Abstract
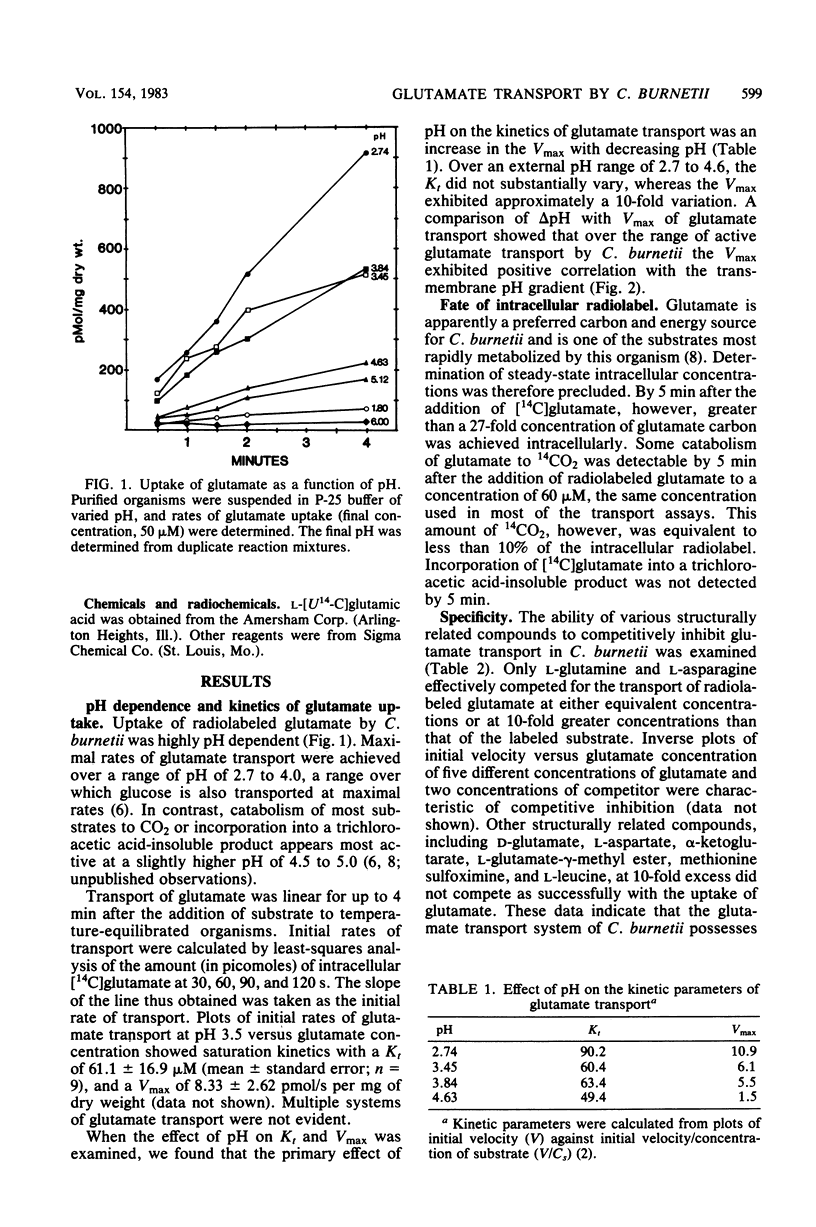
The transport of glutamate, apparently a primary energy source for Coxiella burnetii, has been examined. C. burnetii is shown to possess a pH-dependent active transport system for L-glutamate with an apparent Kt of 61.1 microM and Vmax of 8.33 pmol/s per mg at pH 3.5. Both L-glutamine and L-asparagine competitively inhibited transport of glutamate, but D-glutamate, L-aspartate, L-glutamate-gamma-methyl ester, methionine sulfoximine, or alpha-ketoglutarate did not compete. This transport system is both temperature and energy dependent. Uptake of glutamate is highly sensitive to uncouplers of oxidative phosphorylation such as 2,4-dinitrophenol and carbonyl cyanide-m-chlorophenyl hydrazone that decrease the proton motive force across the cytoplasmic membrane. ATPase inhibitors such as dicyclohexylcarbodiimide or metabolic poisons such as KCN, NaF, or arsenite were much less effective as inhibitors of glutamate transport. Uptake of glutamate did not appear to be coupled to Na+ symport as in Escherichia coli since no monovalent cation requirement could be demonstrated. Instead, the Vmax of glutamate transport showed good correlation with the transmembrane pH gradient (delta pH). From these results, we propose that L-glutamate transport by C. burnetii is energized via a proton motive force.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentaboulet M., Kepes A. Dependence on pH of parameters of lactose transport in Escherichia coli. Evidence for an essential protonated group of the carrier. Eur J Biochem. 1981 Jul;117(2):233–238. doi: 10.1111/j.1432-1033.1981.tb06327.x. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Estimation of the cytoplasmic pH of Coxiella burnetii and effect of substrate oxidation on proton motive force. J Bacteriol. 1983 May;154(2):591–597. doi: 10.1128/jb.154.2.591-597.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Stability of the adenosine 5'-triphosphate pool in Coxiella burnetii: influence of pH and substrate. J Bacteriol. 1981 Nov;148(2):419–425. doi: 10.1128/jb.148.2.419-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poralla K., Kannenberg E., Blume A. A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett. 1980 Apr 21;113(1):107–110. doi: 10.1016/0014-5793(80)80506-0. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


