/j.1432-1033.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Implication of alpha-subunit of Escherichia coli nitrate reductase in catalytic activity [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):329–330. doi: 10.1042/bst0080329a. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hackett C. S., MacGregor C. H. Synthesis and degradation of nitrate reductase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):352–359. doi: 10.1128/jb.146.1.352-359.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lund K., DeMoss J. A. Association-dissociation behavior and subunit structure of heat-released nitrate reductase from Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2207–2216. [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976 Apr;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., McElhaney G. E. New mechanism for post-translational processing during assembly of a cytoplasmic membrane protein? J Bacteriol. 1981 Nov;148(2):551–558. doi: 10.1128/jb.148.2.551-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichinoty F., Piéchaud M. Recherche des nitrate-réductases bactériennes A et B: méthodes. Ann Inst Pasteur (Paris) 1968 Jan;114(1):77–98. [PubMed] [Google Scholar]
- Ramadoss C. S., Shen T. C., Vennesland B. Molybdenum insertion in vitro in demolybdo nitrate reductase of Chlorella vulgaris. J Biol Chem. 1981 Nov 25;256(22):11532–11537. [PubMed] [Google Scholar]
- Scott R. H., DeMoss J. A. Formation of the formate-nitrate electron transport pathway from inactive components in Escherichia coli. J Bacteriol. 1976 Apr;126(1):478–486. doi: 10.1128/jb.126.1.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. P. Oxidation--reduction potentials of molybdenum and iron--sulphur centres in nitrate reductase from Escherichia coli. Biochem J. 1979 Feb 1;177(2):757–759. doi: 10.1042/bj1770757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]