Summary
Supportive social interactions may be protective against stressors and certain mental and physical illness, while social isolation may be a powerful stressor. Prairie voles are socially monogamous rodents that model some of the behavioral and physiological traits displayed by humans, including sensitivity to social isolation. Neuroendocrine and behavioral parameters, selected for their relevance to stress and depression, were measured in adult female and male prairie voles following 4 weeks of social isolation versus paired housing. In Experiment 1, oxytocin-immunoreactive cell density was higher in the hypothalamic paraventricular nucleus (PVN) and plasma oxytocin was elevated in isolated females, but not males. In Experiment 2, sucrose intake, used as an operational definition of hedonia, was reduced in both sexes following 4 weeks of isolation. Animals then received a resident-intruder test, and were sacrificed either 10 minutes later for the analysis of circulating hormones and peptides, or two hours later to examine neural activation, indexed by c-Fos expression in PVN cells immunoreactive for oxytocin or corticotropin-releasing factor (CRF). Compared to paired animals, plasma oxytocin, ACTH and corticosterone were elevated in isolated females and plasma oxytocin was elevated in isolated males, following the resident-intruder test. The proportion of cells double-labeled for c-Fos and oxytocin or c-Fos and CRF was elevated in isolated females, and the proportion of cells double-labeled for c-Fos and oxytocin was elevated in isolated males following this test. These findings suggest that social isolation induces behavioral and neuroendocrine responses relevant to depression in male and female prairie voles, although neuroendocrine responses in females may be especially sensitive to isolation.
Keywords: Adrenocorticotropic hormone, Corticosterone, Corticotropin-releasing factor, Depression, Double labeling, Fos, Hypothalamic-pituitary-adrenal axis, Immunohistochemistry, Oxytocin, Resident-intruder, Social behavior
1. Introduction
Mood disorders, such as depression, are associated with neuroendocrine alterations that are similar to the body's responses to stressors (Glowa & Gold, 1991; Grippo et al., 2002; Garlow & Nemeroff, 2004; Grippo et al., 2005a). Depression is associated with altered corticotropin-releasing factor (CRF), elevated hypothalamic-pituitary-adrenal (HPA) axis hormones, and impaired feedback regulation of the HPA axis (Asnis et al., 1987; Banki et al., 1992; Raadsheer et al., 1995; Sullivan Hanley & Van de Kar, 2003; Young et al., 2004). Similar changes, including alterations in corticosterone and adrenocorticotropic hormone (ACTH), impaired feedback control of HPA axis functioning, impaired glucorticoid receptor binding (increased mRNA expression and density of binding sites) in the hippocampus, cortex and dorsal raphe nucleus, and altered CRF input to the dorsal raphe nucleus, have been observed in several validated animal models of depression (Froger et al., 2004; Maier & Watkins, 2005; Grippo et al., 2005a; Grippo et al., 2005b) [but see (Azpíroz et al., 1999) for negative findings regarding circulating corticosterone levels]. Some of these responses are sexually dimorphic, with female rodents possibly showing increased sensitivity to stressors (Dalla et al., 2005).
The social environment, including increased physiological reactivity to social stressors, influences behavioral and neuroendocrine dysfunction associated with mood disorders (Anisman & Zacharko, 1992; Post, 1992; Sapolsky, 1996; Heinrichs et al., 2003; Ploog, 2003; Tafet & Bernardini, 2003; Adams et al., 2004; Steptoe et al., 2004). In humans, a sense of loneliness is related to symptoms of depression and cardiovascular responses to a psychological stressor (Steptoe et al., 2004), and to increased peripheral resistance (Cacioppo et al., 2002). Oxytocin and CRF are particularly relevant to the consequences and causes of sociality, and possibly to affective disorders. Oxytocin treatment combined with social support attenuates cortisol responses and anxiety reactions to a social stressor in humans (Heinrichs et al., 2003). In rats, intracerebroventricular oxytocin attenuates stressor-induced corticosterone responses and anxiety-like responses (Windle et al., 1997).
The present study investigated the role of a negative social environmental manipulation (social isolation) in mediating both behavioral and neuroendocrine responses related to depression. Female and male prairie voles (Microtus ochrogaster) were selected as subjects based on their display of face-valid social behaviors (e.g., behaviors that model those observed in humans), including an active engagement in and reliance on their social environment (Carter et al., 1995; Carter & Keverne, 2002). These animals typically live either in pairs or family groups in nature (Getz & Carter, 1996). Although there is an extensive literature on regulation of social behavior in prairie voles (see Carter et al., 1995; Insel & Young, 2001), neurobiological effects of social isolation in this species are only recently being investigated. Social isolation in prairie voles may alter HPA axis function (Ruscio et al., 2007) and reduce neurogenesis in hypothalamus and amygdala (Fowler et al., 2002).
Two parallel experiments were conducted in the current study to investigate specifically the influence of isolation on basal and stressor-induced neuroendocrine dysfunction and behaviors relevant to depression. Recent findings from our laboratory have shown that chronic social isolation (2 months) in female prairie voles is associated with behavioral alterations related to depression as well as exaggerated stressor-induced endocrine responses, including elevated circulating levels of oxytocin and corticosterone in isolated animals [versus paired (control) animals] following exposure to a 5-minute acute social stressor (Grippo et al., 2007). However, it is currently unclear whether these changes following exposure to an acute stressor are due to increased basal levels of circulating hormones and peptides as a result of the chronic isolation, or whether the changes are due to increased reactivity to the acute stressor in isolated animals. In addition, possible sex differences in these responses have not previously been examined using the prairie vole model. Therefore, in the current study (Experiment 1) we examined in both female and male prairie voles the effects of 4 weeks of social isolation on basal circulating levels of oxytocin, ACTH and corticosterone, as well as basal oxytocin- and CRF-immunoreactivity in hypothalamic paraventricular nucleus (PVN) neurons. Given previous findings indicating that the social environment mediates behavior and neuroendocrine function related to depressive disorders (Anisman & Zacharko, 1992; Post, 1992; Sapolsky, 1996; Heinrichs et al., 2003; Ploog, 2003; Tafet & Bernardini, 2003; Adams et al., 2004; Steptoe et al., 2004), and those from our laboratory showing specifically that female prairie voles are sensitive to the effects of a combination of chronic social isolation (2 months) and an acute stressor (5-minute resident-intruder test) (Grippo et al., 2007), we hypothesized that isolation alone in the present study would induce some basal neuroendocrine disturbances, especially in female prairie voles.
Similarly, our previous investigation in female prairie voles suggested that 2 months of social isolation (versus social pairing) may be associated with increased numbers of oxytocin- and CRF-immunoreactive cells in the PVN following exposure to a 5-minute resident-intruder stressor (Grippo et al., 2007). However, the mechanisms of central and peripheral changes in response to an acute stressor in isolated prairie voles are not clear. Therefore, in a second experiment in the present study (Experiment 2), we again investigated the effects of 4 weeks of isolation in both female and male prairie voles. Following a 5-minute resident-intruder stressor [using our previously published procedures (Grippo et al., 2007)], Fos expression in cells immunoreactive for either oxytocin or CRF was assessed in the PVN, to investigate whether both female and male isolated animals display increased neural activation in stressor-responsive PVN cells. We also measured circulating oxytocin, ACTH and corticosterone following the resident-intruder stressor, to determine whether 4 weeks of isolation in both female and male animals was sufficient to induce plasma changes in these hormones and peptides after exposure to an acute a stressor. We hypothesized specifically that isolation would increase HPA axis and oxytocin activation both in the brain and in the peripheral nervous system following exposure to the resident-intruder stressor. Finally, in Experiment 2 we also investigated a behavioral index of depression, assessment of sucrose intake; this measure in rodents is sensitive to antidepressant treatment and represents the reduced responsiveness to pleasurable stimuli (anhedonia) that is often observed in human depression (Willner et al., 1987; American Psychiatric Association, 2000; Grippo et al., 2006). We hypothesized that 4 weeks of isolation would induce anhedonia in both male and female prairie voles [similar to the effects of 2 months of isolation that have been previously reported in female animals (Grippo et al., 2007)], and that a progressive deficit in hedonic responses would be observed across 4 weeks of isolation.
2. Methods
2.1 Experiment 1
2.1.1 General Experimental Design
The general experimental design of Experiment 1 is described here; specific methodological procedures are presented in the following sections. Adult, female and male prairie voles were randomly assigned to be socially isolated (4 weeks; n = 8 female and n = 8 male) or pair-housed with a same-sex sibling (control conditions; n = 8 female and n = 8 male). Following this 4-week period, all animals were sacrificed under anesthesia for the collection of blood and brain tissue. Basal circulating hormones and peptides (oxytocin, ACTH, and corticosterone) were analyzed in the plasma in both sexes. Basal central hormones and peptides (oxytocin-immunoreactivity and CRF-immunoreactivity) were analyzed in the PVN in both sexes.
2.1.2 Animals
Animals in this study were adult, reproductively naïve1 prairie voles of both sexes (n = 16 female and n = 16 male) ranging between 60 and 90 days of age, and between 40-50 grams in body weight. Animals were descendants of a wild stock originally caught near Champaign, Illinois. Animals were maintained on a 14/10 h light/dark cycle (lights on at 0600 h), with a temperature of 25 ± 1° C and relative humidity of 21 ± 4 g/m3. All animals were allowed food (Purina rabbit chow; Purina, St. Louis, Missouri) and water ad libitum. Offspring remained in their natal group in large polycarbonate cages (25 × 45 × 60 cm) with cotton nesting material until weaning at 21 days of age. At this time, animals were housed in same-sex sibling pairs in smaller cages (12 × 18 × 28 cm) until the commencement of the study. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were preapproved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
2.1.3 Social Isolation
Animals were randomly divided into paired (control; n = 8 female and n = 8 male) or isolated (n = 8 female and n = 8 male) conditions; only one animal from each sibling pair was studied. Animals were subjected to social isolation (total 4 weeks) after living with a same-sex sibling in a standard-sized cage since weaning. Isolation involved removing the experimental animal from the home cage and placing it into an individual cage (in a separate room, beyond smelling distance from the sibling) for 4 weeks. Paired (control) animals also were moved into new cages at the same time as the isolated animals, and then were continually housed with their same-sex siblings for the length of the respective isolation period. The two groups were matched on handling and cage changing throughout the period of isolation or pairing.
2.1.4 Collection of Plasma
Following the period of social isolation or pairing, all animals were anesthetized with a mixture of ketamine (67 mg/kg, SC; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, SC; NLS Animal Health, Owings Mills, MD) during the light period (3-5 hours after light onset). The animal was anesthetized within 1 minute of being removed from the housing room. Blood was sampled within 2 minutes of the anesthetic injection, from the periorbital sinus via a heparanized capillary tube, and was collected during a period not exceeding 1 minute. The blood was placed immediately on ice, and then centrifuged at 4° C, at 3500 rpm, for 15 minutes to obtain plasma. Plasma aliquots were stored at -80° C until assayed for circulating hormones and peptides.
2.1.5 Circulating Hormone and Peptide Analysis
Plasma levels of oxytocin were determined using a commercially available enzyme-linked immunosorbent assay kit (Assay Designs, Ann Arbor, MI), which has been validated previously for use in prairie voles (Kramer et al., 2004). Inter- and intra-assay coefficients of variation for oxytocin are 10.0% and 10.5%, respectively. The minimum detection limit for this assay is 11.7 pg/ml. The antibody has negligible cross-reactivity (< 0.04%) with similar mammalian peptides.
Plasma levels of ACTH were determined by radioimmunoassay according to procedures described elsewhere (Li et al., 1993). The inter- and intra-assay coefficients of variation are 14.6% and 4.2%, respectively. The sensitivity of this assay is 0.25 pg/tube.
Plasma levels of corticosterone were determined using a commercial radioimmunoassay kit (MP Biomedicals, Irvine, CA). The plasma was diluted in assay buffer as necessary (1:2000) to give results reliably within the linear portion of the standard curve. The inter- and intra-assay coefficients of variation for corticosterone are both less than 5%, and cross reactivity with other steroids is less than 1%. The minimum detectable dose for this assay is 7.7 ng/ml.
2.1.6 Collection of Tissue
Following collection of blood, all animals were sacrificed under anesthesia via cervical dislocation. Brains were carefully removed from the skulls and processed with a passive perfusion (i.e., spin immersion) technique described previously by our laboratory (Cushing et al., 2001; Grippo et al., 2007). Briefly, brains were immersed in a fixative solution consisting of 4% paraformaldehyde containing 5% acrolein (pH 8.6) for a total of 4 hours. Brains were postfixed for 24 hours in 4% paraformaldehyde, and sunk in 25% sucrose. Tissue was stored in 25% sucrose at 4° C until it was sectioned at 40 μm on a freezing sliding microtome. Sliced serial brain sections were stored in wells at -20° C, in cryoprotectant antifreeze solution, until assayed for hormones and peptides in the PVN.
2.1.7 Immunohistochemistry and Image Analysis
Serial brain slices (40 μm) from the PVN were assayed for oxytocin and CRF using standard avidin:biotinylated enzyme complex (ABC) immunohistochemistry. Anti-CRF (generously provided by Dr. Ann-Judith Silverman) was used at a concentration of 1:50,000, and anti-oxytocin (generously provided by Dr. Mariana Morris) was used at a concentration of 1:300,000. Both antibodies were generated in rabbit.
Free-floating sections were rinsed 6 times during a 1 hour period with potassium phosphate buffered saline (KPBS). Sections were then incubated in 1% sodium borohydride for 20 minutes at room temperature. After multiple washes in KPBS, sections were incubated in 0.014% phenylhydrazine for 15 minutes at room temperature. Tissue was rinsed 6 times during a period of 1 hour in KPBS. Sections were then incubated in primary antibody for either oxytocin or CRF diluted in KPBS + 0.4% Triton X-100 for 1 hour at room temperature, and then incubated for 42 hours at 4° C. Following this incubation period, sections were rinsed 10 times during a period of 1 hour with KPBS. Sections were then incubated in anti-rabbit IgG (BA-1000; Vector Laboratories, Burlingame, CA; 1:600) for 1 hour at room temperature. Sections were rinsed 5 times during a period of 50 minutes with KPBS, and then incubated in A/B solution (Vectastain Elite PK-6100; Vector Laboratories, Burlingame, CA; 45 μl A, 45 μl B per 10 ml KPBS + 0.4% Triton X-100) for 1 hour at room temperature. Sections were rinsed 3 times in KPBS and then 3 times in Tris buffered saline. Both oxytocin and CRF were visualized by incubation in diaminobenzadine (DAB) dissolved in Tris buffered saline, for 15 minutes at room temperature, and then sections were rinsed 3 times with Tris buffered saline and 3 times with KPBS.
Stained sections were mounted on gelatin coated slides, air-dried, dehydrated in a series of ethanol dilutions, cleared with Histoclear (National Diagnostics, Atlanta, GA), and then protected with coverslips using Histomount mounting medium (National Diagnostics, Atlanta, GA).
Images were captured using a Nikon Eclipse E 800 microscope, Sensi-cam camera and IPLab software (Scanalytics, Inc., Fairfax, VA). The density of oxytocin- and CRF-immunoreactive cell bodies was determined manually in the PVN using a 20x objective, using a standardized sampling area, according to procedures described previously by our laboratory (Ruscio et al., 2007). Measurements within the PVN were taken in a caudal section of the nucleus where the stained cells take a characteristic shape demonstrated in previous studies (Wang et al., 1996; Ruscio et al., 2007). This section is further characterized by the medial-lateral position of the fornix (relative to the third ventricle) and medial and dorsal location of the optic tract (relative to more central and ventral position in more rostral sections). It is approximate to Fig. 49 in Paxinos and Watson (2005).
Density measurements were taken from sections matched in rostral-caudal orientation to minimize variability. Two to three brain sections were analyzed from each subject, and results were averaged across sections. Counts were performed separately for each hemisphere, and the results were averaged between hemispheres. For all subjects, density measures were conducted by two trained, experimentally-blind raters, and the results were averaged between raters. Therefore, density measures were averaged across multiple brain slices, hemispheres, and raters to provide an accurate estimation of cell density in the PVN. Only cell bodies were counted; stained fibers were excluded from analysis. Damaged sections were excluded from analysis.
2.1.8 Data Analysis
The data are presented as means ± (or +) standard error of the mean (SEM) for all analyses, tables and figures. A probability value of P < 0.05 was considered to be statistically significant. All data were analyzed with two factor between-subject analyses of variance (ANOVA), with sex (male or female) and group (paired or isolated) as the independent variables and (a) body weight, (b) plasma oxytocin levels, (c) plasma ACTH levels, (d) plasma corticosterone levels, (e) oxytocin-immunoreactivity density in the PVN, or (f) CRF-immunoreactivity density in the PVN as dependent variables. Follow-up analyses were conducted using Student's t-tests (hypothesis-driven, statistically justified comparisons only) using a Bonferroni correction for all multiple comparisons; in each case, the adjusted probability value, depending on the total number of comparisons made, was used to determine whether the result was statistically significant (probability values of 0.05 are reported in the text for accuracy).
2.2 Experiment 2
2.2.1 General Experimental Design
The general experimental design of Experiment 2 is described here; specific methodological procedures are presented in the following sections. Adult, female and male prairie voles were allowed ad libitum access to 1% sucrose for 1 week prior to beginning any experimentation. Two baseline fluid intake tests were conducted. Following these tests, animals were randomly assigned to be either socially isolated (4 weeks; n = 16 female and n = 16 male) or pair-housed with a same-sex sibling (control conditions; n = 16 female and n = 16 male). A fluid intake test was conducted following 2 and 4 weeks of isolation or pairing. Following the final fluid intake test, all animals were exposed to a resident-intruder stressor for 5 minutes. Half of the animals in each group (n = 8 female and n = 8 male) were sacrificed under anesthesia, 10 minutes following the end of the resident-intruder stressor, for the collection of blood. Acute circulating stressor-induced hormone and peptide levels (oxytocin, ACTH, and corticosterone) were analyzed in the plasma in both sexes. The other half of the animals in each group (n = 8 female and n = 8 male) were sacrificed under anesthesia, 2 hours following the end of the resident-intruder stressor, for the collection of brain tissue. Acute central stressor-induced hormone and peptide levels (oxytocin-immunoreactivity + c-Fos and CRF-immunoreactivity + c-Fos) were analyzed in the PVN in both sexes.
2.2.2 Animals
Thirty-two adult, reproductively naïve female and 32 adult male prairie voles were randomly assigned to paired or isolated groups for the experimental procedures. Age, body weight and environmental conditions were identical to those specified in Experiment 1. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were preapproved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
2.2.3 Social Isolation
Animals were randomly divided into paired (control; n = 16 female and n = 16 male) or isolated (n = 16 female and n = 16 male) conditions; only one animal from each sibling pair was studied. Animals were subjected to social isolation (total 4 weeks) after living with a same-sex sibling in a standard-sized cage since weaning. The isolation/pairing procedures were identical to those specified in Experiment 1.
2.2.4 Fluid Intake
All animals were allowed ad libitum access to 1% sucrose, along with food and water, for 1 week before beginning the experimental procedures to allow for adaptation to the taste of the sucrose. The intake of water and 1% sucrose were measured according to procedures described elsewhere (see Grippo et al., 2002), as an operational index of anhedonia (reduced sucrose intake and sucrose preference, relative to paired animals and baseline intake), and were similar to the procedures used in our previous study with prairie voles (Grippo et al., 2007) (with the exception that in the current study sucrose intake was measured prior to, during, and following isolation to determine the time course of changes in hedonic responding). Food and water were removed from the animal's cage for a period of 16 hours prior to the fluid intake test. One hour before beginning the test, all animals were moved into clean, individual cages to ensure accurate fluid intake measurements of paired animals. Both groups (paired and isolated) were moved into clean cages for this 1-hour period to avoid potentially differential responses to a novel environment in the two groups. Tap water and 1% sucrose were placed on the cage in premeasured bottles, and fluid intake was monitored for 1 hour. All animals were returned to the home cage immediately following the test. Two baseline fluid intake tests were conducted (prior to isolation), and the results were averaged. Fluid intake was measured following 2 and 4 weeks of social isolation or pairing.
2.2.5 Resident-Intruder Test
Following the period of social isolation or pairing (72 hours following the final fluid intake test), all animals participated in a resident-intruder test during the light period (approximately 3-5 hours after light onset), using procedures identical to those described previously (Grippo et al., 2007). The resident-intruder paradigm has been demonstrated previously to be a stressor in rodents, including prairie voles (Bosch et al., 2004; Grippo et al., 2007). This test consisted of placing the paired or isolated animal (intruder) into the cage of an unrelated and unfamiliar animal of the same sex (resident) for 5 minutes. Residents and intruders had no prior contact before the test, and did not share parentage. Resident animals remained in their respective home cages for the test; the resident's same-sex sibling was removed from the home cage approximately 1 minute prior to the introduction of the intruder (the resident's sibling was placed in a separate cage during the length of the respective resident-intruder test, and then was replaced in the home cage following completion of the test and removal of the intruder from the cage). The behaviors were recorded with a video camera. Aggressive behavior of the intruder was analyzed by two trained, experimentally-blind raters, and was defined as aggressive grooming or posture, swatting, biting, thrusting, pulling and/or attack behavior directed toward the other animal (Mitchell et al., 2003). An overall score of aggressive behavior was determined for each animal by summing the number of episodes of each behavior, and the results were averaged between raters.
2.2.6 Collection of Plasma
Ten minutes following the completion of the resident-intruder test, half of the male and female animals in each group (paired: n = 8 female and 8 male; isolated: n = 8 female and 8 male) were anesthetized with a mixture of ketamine (67 mg/kg, SC; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, SC; NLS Animal Health, Owings Mills, MD). All procedures and materials for blood collection, separation of plasma and sample storage were identical to those specified in Experiment 1.
2.2.7 Stressor-induced Circulating Hormone and Peptide Analysis
Plasma levels of oxytocin, ACTH and corticosterone were determined using procedures and materials identical to those specified in Experiment 1.
2.2.8 Collection of Tissue
Two hours following the end of the resident-intruder test, the other half of the animals in each group (paired: n = 8 female and n = 8 male; isolated: n = 8 female and 8 male) were anesthetized as described above with a mixture of ketamine and xylazine. Animals were sacrificed under anesthesia; brains were removed, processed and prepared for staining using procedures and materials identical to those described in Experiment 1.
2.2.9 Stressor-induced Immunohistochemistry and Image Analysis
Serial brain slices (40 μm) from the PVN were assayed for co-expression of c-Fos + oxytocin-immunoreactivity and c-Fos + CRF-immunoreactivity using standard double-label ABC immunohistochemistry, according to the procedures described in Experiment 1. Anti-c-Fos (Oncogene Science, Cambridge, MA; generated in rabbit) was used at a concentration of 1:100,000, and the target was visualized using nickel-DAB dissolved in 0.175 M sodium acetate. As in Experiment 1, anti-CRF (generously provided by Dr. Ann-Judith Silverman) was used at a concentration of 1:50,000, and anti-oxytocin (generously provided by Dr. Mariana Morris) was used at a concentration of 1:300,000.
Images were captured using procedures and materials identical to those described in Experiment 1. The density of single-labeled (oxytocin, CRF, c-Fos), and the proportion of double-labeled cells (oxytocin-immunoreactive cells + c-Fos and CRF-immunoreactive cells + c-Fos) per analysis area were determined manually in the PVN at a 20x objective, using a standardized sampling area, according to procedures described in Experiment 1. Neurons were considered to be double-labeled if the stained cytoplasm and the stained nucleus were in the same focal plane and if both labels were clearly above background.
As described in Experiment 1, density measurements were taken from sections matched in rostral-caudal orientation to minimize variability, using two to three brain sections from each subject, and results were averaged across sections. Counts were performed separately for each hemisphere, and the results were averaged between hemispheres. For all subjects, density measures were conducted by two trained, experimentally-blind raters, and the results were averaged between raters. Thus, density measures were averaged across multiple brain slices, hemispheres and raters to provide an accurate estimation of cell density in the PVN.
2.2.10 Data Analysis
As in Experiment 1, the data are presented as means ± (or +) standard error of the mean (SEM) for all analyses, tables and figures, and a probability value of P < 0.05 was considered to be statistically significant. Data sets with repeated measures were analyzed with a three factor mixed-design ANOVA, with sex (male or female) and group (paired or isolated) as the independent variables and (a) body weight, (b) absolute sucrose intake, (c) absolute water intake, or (d) percent preference for sucrose compared to water [% preference = (sucrose intake / total fluid intake) × 100] as the dependent (repeated measures) variables. For significant three-way interactions, the data were then partitioned along planes of interest and analyzed using simple mixed-design ANOVAs (hypothesis-driven, statistically justified analyses only) using a Bonferroni correction for multiple ANOVAs (probability values of 0.05 are reported in the text for accuracy). Follow-up analyses were then conducted using Student's t-tests (hypothesis-driven, statistically justified comparisons only) using a Bonferroni correction for all multiple comparisons (probability values of 0.05 are reported in the text for accuracy).
Data sets with independent measures were analyzed with two factor between-subject ANOVAs, as in Experiment 1, with sex (male or female) and group (paired or isolated) as the independent variables and (a) aggression during the resident-intruder test (b) plasma oxytocin levels following the resident-intruder test, (c) plasma ACTH levels following the resident-intruder test, (d) plasma corticosterone levels following the resident-intruder test, (e) oxytocin-immunoreactivity density in the PVN following the resident-intruder test, (f) CRF-immunoreactivity density in the PVN following the resident-intruder test, (g) c-fos-immunoreactivity density in the PVN following the resident-intruder test, (h) neural activation of oxytocin cells in the PVN (i.e., proportion of c-fos-labeled oxytocin cells per analysis area) following the resident-intruder test, or (i) neural activation of CRF cells in the PVN (i.e., proportion of c-fos-labeled CRF cells per analysis area) following the resident-intruder test as dependent variables. Follow-up analyses were conducted with Student's t-tests (hypothesis-driven, statistically justified comparisons only) using a Bonferroni correction for all multiple comparisons (probability values of 0.05 are reported in the text for accuracy).
3. Results
3.1 Experiment 1
3.1.1 Body Weight
Social isolation (versus pairing) did not affect body weight in either female or male groups. Following 4 weeks of social isolation or pairing, body weight was the following: 42 ± 3 g in the paired female group; 40 ± 3 g in the isolated female group; 54 ± 4 g in the paired male group; and 50 ± 2 g in the isolated male group. The ANOVA yielded a significant main effect of sex [F(1,28) = 13.62, P < 0.001], which was due to the fact that males weighed more than females in both paired and isolated groups [paired: t(14) = 2.48, P < 0.05; isolated: t(14) = 2.87, P < 0.05; Student's t-tests with a Bonferroni correction]. There was no difference in body weight between paired and isolated females or between paired and isolated males (P > 0.05 for both comparisons; Student's t-tests with a Bonferroni correction).
3.1.2 Circulating Hormones and Peptides
Social isolation (versus pairing) led to an increase in basal circulating oxytocin in females (but not in males), but did not alter basal circulating ACTH or corticosterone levels in either sex. Fig. 1 displays the circulating hormone and peptide levels following 4 weeks of social isolation or pairing in both female and male groups. The ANOVA for oxytocin yielded a significant group by sex interaction [F(1,28) = 6.43, P < 0.02]. Social isolation induced a significant elevation in plasma oxytocin in females [t(14) = 3.22, P < 0.05], but not in males (P > 0.05). The ANOVA for ACTH and corticosterone yielded no statistically significant effects (P > 0.05 for all main effects and interactions); no follow-up tests were conducted on these variables.
Figure 1.
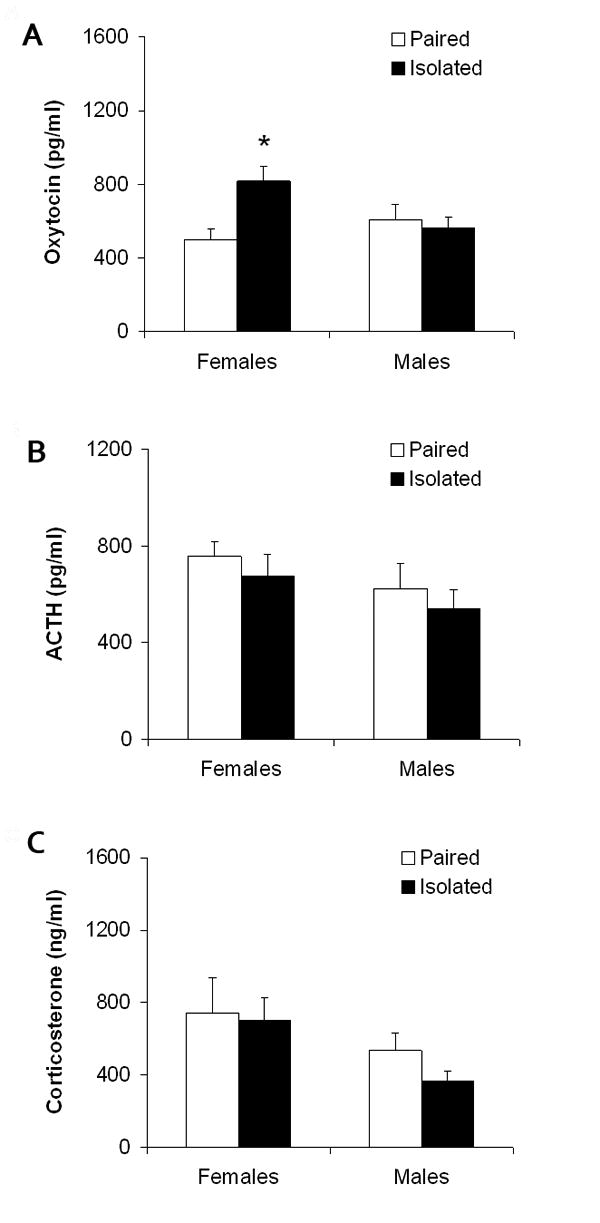
Mean (+ SEM) basal circulating oxytocin (Panel A), adrenocorticotropic hormone (ACTH; Panel B) and corticosterone (Panel C) in female and male prairie voles following 4 weeks of social isolation or pairing. Note the scale differences among the three panels. *P < 0.05 vs. paired value of the same sex.
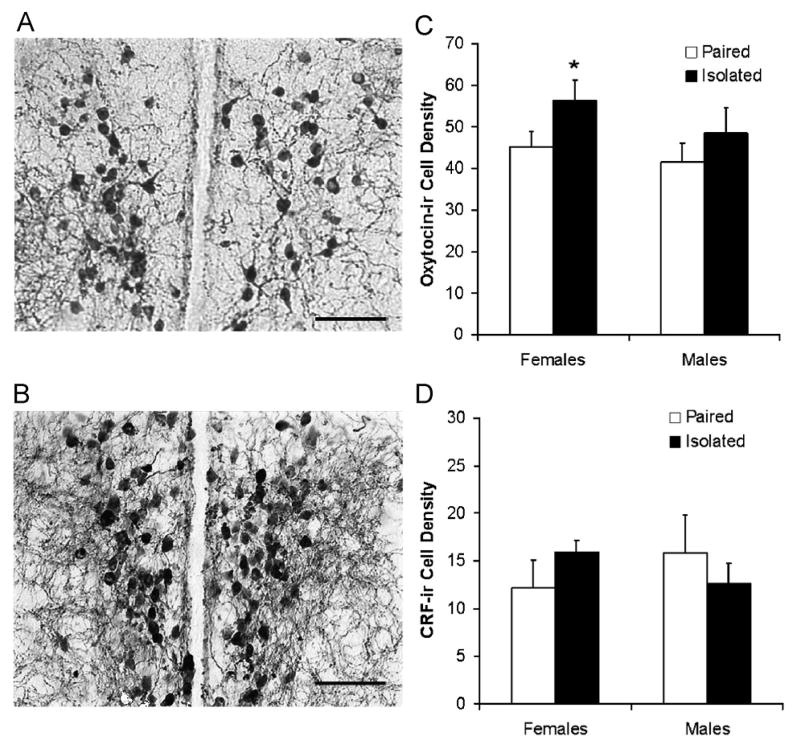
3.1.3 Tissue Hormones and Peptides
Social isolation (versus pairing) led to an increase in oxytocin-immunoreactivity density in the PVN in females (but not in males), but did not alter CRF-immunoreactivity density in the PVN in either sex. Fig. 2 shows an example of oxytocin-immunoreactivity in the PVN of a paired and isolated female (Panels A and B), and the mean number of oxytocin- and CRF-immunoreactive cells in both females and males following social isolation or pairing (Panels C and D). The ANOVA for oxytocin-immunoreactivity density yielded a significant main effect of group [F(1,28) = 7.55, P < 0.01]. Social isolation induced a relatively small, yet significant, increase in the density of oxytocin-immunoreactive cells in the PVN in females [t(14) = 1.74, P < 0.05], but not in males (P > 0.05). The ANOVA for CRH-immunoreactivity density did not yield a statistically significant effect (P > 0.05); no follow-up tests were conducted.
Figure 2.
Brain sections (40 μm) showing oxytocin-immunoreactive (ir) cell density in the hypothalamic paraventricular nucleus of a paired (Panel A) or isolated (Panel B) female prairie vole, and mean (+ SEM) density of oxytocin-ir (Panel C) and corticotropin-releasing factor (CRF)-ir cells (Panel D), averaged across multiple brain slices, hemispheres, and raters, in the hypothalamic paraventricular nucleus in female and male prairie voles following 4 weeks of social isolation or pairing. Stained fibers were excluded from analysis. Scale bars = 100 μm in Panels A and B. Note the scale differences between Panels C and D. *P < 0.05 versus paired value of the same sex.
3.2 Experiment 2
3.2.1 Body Weight
Social isolation (versus pairing) did not affect body weight in either females or males (Table 1). The ANOVA yielded a significant main effect of sex [F(1,60) = 21.07, P < 0.0001], a sex by time interaction [F(2,120) = 5.29, P < 0.006], and a sex by group by time interaction [F(2,120) = 5.10, P < 0.008]. Using a Bonferroni correction, a simple mixed-design ANOVA comparing sex and time yielded a main effect of sex [F(1,62) = 21.28, P < 0.05] and a sex by time interaction [F(2,124) = 5.04, P < 0.05], and a simple mixed-design ANOVA comparing group and time yielded no significant effects (P > 0.05 for both main effects and interaction). Using Student's t-tests with a Bonferroni correction, males weighed more than females following 2 [t(30) = 4.58, P < 0.05] and 4 weeks of isolation [t(30) = 4.18, P < 0.05] in the paired group, and following 2 weeks of isolation [t(30) = 3.63, P < 0.05] in the isolated group. However, there were no significant pairwise differences between paired and isolated groups at baseline (P > 0.05 for both comparisons) or following 4 weeks of isolation or pairing (P > 0.05 for both comparisons), in either females or males.
Table 1.
Body weight (grams) prior to and during social isolation or pairing in female and male prairie voles.
| Baseline | Week 2 | Week 4 | ||
|---|---|---|---|---|
| Females | Paired | 46 ± 2 | 39 ± 2a | 42 ± 2a |
| Isolated | 42 ± 2 | 40 ± 1a | 44 ± 1 | |
| Males | Paired | 50 ± 4 | 54 ± 2 | 55 ± 2 |
| Isolated | 50 ± 2 | 50 ± 2 | 49 ± 3 |
Note: Data are shown as mean ± SEM. There were no significant within- or between-group differences in body weight in either females or males.
P < 0.05 versus respective male group.
3.2.2 Fluid Intake
4 weeks of social isolation produced a reduction in absolute sucrose intake in both male and female prairie voles, relative to these groups' respective baseline consumption and the consumption of the paired groups, however 4 weeks of social isolation produced a reduction in sucrose preference only in females. Fig. 3 (Panels A and B) displays fluid intake in female and male groups at baseline and following 2 and 4 weeks of social isolation or pairing. The ANOVA for absolute water intake yielded a significant sex by time interaction [F(2,120) = 19.24, P < 0.0001]. However, using Student's t-tests with a Bonferroni correction, there were no significant differences in water intake between paired or isolated groups at baseline (P > 0.05 for both comparisons) or following 4 weeks of social isolation or pairing (P > 0.05 for both comparisons), in either females or males.
Figure 3.
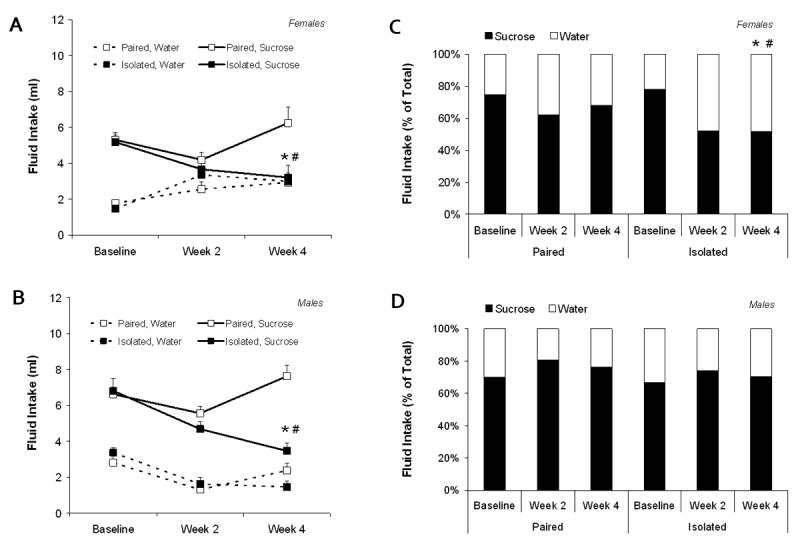
Mean (+ SEM) absolute fluid intake in female and male (Panels A and B, respectively), and mean sucrose preference, compared to water, in female and male (Panels C and D, respectively) paired and isolated prairie voles prior to and during social isolation or pairing. For sucrose intake and sucrose preference: *P < 0.05 versus respective paired value; #P < 0.05 vs. respective baseline value. Only statistically significant comparisons that are relevant to the current hypotheses are noted.
The ANOVA for absolute sucrose intake yielded a significant main effect of group [F(2,60) = 20.22, P < 0.0001], a main effect of sex [F(1,60) = 12.70, P < 0.001], a main effect of time [F(2,120) = 9.38, P < 0.0001], and a significant group by time interaction [F(2,120) = 14.72, P < 0.0001]. Using Student's t-tests with a Bonferroni correction, paired and isolated animals did not differ in their baseline sucrose intake (P > 0.05 for both females and males). Following 4 weeks of isolation or pairing, the isolated groups drank significantly less sucrose compared to the respective baseline sucrose consumption [females: t(15) = 2.37, P < 0.05; males: t(15) = 4.03, P < 0.05] and the consumption of the paired groups [females: t(30) = 2.79, P < 0.05; males: t(30) = 5.53, P < 0.05]. The paired groups did not alter sucrose intake over time (versus baseline intake; P > 0.05 for both females and males).
Fig. 3 (Panels C and D) displays sucrose preference, compared to water, in female and male groups at baseline and following 2 and 4 weeks of social isolation or pairing. The ANOVA for sucrose preference yielded a significant main effect of group [F(1,60) = 7.66, P < 0.007], a main effect of sex [F(1,60) = 12.59, P < 0.001], a main effect of time [F(2,120) = 3.89, P < 0.025], and a significant sex by time interaction [F(2,120) = 11.97, P < 0.0001]. Using Student's t-tests with a Bonferroni correction, the baseline preference for sucrose was not significantly different between paired and isolated groups (P > 0.05 for both females and males). Following 4 weeks of isolation or pairing, the female isolated group showed a significantly reduced preference, relative to its respective baseline preference [t(15) = 3.66, P < 0.05] and that of the female paired group [t(30) = 1.97, P < 0.05]. However, following 4 weeks of social isolation or pairing, sucrose preference in the isolated male group did not differ significantly from either its respective baseline preference or that of the paired male group (P > 0.05 for both comparisons). The paired groups did not alter sucrose preference over time (versus baseline preference; P > 0.05 for both females and males).
3.2.3 Resident-Intruder Test
Social isolation did not alter aggressive behavior during the resident-intruder test in either female or male prairie voles (Table 2). The ANOVA for number of aggressive episodes during the resident-intruder test yielded a significant main effect of sex [F(1,60) = 11.44, P < 0.001], which was due to the fact that the female paired and isolated groups displayed slightly more aggressive episodes during the test versus the male paired and isolated groups [paired: t(30) = 1.77, P < 0.05; isolated: t(30) = 3.71, P < 0.05; Student's t-tests with a Bonferroni correction]. However, there were no significant pairwise differences in number of aggressive episodes between paired or isolated groups (P > 0.05 for both females and males; Student's t-tests with a Bonferroni correction).
Table 2.
Number of aggressive behaviors exhibited during the resident-intruder test in female and male paired and isolated prairie voles.
Note: Data are shown as means ± SEM; all units are absolute values. There were no significant between-group (within-sex) differences in aggressive behaviors.
P < 0.05 vs. respective male group.
3.2.4 Stressor-Induced Circulating Hormones and Peptides
Social isolation (versus pairing) led to a significant increase in resident-intruder-induced circulating oxytocin levels in both female and male prairie voles, but led to a significant increase in resident-intruder-induced ACTH and corticosterone levels only in females. Fig. 4 displays the circulating hormone and peptide levels following the resident-intruder paradigm in both female and male paired and isolated groups. The ANOVA for resident-intruder-induced plasma oxytocin levels yielded a significant main effect of group [F(1,28) = 42.24, P < 0.0001], a main effect of sex [F(1,28) = 15.50, P < 0.0001], and a group by sex interaction [F(1,28) = 29.07, P < 0.0001]. Using Student's t-tests, social isolation was associated with significant elevations in plasma oxytocin following the resident-intruder paradigm in both female and male groups [females: t(7) = 2.39, P < 0.05; these values were compared using a t-test assuming unequal variances; males: t(13) = 2.74, P < 0.05].
Figure 4.
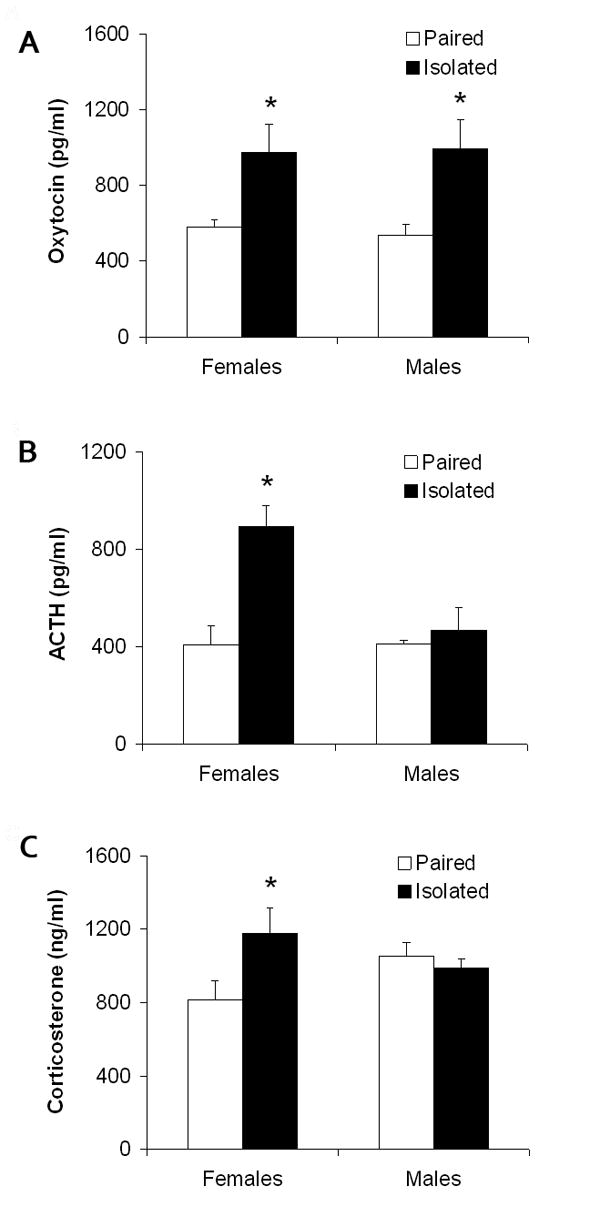
Mean (+ SEM) stressor-induced circulating oxytocin (Panel A), adrenocorticotropic hormone (ACTH; Panel B), and corticosterone (Panel C) in female and male paired and isolated prairie voles 10 minutes following a 5-minute resident-intruder test. Note the scale differences among the three panels. *P < 0.05 vs. paired value of the same sex.
The ANOVA for resident-intruder-induced plasma ACTH levels yielded a significant main effect of group [F(1,28) = 39.52, P < 0.0001]. Using Student's t-tests, social isolation was associated with significant elevations in plasma ACTH in females following the resident-intruder paradigm [t(13) = 5.08, P < 0.05]; resident-intruder-induced ACTH levels in isolated and paired males did not differ significantly (P > 0.05).
The ANOVA for resident-intruder-induced plasma corticosterone levels yielded a significant main effect of group [F(3,28) = 4.72, P < 0.004]. Using Student's t-tests, the isolated females displayed significantly elevated plasma corticosterone levels versus the paired females following the resident-intruder paradigm [t(13) = 2.48, P < 0.05]; resident-intruder-induced corticosterone levels in isolated and paired males did not differ significantly (P > 0.05).
3.2.5 Stressor-Induced Tissue Hormones and Peptides
Social isolation (versus pairing) led to increased neural activation (i.e., increased proportion of double-labeled cells) of oxytocin cells in the PVN in both female and male prairie voles, but led to increased neural activation of CRF cells in the PVN only in females. Fig. 5 shows examples of cells double-labeled for c-Fos and either oxytocin (Panels A and B) or CRF (Panels C and D). Fig. 6 shows the mean percent of oxytocin- (Panel A) and CRF-immunoreactive cells (Panel B) that expressed Fos in the PVN in both female and male paired and isolated groups.
Figure 5.
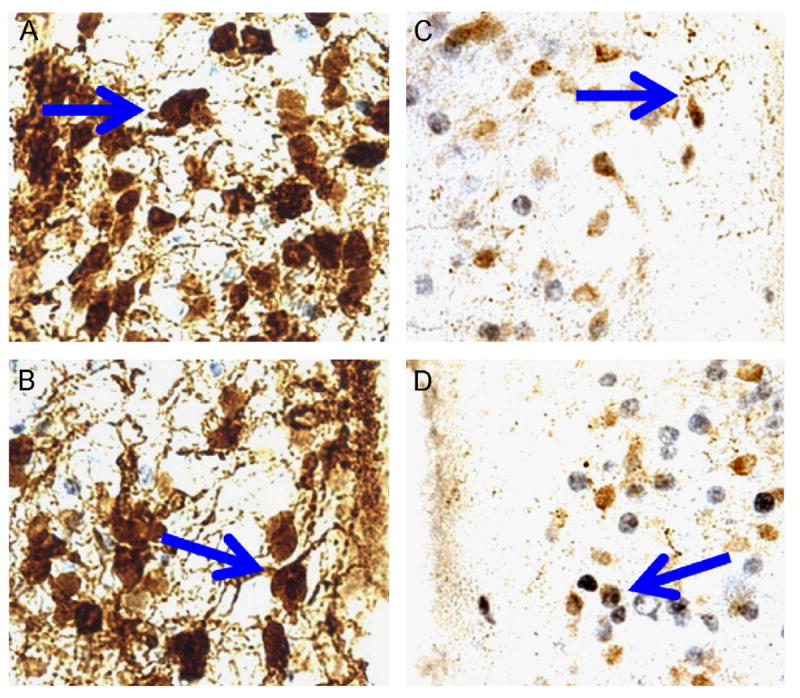
Examples of double-labeling showing immunohistochemistry for c-Fos (black nuclear stain) and either oxytocin (brown cytoplasmic stain; Panels A and B) or corticotropin-releasing factor (brown cytoplasmic stain; Panels C and D). Arrows denote cells that are double-labeled.
Figure 6.
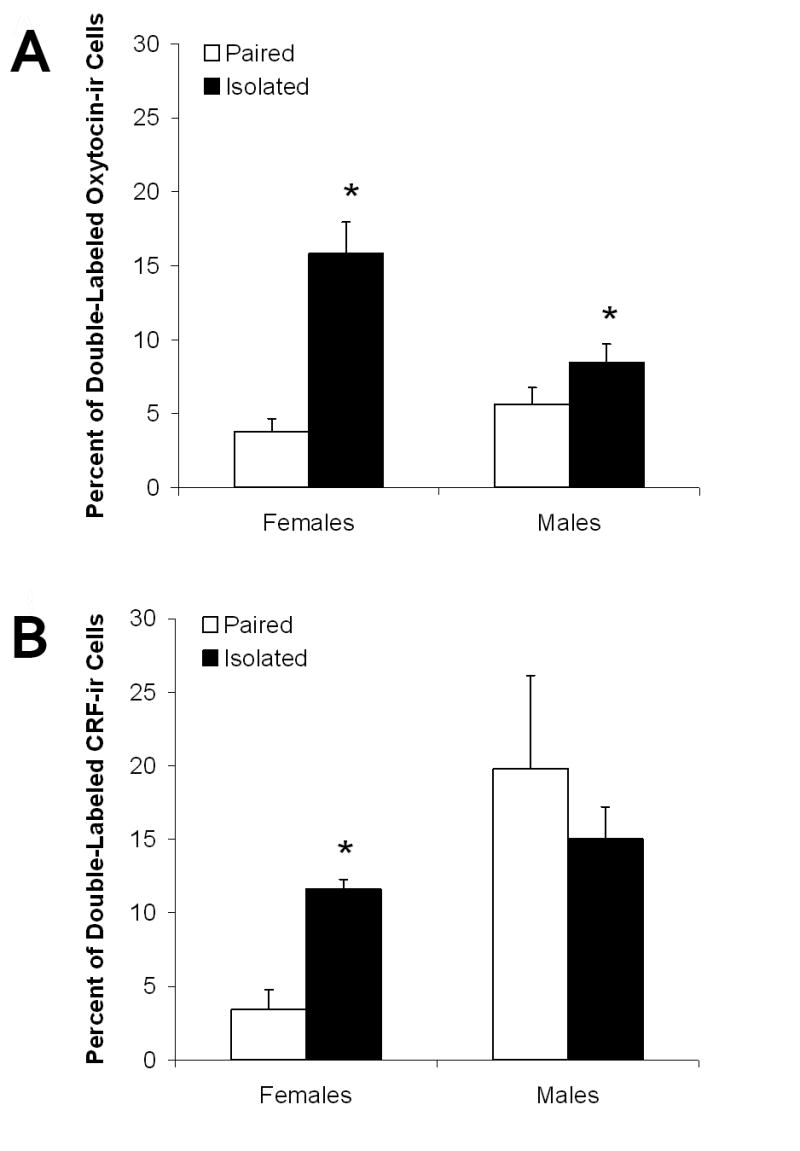
Mean (+ SEM) percentage of c-Fos-labeled oxytocin-immunoreactive (ir; Panel A) and corticotropin-releasing factor (CRF)-ir cells (Panel B) per analysis area, averaged across multiple brain slices, hemispheres, and raters, in the hypothalamic paraventricular nucleus of female and male paired and isolated prairie voles 2 hours following a 5-minute resident-intruder test. *P < 0.05 versus paired value of the same sex.
The ANOVAs for resident-intruder-induced (a) single-labeled oxytocin-immunoreactive cell density, (b) single-labeled CRF-immunoreactive cell density and (c) single-labeled c-Fos-immunoreactive cell density yielded no significant effects (P > 0.05 for all main effects and interactions); no follow-up analyses were conducted on these variables (data not shown).
The ANOVA for neural activation of oxytocin cells in the PVN (i.e., proportion of double-labeled cells) following the resident-intruder test yielded a significant main effect of group [F(1,28) = 35.74, P < 0.0001], a main effect of sex [F(1,28) = 5.77, P < 0.003], and a group by sex interaction [F(1,28) = 15.48, P < 0.001].
The ANOVA for neural activation of CRF cells in the PVN (i.e., proportion of double-labeled cells) following the resident-intruder test yielded a significant main effect of sex [F(1,28) = 11.77, P < 0.02] and a group by sex interaction [F(1,28) = 6.77, P < 0.02]. Isolated females displayed significantly increased proportion of c-Fos-labeled CRF-immunoreactive cells in the PVN after the resident-intruder test versus the paired females [t(12) = 5.41, P < 0.05]; the proportion of c-Fos-labeled CRF-immunoreactive cells in isolated and paired males after the resident-intruder test did not differ significantly (P > 0.05). Isolated males did not differ significantly from isolated males in the proportion of c-Fos-labeled CRF-immunoreactive cells in the PVN following the resident-intruder paradigm (P > 0.05).
4. Discussion
Studies in prairie voles have provided new insights into both the causes and consequences of sociality (Carter et al., 1995; Insel & Young, 2001). For instance, isolation for varying periods has both physiological and behavioral consequences (Kim & Kirkpatrick, 1996; Fowler et al., 2002; Ruscio et al., 2007; Grippo et al., 2007), however the precise central and peripheral mechanisms of social isolation-induced behavioral and neuroendocrine changes are not well elucidated. In the present study, we observed neuroendocrine and behavioral changes as a function of chronic isolation, especially in female prairie voles, including anhedonia, increased basal circulating and central hormone and peptide levels, and exaggerated acute stressor-induced peripheral and central hormone and peptide activation.
Centrally active neuropeptides, including oxytocin and CRF, have been implicated in social behavior and also the management of reactions to stressful experiences (Heinrichs et al., 1995; McCarthy & Altemus, 1997; Sánchez et al., 1998; Neumann, 2002; Windle et al., 2004; Plotsky et al., 2005). In the current study (Experiment 1) females, but not males, living in isolation for four weeks showed increased circulating levels of oxytocin (Fig. 1) and had a higher density of oxytocin-immunoreactive cells in the hypothalamic PVN (Fig. 2). However, plasma levels of ACTH and corticosterone did not differ between isolated and paired animals in either sex.
The effects of isolation on physiology and behavior were especially pronounced following brief exposure to a same-sex stranger (Experiment 2). During the resident-intruder test, aggression was observed in both paired and isolated animals (Table 2), supporting the hypothesis that this paradigm is a social stressor. After the resident-intruder test, isolated females showed increased activation of the oxytocin system as indexed by enhanced co-localization of c-Fos with oxytocin cells in the PVN and elevated plasma oxytocin levels. Additionally, these animals showed HPA axis activation, including increased co-localization of c-Fos with CRF cells in the PVN, and increased circulating ACTH and corticosterone. Males also showed an isolation-related increase in circulating oxytocin following the resident-intruder test, as well as increased co-localization of oxytocin and c-Fos in the PVN. However, males did not show elevations in ACTH or corticosterone, or increased CRF and c-Fos co-localization. The circulating hormone and peptide changes shown here in females are similar to the stressor-induced changes that we previously observed in female prairie voles exposed to 2 months of social isolation and the 5-minute resident-intruder test (Grippo et al., 2007). However, the present results, indicating that the density of single-labeled oxytocin-immunoreactive cells was increased in isolated females following isolation alone (Experiment 1), but not following isolation plus the resident-intruder stressor (Experiment 2), suggest that exposure to an acute stressor may alter oxytocin production and/or release in PVN cells, or possibly gene transcription, in isolated female prairie voles (Wotjak et al., 1998; Liu et al., 2001; Wotjak et al., 2001). Further studies, including those investigating directly oxytocin release in isolated animals exposed to acute social and non-social stressors, will elucidate the precise mechanisms underlying the changes in central and circulating oxytocin.
As shown here, female and male prairie voles appear to be differentially responsive to chronic and acute social stressors. Growing evidence suggests that sexually dimorphic responses exist following environmental and social stressors (Palanza, 2001; Klein & Corwin, 2002; Beck & Luine, 2002; McCormick et al., 2005). For instance, female rats exposed to social stressors show increased locomotor responses to amphetamine and increased corticosterone release relative to controls, whereas no effect of social stressors is found in males (McCormick et al., 2005). Female and male rats also show differential behavioral responses to chronic versus acute stressors (see Kennett et al., 1986), and socially isolated male rats, versus females, exhibit more prominent changes in the density of CRF receptors in cortical brain areas (Ehlers et al., 1993). The current observation that neural activation of CRF cells is increased following the resident-intruder stressor in isolated females (but not males) may suggest that the female HPA axis response is more sensitive to the effects of isolation versus the male response, perhaps representing sexually-dimorphic differences in social stressor responseiveness in prairie voles.
However, oxytocin was elevated following the resident-intruder stressor in both males and female isolated animals; this peptide system may serve a stress-buffering role during isolation. Oxytocin can be released during positive social experiences (Carter, 1998), but also may be increased following stressful events (Lang et al., 1983; Gibbs, 1984; Nishioka et al., 1998; Taylor et al., 2006). The precise consequences of increases in endogenous oxytocin, especially in association with putative stressors, remain to be investigated. However, based on the beneficial effects of exogenous oxytocin, it seems most likely that the release of oxytocin under these conditions is compensatory or protective (Uvnäs-Moberg, 1998). Previous investigations in prairie voles (Carter, 1998), rats (Neumann et al., 2000) and humans (Legros et al., 1987; Chiodera et al., 1991; Heinrichs et al., 2003) have shown that exogenous oxytocin can inhibit behavioral or physiological responses to stressors. Similarly, exogenous oxytocin promotes positive social behaviors in both female (Williams et al., 1994; Insel & Hulihan, 1995) and male (Cho et al., 1999) prairie voles. There is also evidence from rats that oxytocin can down-regulate CRF gene expression in the PVN (Nomura et al., 2003). In addition, mice that are genetically deficient for either oxytocin (Michelini et al., 2003; Mantella et al., 2004) or the oxytocin receptor (Takayanagi et al., 2005) tend to be highly reactive to stressors.
These previous findings suggesting a stress-buffering role for oxytocin may be consistent with the results of the current study, showing that increased oxytocin cell density following chronic isolation in females (Experiment 1) and increased neural activation of oxytocin cells in both isolated females and males after the resident-intruder stressor (Experiment 2) may have differential stress-buffering consequences in female and male prairie voles. Because oxytocin was elevated (but ACTH and corticosterone were not) in isolated males after the resident-intruder stressor, it is possible that activation of the oxytocinergic system in the brain and periphery of male prairie voles was sufficient to reduce the HPA axis response to the resident-intruder stressor; conversely, oxytocinergic activation in isolated female animals may not be sufficient to reduce the HPA axis response to the resident-intruder stressor. Indeed, treatment with exogenous oxytocin is capable of reducing HPA axis activity in male prairie voles (Carter, 1998), as well as in humans (Heinrichs et al., 2003). It is also possible that sex differences exist in the timing of either oxytocin or HPA axis reactions to stressful experiences, which may have not been detected in the present study. That is, ACTH and corticosterone levels may be reduced as a result of oxytocin release over a longer time course in female prairie voles versus males, which may explain why both ACTH and corticosterone were elevated along with oxytocin in isolated females (but not in isolated males) after the resident-intruder stressor. Consistent with this hypothesis are data from rats showing a more pronounced habituation of corticosterone responses to chronic restraint stress in males versus females (Galea et al., 1997). Sex differences in the central synthesis or effects of other neuropeptides, including vasopressin (DeVries & Simerly, 2002; DeVries & Panzica, 2006) or CRF (Lim et al., 2005; Lim et al., 2006), may also mediate the neuroendocrine responses described here.
In addition to altered basal and acute stressor-induced neuroendocrine function in the current study, social isolation was associated with a progressive reduction in sucrose intake in both sexes. However, a significant reduction in the preference for sucrose, versus water, was seen after 4 weeks of isolation only in females. Reduced sucrose intake and preference have been used as indices of anhedonia, which is a behavioral feature of human depression (Keller et al., 1995; American Psychiatric Association, 2000). The present findings are consistent with our previous observation of reduced sucrose preference in female prairie voles exposed to 2 months of isolation (Grippo et al., 2007). Further, these results extend our previous findings by demonstrating that anhedonia develops gradually over the course of isolation in prairie voles, similar to the progression of reduced hedonic responsiveness observed in rodent models of depression (Grippo et al., 2002; Grippo et al., 2003). The reduction in sucrose intake shown here represents a specific hedonic deficit. Water intake was not changed in isolated animals, indicating that there was no generalized deficit in ingestive behavior. Also, body weight was unaffected by the isolation period in both sexes, indicating that the reduction in sucrose intake was not confounded by a reduction in body weight in the isolated groups. A female bias in the occurrence of depression is found in humans (American Psychiatric Association, 2000; Cyranowski et al., 2000), and social interactions may be of particular importance to coping in women (see for review Kiecolt-Glaser & Newton, 2001). Similarly, sex differences in the social regulation of neuropeptides, including oxytocin, vasopressin and CRF, could contribute to the physiological mechanisms underlying female vulnerability to depression (Carter & Altemus, 2005). The precise consequences of increased central and peripheral oxytocin function in prairie voles after isolation, and how these changes relate to decreased hedonic responsiveness, are not yet elucidated and should be a target of future research. To this end, Light and colleagues have reviewed evidence that oxytocin may serve to modulate affective states in human mothers (see Light et al., 2004).
A limitation of the current series of experiments involves studying social environmental manipulations in socially monogamous mammals (such as prairie voles or humans). In particular, the present experimental design may have included uncontrolled variables associated with temporary separation of paired animals from their respective siblings (e.g., during the resident-intruder stressor). However, if this was that case, differences between paired and isolated groups may have been less pronounced or absent; whereas the present results indicate that several robust behavioral and neuroendocrine differences exist between paired and isolated animals. However, future studies should consider the inclusion of additional control groups when investigating behavioral and neuroendocrine responses to acute social manipulations in socially monogamous rodent models, including paired and isolated animals that are temporarily moved into a new cage but not exposed to an additional stressor.
To conclude, the current findings indicate that social isolation induces slight basal neuropeptide changes in female prairie voles (but not in males), as well as anhedonia and an acute stressor-induced neuroendocrine profile in both sexes that are similar to the changes observed in human depression. Some of these responses may be sexually dimorphic, including perhaps a reduced stressor response (both central and peripheral), or a different time course in the stress-buffering consequences of oxytocin, in males versus females. These findings provide further insight into the mechanisms of reactivity to social stressors. Continues research with translational animal models, including studies in highly social mammals such as prairie voles, can provide a greater understanding of the mechanisms through which disorders such as depression are induced or exacerbated by isolation or a perceived lack of social support.
Acknowledgments
This research was funded by MH73233 (AJG), MH67446, MH72935 and HD48390 (CSC). The authors would like to thank Dr. Mariana Morris and Dr. Ann-Judith Silverman for generously donating the antibodies used in these experiments. The authors are grateful to Dr. Bruce Cushing, Ms. Francisca Garcia, Dr. Ralph Johnson, Ms. Rayna Brooks, Ms. Felice Dredze, Ms. Lisa Sanzenbacher, and Ms. Yuyu Song for technical assistance.
Footnotes
The female animals studied in both Experiments 1 and 2 were reproductively naïve. Female prairie voles do not show a spontaneous puberty or estrous cycle; in this species the ovaries remain inactive until the female has physical contact with a male (Carter et al., 1987), which allows for the use of reproductively intact animals without the need for controlling the estrous cycle (e.g., via ovariectomy).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KB, Sanders S, Auth EA. Loneliness and depression in independent living retirement communities: risk and resilience factors. Aging Ment Health. 2004;8:475–485. doi: 10.1080/13607860410001725054. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. fourth. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J Psychiatry. 1992;160 15:36–43. [PubMed] [Google Scholar]
- Asnis GM, Halbreich U, Ryan ND, Rabinowicz H, Puig-Antich J, Nelson B, Novacenko H, Friedman JH. The relationship of the dexamethasone suppression test (1 mg and 2 mg) to basal plasma cortisol levels in endogenous depression. Psychoneuroendocrinology. 1987;12:295–301. doi: 10.1016/0306-4530(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Azpíroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferation response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology. 1999;24:345–361. doi: 10.1016/s0306-4530(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. 1992;2:107–113. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Oxytocin, vasopressin and depression. In: den Boer JA, George MS, Ter Horst GJ, editors. Current and future developments in psychopharmacology. Amsterdam: Benecke N.I.; 2005. pp. 201–216. [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm Brain Behav. 2002;1:299–337. [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Chiodera P, Salvarani C, Bacchi-Modena A, Spallanzani R, Cigarini C, Alboni A, Gardini E, Coiro V. Relationship between plasma profiles of oxytocin and adrenocorticotropic hormone during suckling or breast stimulation in women. Horm Res. 1991;35:119–123. doi: 10.1159/000181886. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1080. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Klein D, Hoffman GE, Carter CS, Le WW, De Vries GJ. Comparison of fixation techniques: immersion versus perfusion. Horm Behav. 2001;39:329. [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries GJ, Simerly RB. Anatomy, development and functions of sexually dimorphic neural circuits in the mammalian brain. In: Arnold AP, Etgen AM, Fharbach SE, Rubin RT, Pfaff D, editors. Hormones, brain and behavior. IV. San Diego: Academic Press; 2002. pp. 137–192. [Google Scholar]
- Ehlers CL, Kaneko WM, Owens MJ, Nemeroff CB. Effects of gender and social isolation on electroencephalogram and neuroendocrine parameters in rats. Biol Psychiatry. 1993;33:358–366. doi: 10.1016/0006-3223(93)90325-8. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez-Casteloot I, Enache M, Maccari S, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of mental illness. 2nd. New York: Oxford University Press; 2004. pp. 440–460. [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am Scientist. 1996;84:56–62. [Google Scholar]
- Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35:487–491. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005a;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, van de Kar LD. Chronic mild stress induces behavioral and physiological changes and may alter serotonin 1A receptor function in male and cycling female rats. Psychopharmacology. 2005b;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Ann NY Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Keller MB, Klein DN, Hirschfeld RM, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, Keitner GI, Marin DB, et al. Results of the DSM-IV mood disorder field trial. Am J Psychiatry. 1995;152:843–849. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiatry. 1996;40:918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ. Seeing the unexpected: how sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep. 2002;4:441–448. doi: 10.1007/s11920-002-0072-z. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can J Zool. 2004;82:1194–1200. [Google Scholar]
- Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Chiodera P, Geenen V, von Frenckell R. Confirmation of the inhibitory influence of exogenous oxytocin in cortisol and ACTH in man: evidence of reproducibility. Acta Endocrinol. 1987;114:345–349. doi: 10.1530/acta.0.1140345. [DOI] [PubMed] [Google Scholar]
- Li Q, Brownfield MS, Battaglia G, Cabrera TM, Levy AD, Rittenhouse PA, van de Kar LD. Long-term treatment with the antidepressants fluoxetine and desipramine potentiates endocrine responses to the serotonin agonist 6-chloro-2-[1-piperazinyl]-pyrazine (MK-212) and (+/-)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCL (DOI) J Pharmacol Exp Ther. 1993;266:836–844. [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J Comp Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, Ryabinin AE. Distribution of corticotropin-releasing factor and urocortin 1 in the vole brain. Brain Behav Evol. 2006;68:229–240. doi: 10.1159/000094360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Fowler CD, Spencer C, Houpt T, Wang ZX. Differential expression of vasopressin, oxytocin and corticotrophin-releasing hormone messenger RNA in the paraventricular nucleus of the prairie vole brain following stress. J Neuroendocrinol. 2001;13:1059–1065. doi: 10.1046/j.1365-2826.2001.00729.x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1495–R1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Altemus M. Central nervous system actions of oxytocin and modulation of behavior in humans. Mol Med Today. 1997;3:269–275. doi: 10.1016/S1357-4310(97)01058-7. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. Am J Physiol Heart Circ Physiol. 2003;284:H2269–H2276. doi: 10.1152/ajpheart.00774.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Fairhall SJ, Fletcher A, Redfern PH. Effects of single and repeated electroconvulsive shock on the social and agonistic behaviour of resident rats. Neuropharmacology. 2003;44:911–925. doi: 10.1016/s0028-3908(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. J Neuroendocrinol. 2003;15:1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression; how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Ploog DW. The place of the Triune Brain in psychiatry. Physiol Behav. 2003;79:487–493. doi: 10.1016/s0031-9384(03)00154-9. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJG, Tilders FJH, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Aguado F, Sánchez-Toscano F, Saphier D. Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology. 1998;139:579–587. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Sullivan Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic-pituitary-adrenal axis in health and disease. Vitam Horm. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression Prog Neuropsychopharmacol. Biol Psychiatry. 2003;27:893–903. doi: 10.1016/S0278-5846(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Oxytocin may mediate benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Zhuo L, Hulihan TJ, Insel TR. Imunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles. J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2001;13:2273–2281. doi: 10.1046/j.0953-816x.2001.01613.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]