Abstract
Sec2p is required for the polarized transport of secretory vesicles in S. cerevisiae. The Sec2p NH2 terminus encodes an exchange factor for the Rab protein Sec4p. Sec2p associates with vesicles and in Sec2p COOH-terminal mutants Sec4p and vesicles no longer accumulate at bud tips. Thus, the Sec2p COOH terminus functions in targeting vesicles, however, the mechanism of function is unknown. We found comparable exchange activity for truncated and full-length Sec2 proteins, implying that the COOH terminus does not alter the exchange rate. Full-length Sec2-GFP, similar to Sec4p, concentrates at bud tips. A COOH-terminal 58–amino acid domain is necessary but not sufficient for localization. Sec2p localization depends on actin, Myo2p and Sec1p, Sec6p, and Sec9p function. Full-length, but not COOH-terminally truncated Sec2 proteins are enriched on membranes. Membrane association of full-length Sec2p is reduced in sec6-4 and sec9-4 backgrounds at 37°C but unaffected at 25°C. Taken together, these data correlate loss of localization of Sec2 proteins with reduced membrane association. In addition, Sec2p membrane attachment is substantially Sec4p independent, supporting the notion that Sec2p interacts with membranes via an unidentified Sec2p receptor, which would increase the accessibility of Sec2p exchange activity for Sec4p.
Keywords: transport, exchange factor, yeast, Rab, vesicles
Introduction
The yeast Saccharomyces cerevisiae is a model system to study the regulation of the polarized delivery of post-Golgi vesicles to the cell surface. Polarized vesicle transport and localized exocytic release of vesicular cargo allows for the deposition of cell surface components at a specific site and thereby helps to regulate cell shape. While post-Golgi transport in mammalian cells uses the microtubule-based cytoskeleton for long-range vesicular movement and the actin-based cytoskeleton for short-range movement and/or capture (for review, see Schliwa 1999), polarized yeast transport is effected solely by the actin cytoskeleton (Adams and Pringle 1984; Novick and Botstein 1985; Pruyne et al. 1998). Vesicles bud from the Golgi complex and then move vectorially towards sites of polarized growth (the bud and mother/daughter neck). Vesicles arriving at the target membrane dock and subsequently fuse. Cells treated with the actin depolymerization drug latrunculin (LAT)-A, or harboring mutations that affect the actin cytoskeleton, accumulate vesicles randomly which leads to isotropic growth of the mother cell and a reduction in the number of budded cells observed (Novick and Botstein 1985; Govindan et al. 1995; Karpova et al., 1999). Vesicles also accumulate in secretory (sec) mutants (Novick and Schekman 1979; Novick et al. 1980, Novick et al. 1981). However, most sec mutants functioning in Golgi to plasma membrane transport (late-acting), accumulate vesicles in a polarized manner, concentrated in the bud (Novick and Brennwald 1993; Walch-Solimena et al. 1997). The sole exception is sec2, which accumulates vesicles in a depolarized fashion, similar to that seen in act1-1 and LAT-A–treated cells. SEC2 is epistatic to other late-acting SEC genes, consistent with a role in the polarized delivery of these vesicles (Walch-Solimena et al. 1997).
Sec2p is a potent exchange factor for Sec4p, the essential post-Golgi Rab protein residing both tightly associated with post-Golgi vesicles and in a soluble complex with Gdi1p. Sec2p catalyzes not only GDP dissociation from Sec4p, but also the Sec4p GTP on-rate (Walch-Solimena et al. 1997). These data suggest that Sec4p activation is required for the polarized transport of post-Golgi vesicles in yeast. Once activated, GTP-bound Sec4p can interact with downstream effector protein(s). Presently, the only effector known for Sec4p is Sec15p, which is a component of the multi-subunit complex, the exocyst (Guo et al. 1999b) and may be the docking effector for Sec4p vesicles that tethers vesicles to the proper sites before interaction with the fusion machinery.
If the sole function of Sec4p were to interact with Sec15p before docking, loss of Sec2p function would not lead to a depolarized accumulation of post-Golgi vesicles. In fact, vesicles accumulate in a strongly polarized fashion in a sec15-1 mutant background (Walch-Solimena et al. 1997) and, hence, the phenotypes for sec2 and sec15 are distinct. It is, however, possible that Sec15p is the only effector of Sec4p and that the sec15-1 mutation does not affect the vesicle transport event. Nevertheless, similar to Rab5, Sec4p may have multiple effectors (Stenmark et al. 1995; Gournier et al. 1998; Pfeffer 1999). Activated Sec4p may interact directly or indirectly, via an effector, with an actin-based motor. Importantly, Rab6 has been shown to interact with Rab-kinesin, a protein involved in Golgi dynamics in mammalian cells (Echard et al. 1998). Indirect evidence places the unconventional, type V, myosin in budding yeast, Myo2p, as a strong candidate for a post-Golgi vesicle motor (Johnston et al. 1991; Govindan et al. 1995; Pruyne et al. 1998; Karpova et al. 2000; Reck-Peterson et al. 1999; Schott et al. 1999). A mutation in the actin-binding face of the Myo2p head domain (Lillie and Brown 1994) or overexpression of the Myo2p tail domain (Karpova et al. 2000; Reck-Peterson et al. 1999; Schott et al. 1999), causes depolarized exocytosis, leading to isotropic growth and finally death. This phenotype is similar to that observed for act1-1 (Novick and Botstein 1985) and LAT-A–treated cells (Karpova et al. 2000).
Sec2p is a large protein with an apparent molecular mass of 105 kD (Nair et al. 1990). The protein can be functionally divided into two domains: the NH2-terminal half contains a large coiled-coil domain necessary for both Sec2p homodimerization and Sec4p-interaction (Nair et al. 1990; Collins, R.N., and P.J. Novick, manuscript in preparation). This domain catalyzes nucleotide exchange on Sec4p (Walch-Solimena et al. 1997). The COOH terminus possesses no predicted structural motifs and has no known function, nonetheless, truncation of this region of Sec2p gives rise to temperature-sensitive defects in protein secretion and growth (Nair et al. 1990).
This study addresses the role of the COOH terminus of Sec2p in the polarized delivery of post-Golgi vesicles. Our results indicate that the COOH terminus is not directly involved in the exchange activity of Sec2p. We find that Sec2p localizes in a polarized manner to small- and medium-sized buds and the mother/daughter neck region. This localization is similar to that of Sec4p in that it depends on the actin cytoskeleton and the production of post-Golgi vesicles. We have characterized a 58–amino acid domain in the COOH terminus of Sec2p required for proper localization. Sec2p is shown to associate with membranes and loss of localization reflects an inability of Sec2 proteins to efficiently bind membranes. We propose that the association of Sec2p with membranes increases the accessibility of Sec2p exchange activity for Sec4p and that when Sec2p membrane attachment is perturbed, Sec4p activation is compromised and the efficiency of vesicle transport is reduced. We will discuss these data in the context of a model whereby Sec2p resides on vesicles with Sec4p during their polarized transport and the requirement for Sec4p activation is either before or during transport.
Materials and Methods
Materials
Oligonucleotides were synthesized by M. Talmor (Yale University, New Haven, CT). Chymostatin, antipain, leupeptin, protein A–Sepharose, and all antibodies, with the exception of Anti-GFP monoclonal and polyclonal antibodies (Clonetech), were from Sigma Chemical Co. Ingredients for SDS-PAGE, 2-mercaptoethanol, DTT, Tween 20, aprotinin, and pepstatin were from American Bioanalytical. Restriction enzymes, VENT polymerase, and 10× ThermoPol buffer were from New England Biolabs. LAT-A was kindly provided by Phil Crews (University of California, Santa Cruz, CA). Glass beads (0.5 mm) and Zirconia/silica beads (0.5 mm) and bead-beater instrument were from Biospec Products, Inc. 35S-labeled GTPγS was from NEN Life Science Products. [32P]-orthophosphate was from Amersham Pharmacia. Optiprep (iodixanol) was from Nycomed Pharma AS. Kodak X-OMAT BMR film was used in conjunction with a Kodak X-OMAT film processor.
Yeast Strains
Construction of sec2 Delete Cells.
To construct various SEC2 alleles expressed as the sole copy in yeast, it was necessary to delete the genomic copy of SEC2. Since SEC2 is essential, it was necessary to construct a SEC2 expression plasmid that would balance the loss of genomic sec2. The plasmid used to knockout the genomic copy of SEC2 was constructed as follows: The HIS3 marker was PCR cloned (using standard methods; Clackson et al. 1996) into pBluescript SK II+. SEC2 5′-noncoding sequences (1,274 base pairs) were amplified using the forward primer 5′-gtatcgatATCGATTGTAAACGTAGAGCG-3′ (#23861) and the reverse primer 5′-ctGATATCggaaatcaacgttgctacagctgc-3′ (#23862). This product was digested with ClaI (5′) and EcoRV (3′) and subcloned upstream to HIS3. Downstream of the HIS3 gene, a PCR product was inserted of the 3′ noncoding SEC2 sequences (664 base pairs) using the forward primer 5′-AGCTACTAGTTAATTTTATGTGTCCTAATGG-3′ (#23863) and the reverse primer 5′-CACCGCGGAAGTATTGAAAGCATAAGAGC-3′ (#23864) digested with SpeI (5′) and SacII (3′). The resulting plasmid was digested with ClaI (5′) and SacII (3′) and transformed into the diploid NY1523 to replace the genomic copy of SEC2 by homologous recombination, which resulted in total deletion of the SEC2 ORF. To create the SEC2 balancer plasmid, the complete SEC2 ORF was PCR cloned into the plasmid vector pTS395, devoid of its GFP insert. The resulting centromeric balancer plasmid is URA3 marked, possesses the strong GAL1-10 promoter driving expression of SEC2 and ACT1 terminator sequences (kindly provided by T. Stearns), and is called pNB761. sec2 delete heterozygous diploid cells (NY2194) transformed with pNB761 (NY2195) were dissected onto YP galactose and the URA3 positive spores, which exhibited conditional growth on galactose, were assayed for balancer plasmid dependence. These cells were tested for death and loss of Sec2p expression in YP glucose media. These cells were then used for integration and expression of various sec2 alleles as the sole copy in yeast.
Construction and Expression of Various sec2-expressing Plasmids.
The sec2-complementing ClaI-BamHI fragment (encoding the SEC2 ORF) was subcloned into the LEU2-marked, integrating plasmid pRS305 producing pNB977. This SEC2-expressing plasmid was digested with EcoRI and introduced into yeast (NY2196) at the LEU2 locus by homologous recombination. Transformed yeast were then plated onto 5-FOA to select for yeast having lost the SEC2 balancer (pNB761). The resultant yeast strain (NY2145) was used as the untagged SEC2 control in all experiments. The plasmid (pNB977) was digested with ApaI and relegated to drop out the 800-bp ApaI-ApaI fragment resulting in pNB978. To rid SEC2 of two 3′ terminal XbaI sites, leaving one XbaI site in the SEC2 ORF, PCR was performed using the forward primer 5′-CCTTTTGTGGAGAATCTAGAGATGAC-3′ (#24797) and the reverse primer 5′-AAA-CTAGTGGATCCCGGGGAGTTGTTTTGAGCCAAGG-3′ (#24796). The product was digested with XbaI and SpeI and subcloned into the XbaI-XbaI sites of pNB978, producing pNB979. The 3′ primer used for the above PCR was engineered with an additional BamHI site. The resultant plasmid was digested with SpeI-BamHI, liberating the SEC2 ORF and surrounding sequences that were then subcloned into pBluescript SK II+ (pNB980). Uracil-modified site-directed mutagenesis was performed in order to produce new restriction enzyme recognition sites in the SEC2 ORF (pNB981). An NcoI site was engineered at the first ATG (first base), SalI, NheI, Bgl, PstI, and EcoRV at the base pair positions of 51, 127, 380, 449, and 606, respectively. The mutagenized SEC2 gene was then subcloned back into pRS305 using the SpeI and BamHI sites as before producing pNB982.
To produce the SEC2(1-759)-GFP fusion, the coding sequence of the mut3 version of A. victoria GFP (S65G, S72A; Cormack et al. 1996) was fused, in frame, to the 3′ end of SEC2 using the splicing by overlap extension methodology (SOE; Clackson et al. 1996). The SEC2-GFP PCR fusion product was subcloned into pNB979 to produce pNB983. The SpeI-XbaI fragment (5′ mutagenized SEC2 sequences) of pNB982 was subcloned into pNB983, producing the pNB984. The 621 base pair SEC2 terminator was PCR cloned using the forward primer 5′-ATCGTTAACTCGAGCGGCCGCatgatgcccaggaacag-3′ (#25689) and the reverse primer 5′-TCAGCGGCCGCGAGCTCCAAGTATTGAAAGCATAAGAGC-3′ (#25358). The resultant PCR product was digested with NotI (5′) and SacI (3′) and cloned into pNB984, downstream of the SEC2 gene, producing pNB985. SEC2(1-759)-GFP (pNB985) was then expressed as for pNB977 (above) as the sole copy in yeast (NY2146).
With the exception of sec2-59(1-374)-GFP (pNB990), all SEC2 COOH-terminal truncations fused to GFP were produced by SOE PCR. The resultant PCR products were subcloned into pRS305-SEC2(1-759)-GFP (pNB985) using the restriction enzymes SnaBI and NotI to produce sec2(1-587)-GFP (pNB986), sec2(1-541)-GFP (pNB987), sec2-70(1-508)-GFP (pNB988), and sec2(1-450)-GFP (pNB989). The sec2-59(1-374)-GFP (pNB990) fusion was produced using standard two primer PCR. The product was subcloned as described above.
To produce the sec2-78 point mutation in the context of full-length SEC2-GFP, the region surrounding the point mutation (see Characterization and Reconfirmation of the sec2-78 Mutation below) was amplified on a template of sec2-78 genomic DNA (NY1529) and subcloned into the plasmid pNB985 to generate pRS305-sec2-78-GFP (991). This plasmid was expressed as the sole copy in yeast as above.
To overexpress various SEC2 sequences fused to GFP behind the GAL1-10 promoter, the SEC2-GFP fusion PCR product (described above) was digested with XbaI and NotI and ligated into pNB761. The resultant plasmid pTS395-SEC2(1-759)-GFP (pNB994) was transformed into the wild-type yeast, NY451, producing NY2155. To produce sec2(1-160)-GFP (pNB996), the NH2-terminal SEC2 sequences were PCR cloned using the SOE methodology with the sec2 forward #23795 and the reverse 5′-GTTCTTCTCCTTTACTCATactatgcatcacttttttcagattcttgagc-3′ (#26559) primers and the GFP forward 5′-gtgatgcatagtATGAGTAAAGGAGAAGAACTTTTCACTGGAG-3′ (#26560) and reverse #24968 primers. The PCR product and pNB994 were digested with HindIII and NotI and ligated. Yeast expressing sec2(1-160)-GFP in a wild-type SEC2 background are called NY2157. The plasmid pTS395-sec2(161-759)-GFP (pNB995) was produced by amplifying the first 18 base pairs of SEC2 fused to base pair 483 (fused in the 5′ primer), through to base pair 1107 (SnaBI; MDASEE/LDNEST). This product was digested with HindIII and SnaBI and ligated into pNB994, similarly digested and purified from SEC2 NH2-terminal sequences. Yeast expressing sec2(161-759)-GFP (pNB995) in a wild-type SEC2 background are called NY2156.
The expression of all Sec2 proteins encoded by the alleles constructed above was verified by Western blot analysis using standard protocols (Sambrook et al. 1989). In brief, yeast cells (2 ODU) were harvested in an Eppendorf and decanted of excess media. Pellets were resuspended in TE (10 mM Tris-HCl, pH 8.0, 1 mM EDTA) plus 1×PIC (60 μg/ml chymostatin, 50 μg/ml antipain, 5 μg/ml leupeptin, 2 μg/ml aprotinin, 0.7 μg/ml pepstatin, and 174 μg/ml PMSF). An equal volume of acid-washed glass beads was added and tubes were vortexed for three 20-s intervals with intermittent cooling on ice. Samples were then boiled in DTT-containing sample buffer and loaded onto 10% SDS–polyacrylamide gels. Gels were transferred to nitrocellulose (600 mA*h). Filters were blocked with 7% milk in PBST and probed by Western analysis with the anti-GFP monoclonal antibody (1:500) and then the anti–mouse IgG-horseradish peroxidase (1:10,000). Incubations and washes were performed at room temperature for 30 min. Filters were developed for ECL and exposed to film for various times.
Production of the sec2(1-450) Mutation at the SEC2 Locus.
The sec2 COOH-terminal truncation sec2(1-450) was reconstituted at the genomic copy of SEC2 by homologous recombination. sec2 sequences with an engineered stop codon at position 451 was PCR cloned using the forward primer #40497 and the reverse primer 5′-cagtGGATCCttatgaagaattgataccaagtcctgcc-3′ (#40499). The product and the URA3-marked integrating vector, yIP5, were digested with ClaI and BamHI and ligated together. This plasmid [yIP5-sec2(1-450)] was linearized with XbaI and transformed into the diploid NY1523. Diploids were sporulated and tetrad analysis revealed two live Ura− and two dead spores, confirming that the sec2(1-450) mutation is indeed temperature sensitive.
Characterization and Reconfirmation of the sec2-78 Mutation.
The SacI-KpnI insert derived from pRS305-SEC2(1-759)-GFP, encoding the SEC2-GFP ORF and regulatory sequences, was subcloned into the URA3-marked, centromeric plasmid pRS316. This plasmid was digested with SalI and SphI and transformed into sec2-78 yeast (NY1529). Genomic sec2-78 sequences were recovered through the gap repair mechanism onto the centromeric pRS316-sec2 plasmid which was then shuffled into bacteria. Limited genetic gap repair data using overlapping restriction digest pairs revealed that the COOH terminus of sec-78 most likely harbors the mutation(s). We then carried out sequence analysis of the COOH terminus and found a single point mutation converting a G→A, which leads to a cysteine to tyrosine amino acid substitution at position 483.
This mutation was then reproduced in wild-type yeast by homologous recombination at the SEC2 locus to confirm that sec2-78 is indeed the result of only one point mutation. The primer pair #40497 and #40498, was used to PCR amplify sec2-78 sequence. The procedure for producing the sec2-78 mutation is similar to that described above, used in the production of the sec2(1-450) mutation.
Yeast strains are listed in Table .
Table 1.
Strain List
| Strain | Genotype |
|---|---|
| NY451 | MATa ura3-52 Gal+ |
| NY1529 | MATα ura3-52 sec2-78 |
| NY1523 | MATa/MATα ura3-52/ura3-52 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 Gal+/ Gal+ |
| NY2145 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2; pNB977] his3-Δ200 ura3-52 Gal+ |
| NY2146 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 Gal+ |
| NY2147 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-59(1-374)-GFP; pNB990] his3-Δ200 ura3-52 Gal+ |
| NY2148 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-70(1-508)-GFP; pNB988] his3-Δ200 ura3-52 Gal+ |
| NY2149 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2(1-587)-GFP; pNB986] his3-Δ200 ura3-52 Gal+ |
| NY2150 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2(1-541)-GFP; pNB987] his3-Δ200 ura3-52 Gal+ |
| NY2151 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2(1-450)-GFP; pNB989] his3-Δ200 ura3-52 Gal+ |
| NY2152 | MATa sec2-Δ1::HIS3 leu2-3,112::[LEU2 sec2-78-GFP; pNB991] his3-Δ200 ura3-52 Gal+ |
| NY2153 | MATα sec2::[sec2-78 URA3; pNB992] his4-619 ura3-52 Gal+ |
| NY2154 | MATa sec2::[sec2(1-450) URA3; pNB993] his3-Δ200 ura3-52 leu2-3,112 Gal+ |
| NY2155 | MATa ura3-52 + [GALp-SEC2-GFP CEN URA3; pNB994] Gal+ |
| NY2156 | MATa ura3-52 + [GALp-sec2(161-759)-GFP CEN URA3; pNB995] Gal+ |
| NY2157 | MATa ura3-52 + [GALp-sec2(1-160)-GFP CEN URA3; pNB996] Gal+ |
| NY2158 | MATa act1-1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2159 | MATa cdc42-1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2160 | MATa cdc24-4 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2161 | MATα smy1-Δ1::URA3 sec2-1Δ::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 his4-619 trp1-289 Gal+ |
| NY2162 | MATa myo2-66 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2163 | MATα myo2-2 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 lys2-801 Gal+ |
| NY2164 | MATa sec1-1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2165 | MATa sec6-4 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2166 | MATa sec8-9 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2167 | MATα sec5-24 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2168 | MATa sec10-2 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2169 | MATa sec15-1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2170 | MATa sec3-2 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2171 | MATa sec9-4 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2172 | MATa sec4-8 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2173 | MATα DSS4-Δ1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2174 | MATa sec19-1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2175 | MATa sec16-2 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2176 | MATα sec12-4 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2177 | MATa sec7-1 sec2-Δ1::HIS3 leu2-3,112::[LEU2 SEC2-GFP; pNB985] his3-Δ200 ura3-52 |
| NY2194 | MATa/MATα SEC2/sec2-Δ1::HIS3 ura3-52/ura3-52 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 Gal+/ Gal+ |
| NY2195 | MATa/MATα SEC2/sec2-Δ1::HIS3 ura3-52/ura3-52 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 Gal+/ Gal+ + [GALp-SEC2 URA3 CEN; pNB761] |
| NY2196 | MATa ura3-52 his3-Δ200 leu2-3,112 Gal+ +[GALp -SEC2 URA3 CEN; pNB761] |
Exchange Assay
Exchange reactions were performed to quantitate the Sec4p GTPγS on-rate in the presence of immune complexes of Sec2-GFP, Sec2-59(1-374)-GFP, or control complexes. The exchange reaction buffer that consisted of His6-tagged Sec4p (0.8 μM; His6-tagged SEC4 construct was engineered by Adam Lauring, University of Washington School of Medicine, Seattle, WA) was incubated on ice in the presence of 10 μM cold GTPγS spiked with 0.1 μM [35S]GTPγS (12.5 μCi/μl, 1,250 μCi/nmol; NEN Life Science Products) in exchange buffer that consisted of 64 mM Hepes-NaOH (pH 8.0), 100 mM NaCl, 8 mM MgCl2, 2 mM EDTA, 0.2 mM DTT, and 1 mg/ml BSA. Each time point contained a 10-μl aliquot of exchange reaction buffer added to 10 μl protein A–Sepharose anti-GFP antibody immune complexes devoid of excess buffer on ice. Tubes for each time point were incubated at 14°C for various times at which they were quickly diluted in 1 ml ice exchange buffer. At the end of the time course, all time points were loaded onto a suction filtering apparatus and filtered at once through Millipore nitrocellulose filters (Millipore Corp.). Filters were dried and scintillation counted in Opti-fluor (Packard Instrument Co.) to determine the amount of protein-associated radioactivity. Due to the nature of this kind of experiment, we did not carefully quantitate the amount of Sec2-GFP present in the immune complexes, but a rough upper limit estimate shows not >50 nM-specific protein per 20-μl time point. An equal amount of each immune complex was used to determine the protein immunoprecipitated from the lysate and present in one time point. Exchange rates were calculated from fitting the curves to first order rate equations. Experiments were repeated five times and the ratio of rates of exchange for Sec2-GFP/Sec2-59(1-374)-GFP were used to calculate the mean and SEM.
Localization Studies
Cells expressing various Sec2 proteins fused to GFP were grown to mid-log phase (A600 = 0.5–1.0) in YPD at 25°C overnight. For temperature-sensitive studies, cells were shifted to 37°C for 1 h. When Gal induction was required, cells were grown to mid-log phase in YP raffinose and then pelleted and resuspended in YP galactose for the induction. In some experiments, LAT-A was added to cells at 25°C at a final concentration of 200 mM. An equal volume of DMSO (the solvent for LAT-A) was added to control cells. Cycloheximide and sodium azide were used at final concentrations of 0.35 and 10 mM, respectively. For visualization of Sec2-GFP in smy1-1Δ cells, diploids, heterozygous for both SEC2-GFP and smy1-1Δ (Catlett and Weisman 1998) were sporulated. Small spores were added to 1 ml YPD and grown at 30°C until enough cells were obtained to fix and visualize. Cells were fixed in methanol at −20°C for 10 min, washed with acetone at −20°C, and washed an additional five times in ice-cold PBS, pH 7.4. Cells were stored in the dark on ice until used. Sec4p was localized by indirect immunofluorescence as previously described (Walch-Solimena et al. 1997).
Fluorescence was viewed with a Zeiss Axiophot 2 microscope using a 100× oil-immersion objective (NA 1.4). Images were acquired with a Photometrics Quantix CCD camera cooled to −35°C.
Differential Centrifugation and Immunoprecipitation
Differential centrifugation analysis of yeast lysates was previously described (Walch-Solimena et al. 1997), with the exception that cells were washed twice with spheroplast salts devoid of zymolyase subsequent to zymolyase treatment. Lysates were generated in lysis buffer (0.8 M sorbitol, 10 mM triethanolamine, 1 mM EDTA, and 1×PIC) to slow the degradation of the protease sensitive Sec2 proteins. Equal volumes of supernatants and resuspended pellets were subjected to Western analysis using anti-GFP rabbit polyclonal antibody (1:1,500 in PBS plus 0.05% Tween 20 = PBST) or affinity purified anti-Sec4p polyclonal antibodies (1:400).
The detection of phosphorylated Sec2p was achieved by growing cells to mid-log phase in phosphate-depleted YPD (Guthrie and Fink 1991) to an absorbance at 600 nm (A600) of 0.7–1.0. Cells (20 OD600 units) were then concentrated into 1 ml phosphate-depleted YP dextrose or galactose and metabolically labeled with 550 mCi 32P-orthophosphate at 25°C for 2 h. Cells were harvested and washed twice in ice-cold 50 mM Tris (pH 7.5) and 10 mM NaN3. Cells in IP buffer (20 mM Hepes, pH 7.4, 150 mM KCl, 0.5 mM DTT, 2 mM EDTA, 0.5% Triton X-100, 1XPIC, 200 μg RNAase A, and the phosphatase inhibitors Na3VO4 (0.1 mM), 0.2 mM Na4P2O7, and 1 mM NaF) were transferred to a 2-ml conical screw-capped tube with 2 g of 1-mm zirconia-silica beads and lysed in a Mini-beadbeater-8 at full power for 4 min in the cold. Lysates were clarified at 100,000 g for 20 min at 4°C. Supernatants were precleared by incubation with 60 μl protein A–Sepharose (50% slurry) for 30 min rocking at 4°C. Sec2-specific proteins were quantitatively immunoprecipitated with either anti-Sec2p rabbit polyclonal (1.5 μl affinity-purified/ml lysate) or anti-GFP rabbit polyclonal (1 mg/ml) antibodies for 1 h at 4°C. Protein A–Sepharose (45 μl of a 50% slurry/ml lysate) was added to the lysates for 30 min at 4°C. Immune complexes were then washed three times in IP buffer plus an additional 350 mM KCl and one additional wash in IP buffer (low salt, as above). Proteins were resolved by SDS-PAGE and Sec2-GFP was detected by both anti-GFP monoclonal antibody and fluorography.
Iodixanol Gradient Centrifugation
Cells were grown at 25°C, overnight to mid-log phase. For temperature shift experiments, cells were incubated for 1 h at 37°C. Pellets (100,000 g) generated from 200 ODU of cells were resuspended in lysis buffer (above) and thoroughly mixed with 1 vol of iodixanol making a final volume of 1.3 ml. Lysates were centrifuged for 1 h at 625,000 g (rmax) at 4°C and then split into equal volume fractions which were boiled and subjected to SDS-PAGE. It should be noted that under these conditions these gradients were a combination of both density and velocity gradient centrifugation. Consistent with this, most but not all abundant proteins detected with Ponceau (nitrocellulose filters) were distributed in all fractions. Despite this, Sec2-GFP (under wild-type conditions), Sec4p, and Pma1p (kindly provided by C. Slayman) were detected in membrane fractions by Western blot analysis.
Gdi1p-mediated Solubilization of Sec4p
Gdi1p was expressed in E. coli and purified by ion exchange followed by monoQ as previously described (Garrett and Novick 1995). Membranes (10,000 g pellets, P10) were enriched by differential centrifugation, diluted to 2 mg/ml and frozen in liquid nitrogen and stored at −80°C. The Sec4p solubilization reaction was performed at 30°C for 30 min, followed by separation of supernatant from pellet (membranes) through a sorbitol cushion as described (Garrett et al. 1994). Equal volumes of supernatants and pellets were resolved on SDS-PAGE. Sec2-GFP and Sec4p were detected by Western analysis as described above. In addition, similar reactions were mixed 1:1 with iodixanol and centrifuged as described above (Iodixanol Gradient Centrifugation). Fractions (three) were collected and analyzed for Sec2-GFP and Sec4p, to show that Sec2-GFP, under these conditions was in association with membranes. Membranes (100,000 g pellets, P100) were treated as above except that the P100 was generated upon a 60% sucrose cushion. In addition, subsequent to the Gdi1p reaction, the supernatant and pellet were fractionated through a sorbitol cushion (as above) but centrifuged at 500,000 g to ensure that the vesicles pellet through the cushion.
Results
Sec2p functions as a potent exchange factor for Sec4p and mutations in SEC2 cause an accumulation of post-Golgi vesicles randomly distributed between the mother cell and the bud (Walch-Solimena et al. 1997). Since all other late-acting sec mutants lead to a polarized accumulation of vesicles preferentially in the bud, Sec2p function may be required for the directed transport of vesicles to sites of exocytosis. We initiated studies of Sec2p localization to understand where the activation of Sec4p by Sec2p may occur along the route taken by post-Golgi transport vesicles.
Full-Length and Truncated Sec2 Proteins Function as Exchange Factors for Sec4p
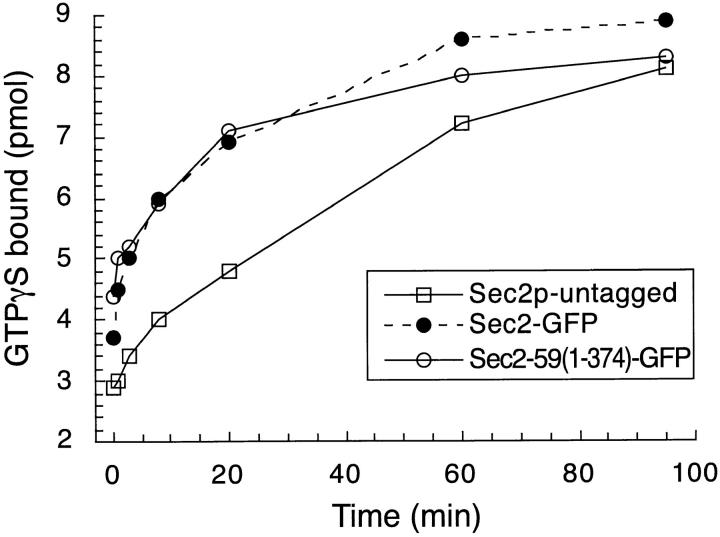
Previous data have shown that Sec2p accelerates both the GDP off-rate and the GTP on-rate of Sec4p. Due to difficulties in expressing full-length Sec2p in bacteria, those studies were performed using the truncated protein encoded by the temperature-sensitive allele sec2-59(1-374; Walch-Solimena et al. 1997). In this study, we compared the enzymatic exchange activity of full-length Sec2(1-759)p and Sec2-59(1-374)p in order to determine if the defect exhibited by truncated Sec2 proteins reflects an altered exchange activity. We measured the nucleotide on-rate by reacting recombinant Sec4p and GTPγS at 14°C with either full-length Sec2(1-759)–green fluorescent protein (GFP) or Sec2-59(1-374)-GFP immunopurified from yeast lysates with anti-GFP antibody. As a measure of the intrinsic on rate of Sec4p, complexes generated from an untagged lysate were assayed for exchange activity. Both full-length and truncated Sec2 proteins enhance the GTPγS on-rate as compared with the Sec4p intrinsic rate (Fig. 1). Sec2-59(1-374)-GFP was found to exchange with 80% efficiency (6.9 SEM, n = 5) relative to full-length Sec2p. This difference in exchange activity is most likely too small to cause the temperature-sensitive phenotype of sec2-59(1-374) since expression of SEC2 behind a weak promoter (from the gene associated with the SAGE tag sequence #778101; Velculescu et al. 1997), produced a level of Sec2p undetectable by Western blot analysis, yet did not cause a growth phenotype (data not shown). Therefore, much less Sec2p function is needed than is normally present in the cell. It is possible that the exchange activity of Sec2-59(1-374)-GFP is temperature sensitive and that the reaction, if carried out at temperatures higher than 14°C would result in decreased activity. However, this possibility has been explored using bacterially expressed Sec2-59(1-374) and the activity was higher at the elevated temperature (Collins, R.N., and P.J. Novick, manuscript in preparation). We conclude that both full-length and truncated Sec2 proteins exchange at comparable rates and that the COOH terminus of Sec2p is not directly involved in exchange.
Figure 1.
Full-length Sec2-GFP and truncated Sec2-59(1-374)-GFP exchange GDP for GTP on Sec4p with similar rates. Exchange reactions were performed to quantitate the Sec4p GTPγS on-rate. Anti-GFP immune complexes were generated from yeast expressing Sec2-GFP (NY2146), Sec2-59(1-374)-GFP (NY2147), or Sec2p (NY2145, control) and incubated with recombinant, partially purified, Sec4p and GTP/[35S]GTPγS for various time points at 14°C. Reactions were stopped with ice cold exchange buffer and protein-associated radioactivity was quantitated on filters by scintillation counting. Exchange data were normalized to protein present in the immune complexes by subjecting immunoprecipitated material to Western analysis using the anti-GFP antibody. First order rate equations were used to calculate the enzymatic exchange rates. Sec2-GFP exchange rate was set at 100% (black circles). Sec2-59(1-374)-GFP (open circles) was found to exchange with 80% the activity of full-length Sec2-GFP (SEM = 6.9). Untagged Sec2p immune complexes were generated as a control (open squares). This reflects the intrinsic rate of exchange of Sec4p and was similar to protein A–Sepharose beads alone. Truncation of the COOH terminus of Sec2p does not dramatically affect its exchange activity and most likely does not account for the defect observed for Sec2-59(1-374)p expressing yeast.
Sec2-GFP Localizes to Sites of Polarized Growth
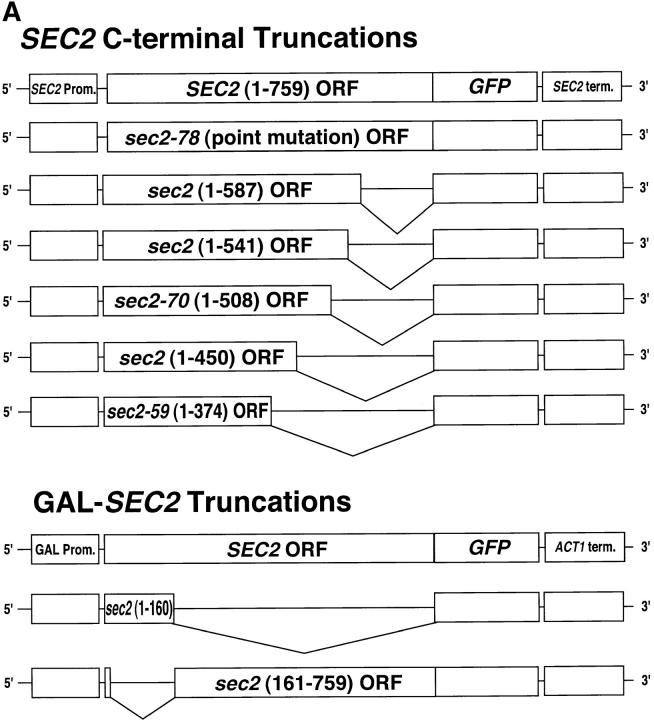
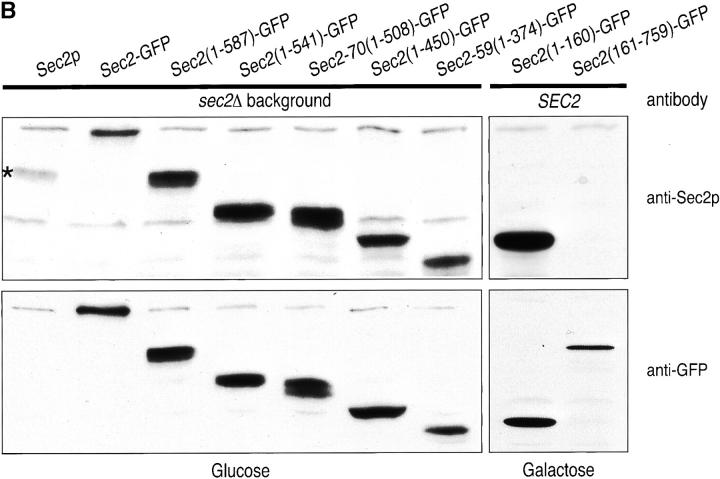
Sec4p localizes to the tips of small- and medium-sized buds and to the mother/daughter neck and this localization reflects the concentration of vesicles carrying Sec4p at exocytic sites (Novick and Brennwald 1993; Walch-Solimena et al. 1997). Establishing the localization of Sec2p could lead to an understanding of the spatial requirement for Sec4p activation during vesicle transport. To this end, GFP was fused to the COOH terminus of Sec2p and expressed under the control of the SEC2 promoter and terminator as the sole copy in yeast (Fig. 2 A). Fluorescence microscopy showed that Sec2-GFP localized to the tips of small- and medium-sized buds and the double ring of the mother/daughter neck with few cytoplasmic punctae observed (Fig. 3). Very little autofluorescence is detected in cells expressing untagged Sec2p (Fig. 3). Sec2p expression was monitored by Western blot analysis (Fig. 2 B). The GFP tag appears to confer stability to Sec2p as several fold higher levels of Sec2-GFP are detected at steady-state (Fig. 2 B, compare Sec2p[*] and Sec2-GFP). The observed localization of Sec2-GFP is reminiscent of the localization of Sec4p. This may indicate that Sec2p is transported together with Sec4p on post-Golgi vesicles, yet it is also possible that Sec2p is localized to sites of polarized growth independent of both Sec4p and vesicles and that Sec4p activation occurs upon the arrival of Sec4p vesicles to sites of exocytosis. This question is addressed in a later section.
Figure 2.
(A) Various sec2-GFP fusion alleles were constructed and expressed in haploid yeast. GFP was fused to the COOH terminus of full-length and mutated sec2 alleles and then integrated into the genome of sec2 delete cells and expressed as the sole copy. SEC2 and SEC2-GFP are under the control of its own promoter and terminator. SEC2-GFP, sec2(1-160)-GFP and sec2(161-759)-GFP were expressed in wild-type cells under the control of the GAL1-10 promoter. (B) Expression of Sec2-fusion proteins in yeast was detected in lysates by Western analysis using either the anti-Sec2p Ab or anti-GFP Ab. As expected, Sec2p (NY2145) is detected (*) with anti-Sec2p but not anti-GFP antibodies. Sec2-GFP (NY2146), Sec2(1-587)-GFP (NY2149), Sec2(1-541)-GFP (NY2150), Sec2-70(1-508)-GFP (NY2148), Sec2(1-450)-GFP (NY2154), and Sec2-59(1-374)-GFP (NY2147), are detected with both antibodies. An increase in the steady-state levels of GFP-tagged protein is observed as compared with untagged Sec2p (*). Double bands can be detected for Sec2(1-587)-GFP, Sec2-70(1-508)-GFP. As Sec2p is a labile protein, these probably reflect degradation products. Yeast expressing sec2(1-160)-GFP (NY2157), and sec2(161-759)-GFP (NY2156), behind the GAL1-10 promoter were grown in galactose and lysates were probed by Western analysis as above. The endogenous wild-type copy of SEC2 cannot be detected when probed with the anti-Sec2p antibody due to insufficient exposure time. The anti-Sec2p antibody does not recognize epitopes in Sec2(161-759)-GFP.
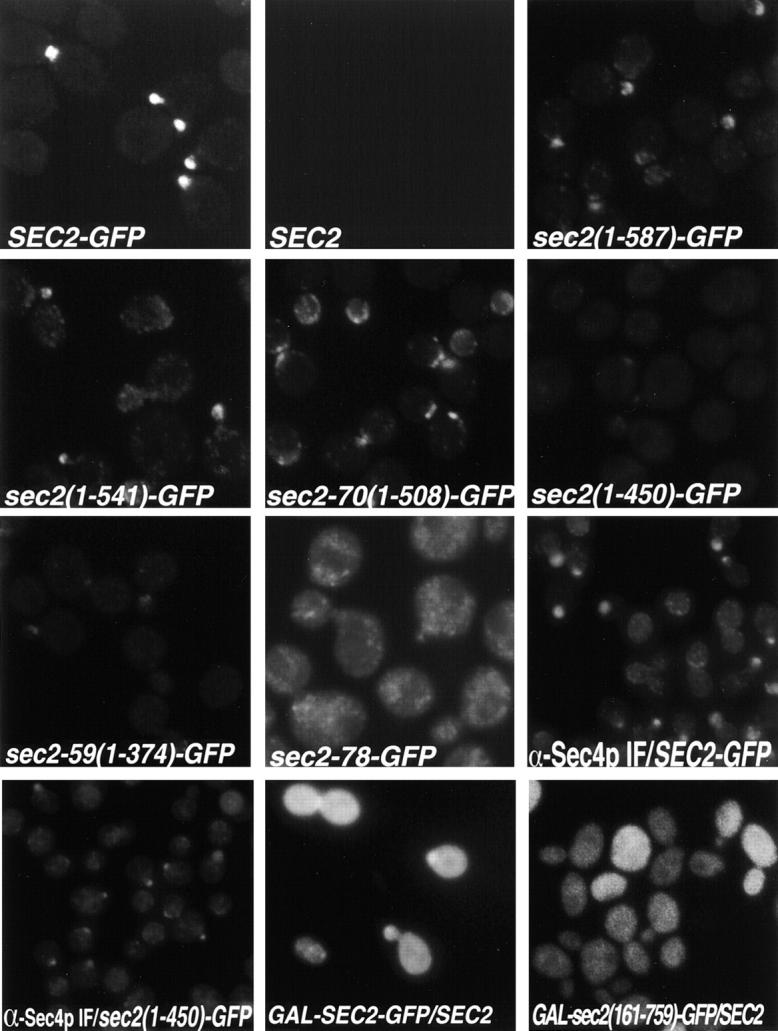
Figure 3.
A COOH-terminal domain of Sec2p is necessary but not sufficient for proper polarized localization. Yeast were grown from stationary cultures overnight at 24°C to early-log phase. All strains were grown in YPD except GAL-sec2(1-160)-GFP and GAL-sec2 (161-759)-GFP which were grown in galactose. For GFP visualization, cells were fixed, permeabilized, washed in PBS and directly observed (SEC2, NY2145; SEC2-GFP, NY2146; sec2(1-587)-GFP, NY2149; sec2(1-541)-GFP, NY2150; sec2-70(1-508)-GFP, NY2148; sec2(1-450)-GFP NY2151; and sec2-59(1-374)-GFP, NY2147; sec2-78-GFP, NY2153; GAL-SEC2-GFP, NY2155; GAL-sec2(161-759)-GFP, NY2156). Sec4p was detected by indirect immunofluorescence using anti-Sec4p hybridoma supernatant. Cells were fixed, permeabilized and incubated with anti-Sec4p Ab followed by Cy3-goat anti–mouse (α-Sec4p/sec2(1-450)-GFP and α-Sec4p/SEC2-GFP).
The COOH Terminus of Sec2p Is Necessary but Not Sufficient for Sec2-GFP Localization
Since the COOH terminus of Sec2p is important for function in vivo (Nair et al. 1990), but not for exchange activity in vitro, it may play a role in regulating the polarized localization of Sec2p. To address this question, we studied the localization of COOH-terminally truncated Sec2 proteins. As for full-length Sec2-GFP, various Sec2 COOH-terminally truncated GFP fusion alleles were expressed as the sole copy in yeast (Fig. 2a and Fig. b) and the cells were observed in the microscope (Fig. 3). When either 172, 218, or 251 amino acids were truncated from the COOH terminus, Sec2p maintained a polarized localization, albeit not as tightly localized as full-length Sec2p (Fig. 3, compare Sec2-GFP with Sec2(1-587)-GFP, Sec2(1-541)-GFP, Sec-70(1-508)-GFP). In cells expressing these truncations, fluorescence was seen to fill medium-sized buds rather than just the tips of buds. Also, more punctate fluorescence was observed throughout the cytoplasm for the truncated Sec2 proteins as compared with full-length Sec2-GFP. However, truncating as little as 58 additional amino acids from Sec-70(1-508)-GFP leads to a dramatic loss of both polarized localization and cytoplasmic punctae (Sec2(1-450)-GFP and Sec2-59(1-374)-GFP). Therefore, we have defined a 58–amino acid domain in the COOH terminus of Sec2p that is required for the polarized localization of Sec2p.
We identified the point mutation responsible for the sec2-78 temperature-sensitive phenotype (Walch-Solimena et al. 1997) and found a cysteine to tyrosine change at amino acid 483, a site located within this 58–amino acid domain. This mutation was reconstituted into a GFP fusion (Sec2-78-GFP) and the encoded protein was found to be mislocalized with the cell displaying many cytoplasmic punctae.
It is important to note that, although cells expressing Sec2-59(1-374)-GFP are not temperature sensitive, expression of such a truncation in the context of the normal SEC2 locus, and hence lacking the stabilizing effect of GFP, does cause a severe growth phenotype (Nair et al. 1990). It is known that the expression of just one additional copy of sec2-59(1-374) allows yeast to survive at elevated temperatures. Since in the expression system employed here, mutant Sec2 fused to GFP and integrated at the LEU2 locus, there is at least a threefold increase in steady-state protein levels, it is not surprising that these strains are not temperature sensitive for growth.
We observed the localization of Sec4p by indirect immunofluorescence in Sec2-GFP– and Sec2(1-450)-GFP–expressing cells (Fig. 3). Sec4p displayed a correctly polarized localization in these cells, despite the mislocalization of Sec2(1-450)-GFP. This is consistent with the observation that these cells grow well and is in agreement with previous work where Sec4p is properly localized in sec2-78 expressing cells at the permissive, but not at the restrictive temperature (Walch-Solimena et al. 1997). An important implication of this result is that the localization of Sec2p does not directly dictate the localization of Sec4p.
We generated the sec2(1-450) and sec2-78 mutations at the endogenous SEC2 locus and found that these mutations lead to a temperature-sensitive growth phenotype (data not shown). Therefore, loss of polarized localization of the GFP fusions correlates with the temperature-sensitive phenotype observed for cells expressing normal levels of either sec2-59(1-374), sec2(1-450), or sec2-78. This suggests that localization of Sec2p is required for its full function, but that overexpression can bypass the need for its localization.
We have determined that the 58–amino acid domain present in the COOH terminus of Sec2p is required for the polarized localization of Sec2p. To address whether this domain is sufficient for localization, we expressed the SEC2 COOH terminus (amino acids 161-759) fused to GFP under the control of the strong inducible GAL1-10 promoter in a wild-type background. We observed that the COOH terminus of Sec2p could not direct GFP to sites of exocytosis (Fig. 3, GAL-sec2(161-759)-GFP/SEC2). Sec2(161-759)-GFP instead displayed a general cytoplasmic localization. Sec2(161-759)-GFP was detected at the expected molecular mass by Western analysis subsequent to GAL induction (Fig. 2 B, Sec2(161-759)-GFP, anti-GFP Ab). The anti-Sec2p antibody does not detect the COOH terminus. When Sec2-GFP was overexpressed in a wild-type background, it was found to localize to the tips of small and medium buds and necks. However, these sites appear to become saturated due to the overexpression and general cytoplasmic staining became enhanced (Fig. 3 , GAL-sec2-GFP/SEC2). Therefore, the COOH terminus of Sec2p is necessary but not sufficient for localization.
Sec2p Localization Is Dependent on the Actin Cytoskeleton, MYO2, SMY1, the Production of Vesicles, and SEC-1, -6, and -9
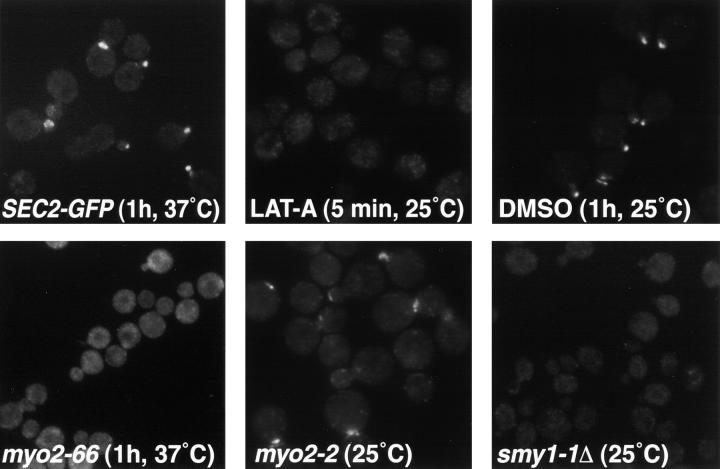
Previous work has investigated the effects of various conditions and mutant backgrounds on the polarized localization of Sec4p (Walch-Solimena et al. 1997). By repeating this strategy for Sec2p and comparing the results to the Sec4p data, we hoped to understand the interrelationship of these proteins. We studied the effects of several sec and morphogenetic mutant backgrounds on Sec2-GFP localization. We also observed the effects of cycloheximide, azide, and latrunculin (LAT)-A on Sec2-GFP localization. We found that treatment of growing yeast with LAT-A caused a very rapid mislocalization of Sec2-GFP at 25°C (Fig. 4). LAT-A sequesters actin monomers leading to the loss of F-actin (Spector et al. 1983; Coue et al. 1987; Ayscough et al. 1997). In contrast, a 1-h incubation with the solvent used for LAT-A, DMSO, did not perturb the polarized localization of Sec2-GFP. Sec2-GFP was also mislocalized in act1-1, cdc42-1, and cdc24-4 at the restrictive temperature (data not shown). Full-length Sec2-GFP in a wild-type background was correctly localized at 10-, 30-, and 60-min time points subsequent to a shift to the restrictive temperature (Fig. 4 and Fig. 5). ACT1 is the essential actin gene in yeast, CDC42 encodes the Rho GTPase required for polarized actin distribution and CDC24 encodes the Cdc42p exchange factor. These data are consistent with the idea that the localization of Sec2-GFP is dependent on a polarized actin cytoskeleton. Sec4p is also mislocalized in an actin mutant. This is thought to reflect the fact that vesicles carrying Sec4p accumulate in a depolarized fashion when actin function is disrupted. We have recently confirmed by electron microscopy that vesicles accumulate in a random manner in LAT-A–treated cells (Karpova et al. 2000).
Figure 4.
The localization of Sec2-GFP is dependent on the actin cytoskeleton, Myo2p, and Smy1p. Haploid yeast were engineered to express Sec2-GFP in wild-type (panels SEC2-GFP, LAT-A, and DMSO) or mutant backgrounds (myo2-66, NY2162; myo2-2, NY2163; smy1-1Δ, NY2161). Cells were grown as in Fig. 3. Temperature shifts were carried out for 1 h at 37°C. Treatment with the drug LAT-A was at a concentration of 200 μm. DMSO was the solvent used to solubilize LAT-A. All cells were fixed and permeabilized before direct visualization of GFP.
Figure 5.
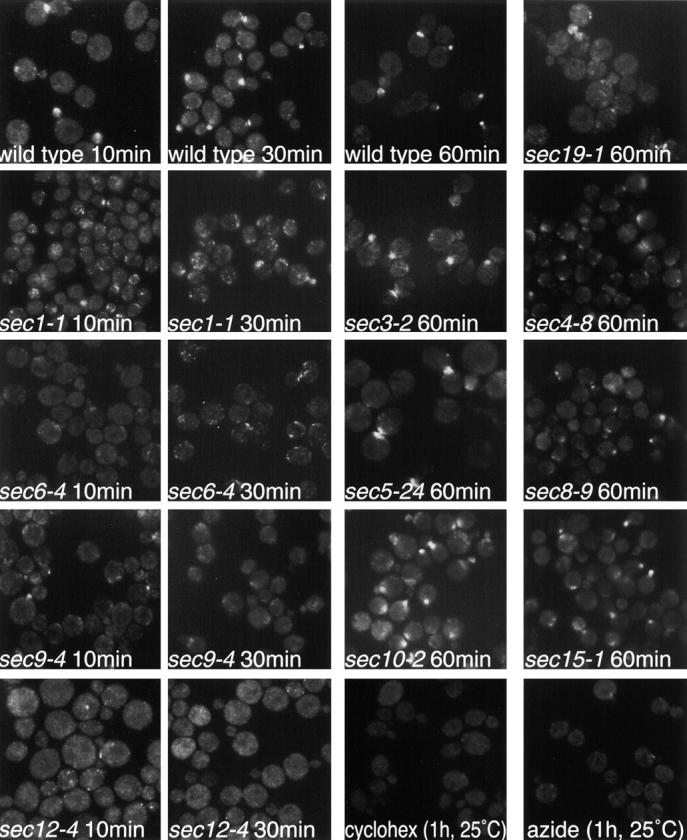
Polarized localization of Sec2-GFP requires the production of post-Golgi vesicles and functional Sec1p, Sec6p, and Sec9p but not Sec3p, Sec5p, Sec8p, Sec10p, or Sec15p. Cells were engineered expressing Sec2-GFP in various mutant backgrounds. Haploid yeast were grown to mid-log phase and half the culture was shifted to 37°C for various times. The rest of the culture was grown at 24°C and visualized as a control (data not shown). Wild-type, NY2146; sec1-1, NY2164; sec3-2, NY2170; sec4-8, NY2172; sec5-24, NY2167; sec6-4, NY2165; sec8-9, NY2166; sec9-4, NY2171; sec10-2, NY2168; sec12-4, NY2176; sec15-1, NY2169; sec19-1, NY2174. Cycloheximide and azide were used at a concentration of 0.35 and 10 mM, respectively, for 1 h at 25°C. Cells were fixed, permeabilized, and visualized directly. Sec2-GFP is properly localized in all genetic backgrounds with the exception of sec1-1, sec6-4, sec9-4, sec12-4, and sec19-1. Sec2-GFP is also mislocalized in yeast treated with cycloheximide and azide. Sec2-GFP is partially mislocalized in a sec4-8 mutant background.
Sec2-GFP localization is also dependent on Myo2p. MYO2 encodes a type V myosin motor for which indirect evidence supports a role in the polarized transport of post-Golgi vesicle (Johnston et al. 1991; Govindan et al. 1995; Walch-Solimena et al. 1997; Reck-Peterson et al. 1999). In myo2-66 cells at the restrictive temperature Sec2-GFP is mislocalized and can be observed as random punctae in the cell (Fig. 4). However, the mutation in myo2-66 is in the actin binding face of the head domain of Myo2p and it is possible that the mislocalization of Sec2-GFP may be an indirect effect of this mutation.
In contrast to myo2-66, myo2-2 (vac15-1; Catlett and Weisman 1998) is a vacuolar inheritance mutant allele, possessing a point mutation in the globular tail domain. This mutation causes the accumulation of vacuoles in the mother cell at 25°C. myo2-2 is not temperature sensitive and is not synthetically lethal with sec4-8, sec2-41, or smy1-Δ1 (Catlett and Weisman 1998) unlike myo2-66. Interestingly and in contrast to Myo2p, Myo2-2p is not localized to bud tips at 25°C. In myo2-2 cells, Sec2-GFP has a slight polarity defect at 25°C (Fig. 4). When localization of Sec2-GFP to buds and necks fluorescence was quantitated in wild-type and myo2-2 cells (sister spores), we found a statistically significant mislocalization of Sec2-GFP. The mean percentages of total number of buds and necks for myo2-2 and MYO2 are 16.6(8.66) and 35.7(8.25), respectively. With α = 0.05, the two percentages are significantly different, t(18) = −5.05, standard error = 3.78. Sec4p appears properly localized in myo2-2 cells consistent with the fact that these cells grow well (data not shown). It would appear that Sec2-GFP localization depends on a fully functional Myo2p. A mutation in the tail domain that strongly affects the Myo2p-vacuole interaction may also confer a mild phenotype for other Myo2p cargo such as vesicles, to the extent that we can detect significant mislocalization of Sec2p, but not Sec4p.
SMY1 encodes a protein distantly related to kinesin (Smy1p) which binds Myo2p and, when overexpressed, suppresses the myo2-66 phenotype (Lillie and Brown 1992) and a dominant negative SEC4 allele (Shannon, J., unpublished results). Deletion of SMY1 causes a transient mislocalization of Sec2-GFP. Freshly dissected spores harboring a smy1 deletion and the SEC2-GFP allele, displayed mislocalization of Sec2-GFP. Sister spores possessing an intact SMY1 gene localized Sec2-GFP correctly. However, upon growth at 25°C for ∼30 h, Sec2-GFP regains localization in the smy1Δ cells, probably through some adaptation event. Therefore, Smy1p is not essential for the localization of Sec2-GFP or for the transport of vesicles in yeast, but may normally play a role, nonetheless. smy1-Δ1 and sec2-41 are synthetically lethal (Lillie and Brown 1998), as are all combinations of sec4-8, sec2-41, smy1-Δ1, and myo2-66 (Salminen and Novick 1987; Lillie and Brown 1992; Govindan et al. 1995; Lillie and Brown 1998). This further adds to the body of indirect evidence that links the functions of Sec4p, Sec2p, Smy1p, and Myo2p in the vectorial transport of post-Golgi vesicles.
We next observed the localization of Sec2-GFP under conditions that block the production of post-Golgi vesicles. Sec12p is required for exit of cargo from the ER. In a sec12-4 background Sec2-GFP localized normally at the permissive temperature, however, within 10 min of a shift to the restrictive temperature Sec2-GFP mislocalized into cytoplasmic punctae (Fig. 5). Sec2-GFP is also mislocalized at 37°C in sec19-1, a mutant affecting the Rab GDI protein. Treatment of cells with either cycloheximide or azide also caused mislocalization of Sec2-GFP. Production of post-Golgi vesicles may be needed for the proper localization of Sec2p because it rides these vesicles to sites of exocytosis. This model will be addressed below.
Of the late-acting sec mutants Sec2-GFP is correctly localized in mutants affecting various components of the exocyst, namely sec3-2, sec5-24, sec8-9, sec10-2, and sec15-1 backgrounds at 37°C. In these mutants, the accumulated vesicles are concentrated primarily in the bud after a short shift to the restrictive temperature. The polarized localization observed for Sec2-GFP in these mutants may reflect the interaction between Sec2p and the vesicles which are accumulated in the bud.
The Sec4-8 mutant protein does not efficiently bind nucleotide and is less abundant than the wild-type protein. In sec4-8 cells, vesicles accumulate randomly, cells grow slowly and Sec4p is not easily detected at sites of polarized growth at the permissive temperature. Consistent with the above, Sec2-GFP is partially mislocalized when expressed in a sec4-8 mutant at the restrictive temperature.
Sec2-GFP mislocalizes upon a shift to the restrictive temperature in the late-acting secretory mutants sec1-1, sec6-4, and sec9-4. SEC6 encodes a component of the exocyst that localizes to sites of polarized growth and has been hypothesized to be the docking complex for incoming post-Golgi vesicles (TerBush and Novick 1995; TerBush et al. 1996). Since mutants affecting other components of the exocyst do not mislocalize Sec2p, Sec6p may have a specific relationship with Sec2p not shared by the other components. Sec9p is a t-SNARE (Brennwald et al. 1994), while Sec1p interacts with assembled Snc/Sso/Sec9p SNARE complexes (Carr et al. 1999). The Sec2-GFP was observed to be polarized in all mutant backgrounds at 25°C (data not shown) and at 37°C in wild-type cells (Fig. 5, wild-type 10, 30, and 60 min).
In conclusion, the localization of Sec2p is mediated by post-Golgi vesicles; if they are not produced (cycloheximide, azide, or early ER→Golgi blocks), or accumulate randomly in the mother cell (LAT-A, act1-1, and sec4-8), Sec2-GFP is mislocalized. Sec2-GFP localization also depends in part, on Myo2p and Smy1p function, consistent with indirect evidence that Myo2p is the motor transporting post-Golgi vesicles in yeast. SEC1, SEC6, and SEC9 are also required for the polarized localization of Sec2-GFP. The fact that only these late-acting SEC mutants mislocalize Sec2-GFP is suggestive of a Sec2p regulatory event not yet understood (see Discussion).
Truncated Sec2 Proteins Are Enriched in a High-Speed Pellet Fraction
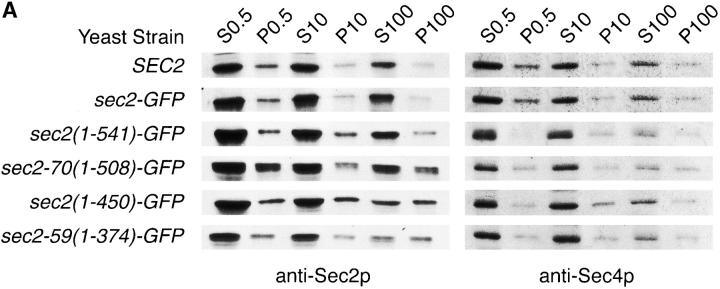
To understand the decrease in polarized fluorescence observed for truncated Sec2 proteins, differential centrifugation was performed on lysates from cells expressing full-length Sec2 and various Sec2p-truncations. Fig. 6 A shows these results. Full-length Sec2p and Sec2-GFP are found in greater amounts in the 100,000-g supernatant (S100) than in the 100,000-g pellet (P100). The P100 is the microsomal pellet which contains post-Golgi vesicles as well as other small cellular components (Walworth and Novick 1987). In lysates possessing COOH-terminally truncated Sec2 proteins (Fig. 6 A, Sec2-70(1-508)-GFP), the ratio is shifted from the S100 to the P100. This shift is maximal for those truncations that display the most diminished localization as monitored by GFP fluorescence. Sec2(1-450)-GFP and Sec-59(1-374)-GFP expressing lysates have similar amounts of protein in the 100,000-g supernatant and pellet. Therefore, truncation of the COOH terminus of Sec2p leads to a redistribution of protein from the S100 to the small microsomal fraction (P100). All lysates were assayed for Sec4p as a control. The Sec4p S100/P100 ratio was similar in each case (Fig. 6 A, anti-Sec4p blots).
Figure 6.
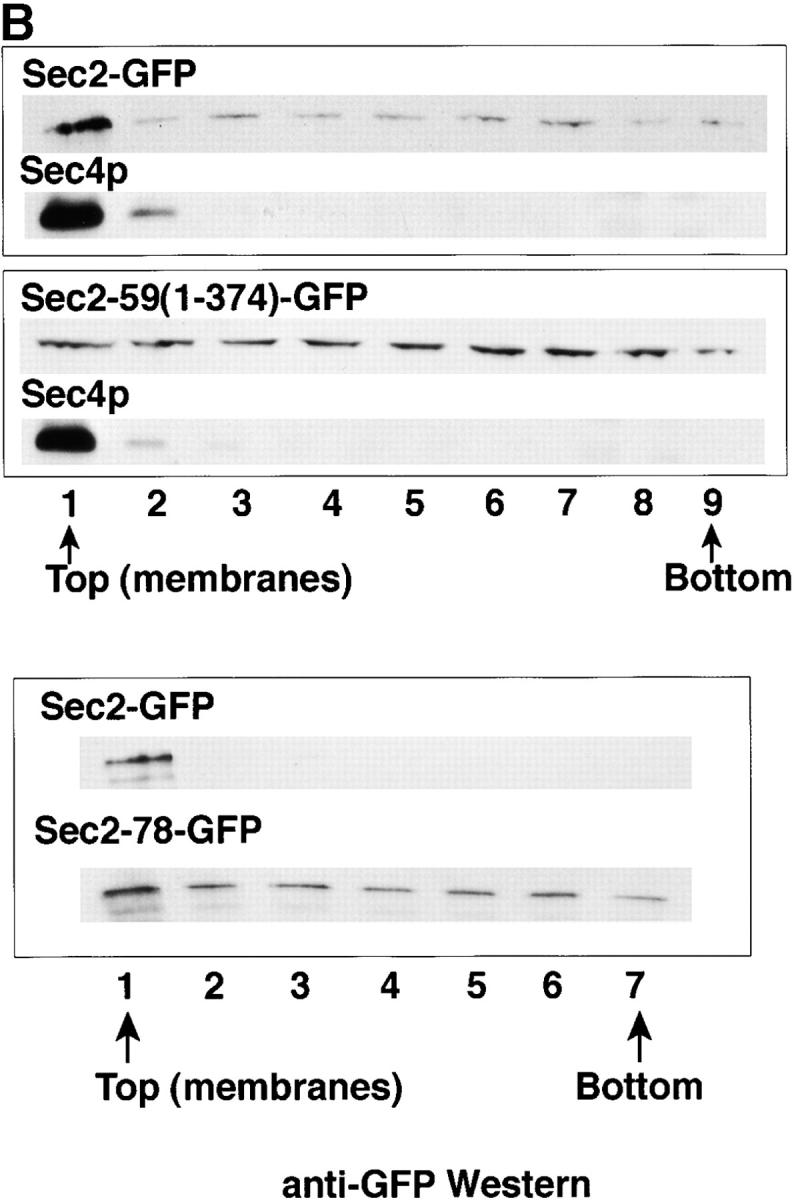
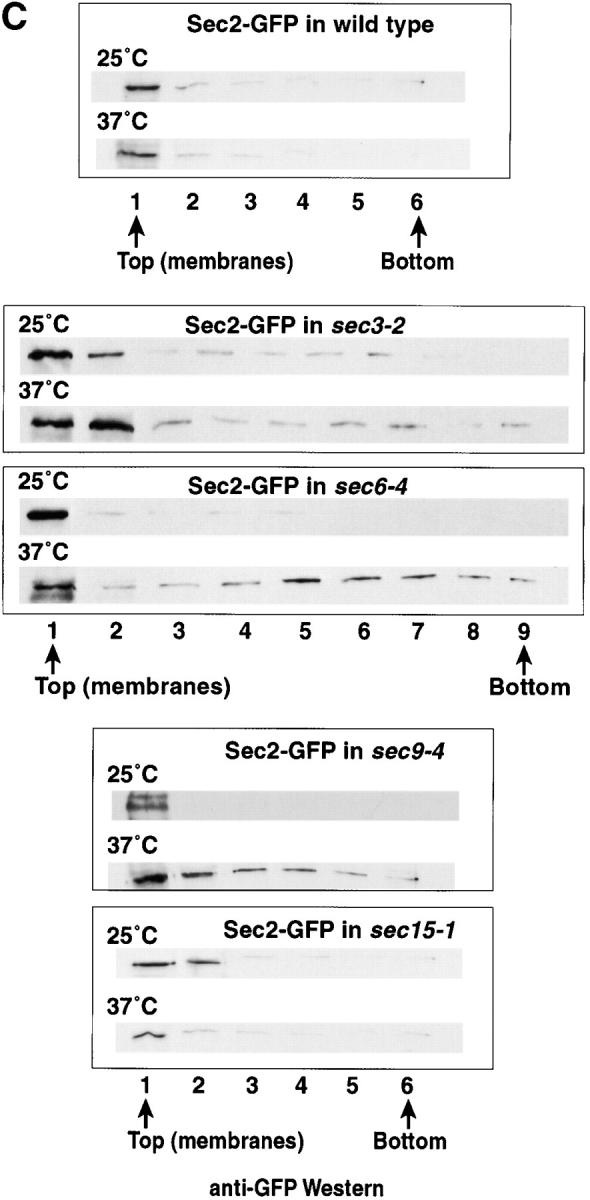
The COOH-terminal domain of Sec2p is required for membrane attachment. (A) Truncation of the COOH terminus of Sec2p causes redistribution from the soluble to the small microsomal fraction. Cells were grown to mid-log phase, harvested, and lysed as described in the Materials and Methods. Differential centrifugation was performed on lysates from Sec2p (NY2145), Sec2-GFP (NY2146), Sec2(1-541)-GFP (NY2150), Sec2-70(1-508)-GFP (NY2148), Sec2(1-450)-GFP (NY2154), and Sec2-59(1-374)-GFP (NY2147). S0.5 supernatants were centrifuged at 10,000 g, producing the S10 and P10. The S10 supernatants were centrifuged at 100,000 g, producing S100 and P100. Equal volumes of samples were prepared for SDS-PAGE and subsequently analyzed by Western analysis with either anti-Sec2p or anti-Sec4p followed by goat anti–rabbit HRP. Upon truncation of the COOH terminus Sec2p redistributes from the soluble fraction (S100) to the microsomal pellet (P100). Hence, Sec2(1-450)-GFP, and Sec2-59(1-374)-GFP are distributed equally between the S100 and P100 while full-length Sec2p is largely soluble. (B) Sec2p membrane association is dependent on the COOH-terminal domain and this association correlates with Sec2p polarized localization. The small microsomal membrane fraction (P100, prepared as above) was resuspended and mixed at a 1:1 (vol/vol) ratio with iodixanol and centrifuged for 1 h at 625,000 g. Fractions (1–9) were collected and equal volumes were subjected to SDS-PAGE, transferred to a filter and probed with anti-GFP (Sec2 proteins, anti-Sec4p and Pma1p antibodies. Fraction 1 (and sometimes 2, top of gradient) contained the post-Golgi marker Sec4p and the plasma membrane ATPase, Pma1p (data not shown). Sec2-GFP, but not COOH-terminally truncated Sec2-59(1-374)-GFP nor the point mutant Sec2-78-GFP was enriched on membranes. (C) In addition, full-length Sec2-GFP membrane enrichment was perturbed in both sec6-4 and sec9-4 mutant backgrounds at 37°C but not the permissive temperature (25°C). This correlates with the loss of Sec2-GFP localization observed in sec6-4 at the restrictive temperature (refer to Fig. 5). As a control, Sec2-GFP was assayed in both sec3-2 and sec15-1 mutant backgrounds and found to be enriched on membranes at both temperatures. As an additional control, Sec2-GFP was assayed at 37°C in a wild-type background where it is enriched on membranes. Sec2-GFP is localized in both sec3-2 and sec15-1 backgrounds at the restrictive temperature (refer to Fig. 5).
To determine the ratio of soluble to pelletable Sec2-GFP in lysates from various sec mutant backgrounds (at 37°C), fractionation experiments similar to those performed above were done. S100/P100 ratios were similar to those seen for Sec2-GFP expressed in a wild-type background (Fig. 6 A); that is, much more of the Sec2-GFP is present in the S100 than in the P100 (data not shown).
The above result indicate that truncated Sec2 proteins that do not localize in a polarized manner by fluorescence are associated to a greater extent with the small microsomal fraction than longer Sec2 proteins.
Full-Length, Localized Sec2 Proteins Are Enriched on Membranes while Truncated or Mislocalized Sec2 Proteins Are Not
Our localization data suggests that Sec2p, like Sec4p, is detected at regions of polarized growth through its association with vesicles that are concentrated at exocytic sites. Why then are truncated Sec2 proteins, which fail to properly localize, found enriched in the microsomal pellet-containing secretory vesicles? One possibility is that the truncated proteins might be pelleting through the formation of a protein complex or aggregate, rather than through association with vesicles. This was addressed by subjecting resuspended P100 fractions from yeast lysates possessing either Sec2-GFP, Sec2-59(1-374)-GFP or Sec2-78-GFP (Fig. 6 B), to iodixanol non-equilibrium density gradients. Subsequent to centrifugation, supernatants were split into equal volume fractions. Membranes were isolated from the first (and sometimes second) fractions, floating at the top of the gradient. Fractions were assayed for Sec2 proteins (anti-GFP), Sec4p, and/or the plasma membrane ATPase, Pma1p (controls for membrane), by Western analysis (Fig. 6 B). Sec2-GFP is found in fraction 1 of the gradient, which contains the bulk of the Pma1p (data not shown) and Sec4p (Fig. 6 B, top two panels). There is ∼20-fold more Sec2-GFP in fraction 1 than in the other eight fractions collected. Sec2-59(1-374)-GFP, on the other hand, is distributed similarly in all fractions. Sec2-78-GFP was distributed throughout all fractions with a minimal enrichment in the membrane fraction. The slight enrichment of Sec2-78-GFP on membranes makes sense in view of the fact that sec2-78 is a temperature-sensitive allele that is healthier than sec2-59 at 25°C and sicker than the wild-type SEC2 allele at elevated temperatures. Gradients were centrifuged for 1.5 h at 625,000 g, which generates enough force for membranes to float but not enough for most proteins to reach their density in the gradients (hence, non-equilibrium). It follows that both Sec2-78-GFP and Sec2-59(1-374)-GFP are in pelletable protein complexes (or possible aggregates) and are not highly enriched on membranes as is full-length Sec2-GFP. Loss of polarized localization of Sec2-78 and Sec2-59(1-374)-GFP reflects a weaker association with microsomal membranes as compared with full-length Sec2-GFP.
The data presented above correlate polarized localization of full-length Sec2-GFP with COOH-terminal domain mediated membrane attachment. It was therefore enticing to postulate that the mislocalization observed for Sec2-GFP in sec1-1, sec6-4, and sec9-4 mutant backgrounds might reflect a lower association of full-length Sec2-GFP with membranes at the restrictive temperature. To address this, iodixanol density gradient fractionation was performed on lysates from yeast expressing Sec2-GFP in either sec6-4 or sec9-4 backgrounds at both the permissive and restrictive temperatures. In Fig. 6 C Sec2-GFP is enriched in the membrane fraction (fraction 1) at 25°C in both sec6-4 and sec9-4 mutant backgrounds. However, at 37°C, Sec2-GFP associates only weakly with membranes and is found redistributed to the other fractions. As a control, the same experiment was performed in sec3-2 and sec15-1 mutant backgrounds. As shown above (Fig. 5), Sec2-GFP localized in a polarized manner in both sec3-2 and sec15-1 at the restrictive temperature. Sec2-GFP is enriched in the membrane-containing fractions (in this case membranes were collected in fraction 1 and 2) at both permissive and restrictive temperatures in sec3-2 and sec15-1 backgrounds. Sec2-GFP fractionates with membranes from wild-type yeast at both 25°C and 37°C. It is also important to mention that the Sec2-GFP 100,000-g supernatant (S100) to pellet (P100) ratios were similar for both wild-type and mutants at 25 and 37°C, and to those shown for SEC2 and sec2-GFP in Fig. 6 A (data not shown). In conclusion, we have established that the polarized localization of Sec2-GFP reflects a tight association with membranes of the small microsomal fraction (P100). Loss of localization of Sec2 proteins due to either truncation of their COOH-terminal domain or expression of full-length Sec2p in sec6-4 or sec9-4 mutant backgrounds lead to weaker association of Sec2 proteins with membranes and redistribution into other protein-containing fractions of the iodixanol gradient.
Sec2p Associates with Membranes in both a Sec4p-dependent and -independent Manner
We have established above that the COOH-terminal domain of Sec2p is required for the association of Sec2p with membranes. Sec4p is also associated with the P100 and floats with membranes in iodixanol gradients (Fig. 6 B, top panels). Does Sec2p associate with membranes via its affinity for Sec4p? To address this question, Sec4p was extracted from membranes with Gdi1p (Garrett et al. 1993, Garrett et al. 1994) and Sec2-GFP was assayed for membrane attachment by Western analysis. Membranes (10,000 g) were prepared and incubated with Gdi1p (or mock treated) for 30 min at 30°C. Membranes were then centrifuged through a sucrose cushion and supernatant and pellet were separated. Equal volumes of supernatant and pellet were subjected to SDS-PAGE and Sec4p and Sec2p were detected by Western analysis (Fig. 7 A). Sec4p is almost completely solubilized by Gdi1p (Fig. 7 A, +Gdi1p) as compared with the mock reaction (−Gdi1p). Sec2-GFP on the other hand, remains in the pellet fraction subsequent to centrifugation (Fig. 7 A, *). Sec2p is a labile protein and some breakdown products are detected (Fig. 7 A, Sec2-GFP, lower bands). It is possible that the Sec2-GFP present in the pellet reflects higher order protein complexes that are not associated with membranes. To rule out this possibility, Gdi1p-treated membranes were floated on iodixanol. Sec2-GFP cofractionates with membranes (Fig. 7 B, fraction 1, Sec2-GFP) and not as a protein complex, while Sec4p is distributed throughout the iodixanol fraction upon Gdi1p treatment (Fig. 7 B, fractions 1–3, +Gdi1p, Sec4p). In conclusion, full-length Sec2p remains bound to membranes in the absence of Sec4p indicative of the existence of a novel Sec2p receptor on membranes.
Figure 7.
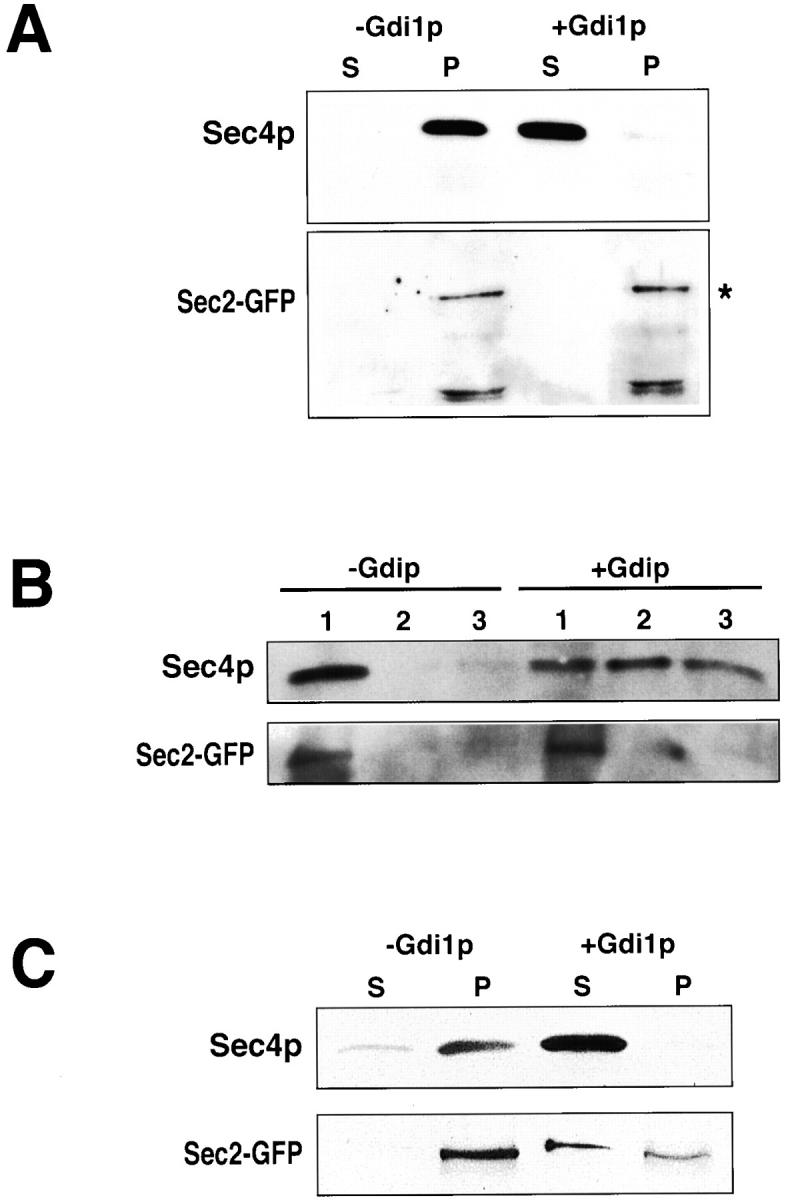
Sec2-GFP is associated with membranes both in a Sec4p-dependent and -independent manner. (A) Membranes (10,000 g pellet, P10) were washed in reaction buffer and incubated at 30°C, 30 min in the presence (+Gdi1p) or absence (−Gdi1p) of partially purified, bacterially expressed, Gdi1p. The reaction was then layered onto a sorbitol cushion and spun at 10,000 g for 20 min at 4°C. Supernatant was removed and the pellet was resuspended to the original reaction volume. Equal volumes of supernatant (S) and pellet (P) were subjected to SDS-PAGE and Western analysis. Sec4p and Sec2-GFP were detected. Sec4p is efficiently solubilized by Gdi1p resulting in a shift of Sec4p from the pellet (P, −Gdi1p) to the supernatant (S, +Gdi1p). Sec2p remains in the pellet in mock- and Gdi1p-treated samples (*). Sec2p breakdown products appear toward the bottom of the Sec2-GFP panel. (B) To ensure that Sec2-GFP is not pelleting independent of membranes upon treatment with Gdi1p, the reactions were repeated and subjected to iodixanol floatation gradients (as in Fig. 6 B). Sec2-GFP indeed migrates in the gradient with membranes. (C) Membranes (100,000 g, P100) were treated as for (A) above, but spun onto a 60% sucrose cushion before the Gdi1p reaction. In addition, the supernatant and pellet were fractionated through a sorbitol cushion (as above) but centrifuged at 500,000 g to ensure that the vesicles pellet through the cushion.
Gdi1p-mediated Sec4p solubilization was also performed on the small microsomal fraction (100,000 g, P100) which is enriched for small vesicles (Walworth and Novick 1987). Sec2-GFP was observed distributed in both the supernatant and the membrane pellet (Fig. 7 C). In the mock experiment (−Gdi1p) some solubilization of Sec4p can be observed. This may reflect endogenous levels of Gdi1p found in the P100. In any case, Sec2-GFP is detected solely on membranes. As a control for membrane pelleting efficiency, the distribution of the post-Golgi vesicle marker Snc1/2p was assayed and found exclusively in the pellet fraction (data not shown). It appears that Sec2p associates with P100 membranes (enriched for vesicles), in part, by directly binding to Sec4p. This is consistent with previous data where Sec2-59(1-374)p was found to be associated with vesicles to a higher degree upon increased expression of Sec4p in the cell (Walch-Solimena et al. 1997). The remainder of the Sec2-GFP associates with vesicles in a Sec4p-independent manner. In conclusion, Sec2-GFP associates with membranes largely by a Sec4p-independent manner. This would imply the existence of an unidentified Sec2p receptor required for membrane attachment.
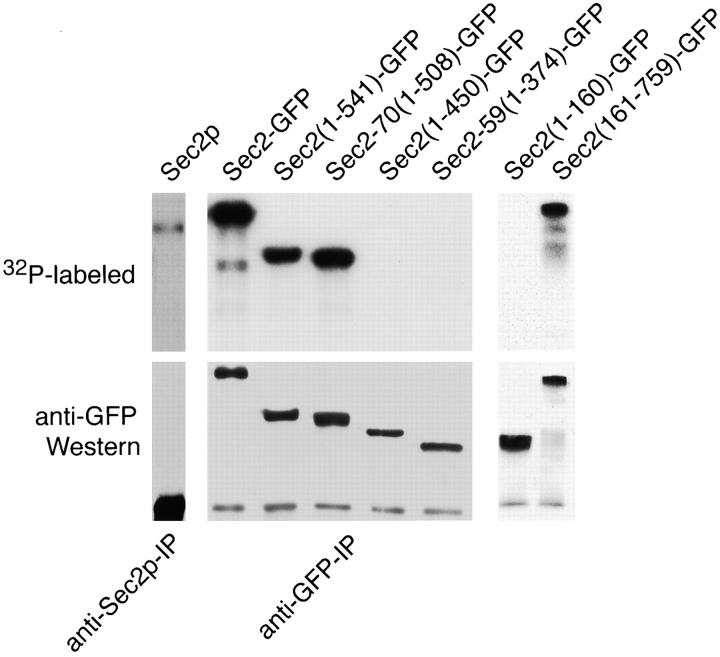
A Sec2p COOH-terminal Domain Is Required for Its Phosphorylation
To explore mechanisms by which Sec2p may be regulated we determined if Sec2p is phosphorylated. Various Sec2 proteins were immunoprecipitated from lysates of metabolically labeled yeast, separated by SDS-PAGE, transferred to nitrocellulose and exposed to film. Sec2p was detected as a phospho-protein of the expected molecular weight when immunoprecipitated using anti-Sec2p antibodies (32P-labeled panel). No mobility shift for Sec2p was detected between steady-state levels of Sec2p and the phosphorylated protein (data not shown). Little phosphorylated Sec2p is detected due to low efficiency of immunoprecipitation with this antibody. Sec2-GFP, Sec2(1-541)-GFP and Sec2-70(1-508)-GFP are also phosphorylated (Fig. 8, 32-labeled panel). Immunoprecipitated Sec2 proteins are detected by Western blot using anti-GFP antibodies. As expected, Western blot analysis using anti-GFP antibodies did not detect untagged Sec2p. Two-dimensional analysis showed that Sec2-70(1-508)-GFP is quantitatively phosphorylated since Sec2-70(1-508)-GFP–specific spots comigrated when detected by either silver stain or radiography (data not shown). Furthermore, Sec2(1-450)-GFP and Sec2-59(1-374)-GFP, although quantitatively immunoprecipitated via their GFP tags (Fig. 8, anti-GFP Western), are not detected as phosphorylated bands (32P-labeled panel). Also, the point mutant Sec2-78-GFP was found to be phosphorylated to the same extent as full-length Sec2p (data not shown).
Figure 8.
Sec2-GFP phosphorylation is dependent on the COOH-terminal domain. Sec2 proteins are phosphorylated. Yeast cells expressing various Sec2 GFP fusion proteins were grown overnight to mid-log phase in phosphate-free glucose or galactose (sec2(1-160)-GFP and sec2(161-759)-GFP) media at 24°C, metabolically labeled with 32P-orthophosphate and harvested. Lysates were generated and post–100,000-g supernatants were immunoprecipitated with either anti-Sec2p (Sec2p) or anti-GFP polyclonal (Sec2p, Sec2-GFP, Sec2(1-541)-GFP, Sec2-70(1-508)-GFP, Sec2(1-450)-GFP, Sec2-59(1-374)-GFP, Sec2(1-160)-GFP, and Sec2(161-759)-GFP) antibodies. Immune complexes were resolved on SDS-PAGE, proteins were transferred to nitrocellulose filters and both exposed to film at −80°C and probed with anti-GFP monoclonal antibodies by Western analysis. Sec2p, Sec2-GFP, Sec2(1-541)-GFP, Sec2-70(1-508)-GFP, and Sec2(161-759)-GFP are phosphorylated while Sec2(1-450)-GFP, Sec2-59(1-374)-GFP, and Sec2(1-160)-GFP are not. The point mutant Sec2-78-GFP is phosphorylated as well (data not shown). Western analysis detects the Sec2-specific proteins present in the lysates (anti-GFP Western). As expected, untagged Sec2p is not detected by the anti-GFP antibodies. Also, only low levels of Sec2p are observed by radiography (32P-labeled) since lower levels of Sec2p are expressed (Fig. 2 B) and the anti-Sec2p polyclonal antibodies are not efficient for immunoprecipitation.
To further characterize the phosphorylation site(s), Sec2(1-160)-GFP– and Sec2(161-759)-GFP–expressing cell lysates were metabolically labeled with radioactive phosphate. Sec2(161-759)-GFP, but not Sec2(1-160)-GFP, was found to be phosphorylated, lending support to the possibility that the phospho-receptor lies within the 58–amino acid domain required for phosphorylation of the COOH-terminally truncated Sec2 proteins. Thus, the same 58–amino acid domain required for Sec2p polarized localization, growth at elevated temperatures and membrane attachment, is also required for phosphorylation.
Discussion
Sec4p, the Rab protein required for post-Golgi transport in budding yeast, is associated with secretory vesicles (Goud et al., 1988). Sec2p is the essential nucleotide exchange factor for Sec4p and is required for the polarized delivery of Sec4p-vesicles to exocytic sites. Sec2p activates Sec4p by increasing both the GDP off-rate and the binding of GTP, thus promoting the interaction of GTP-Sec4p with effectors required for vesicular transport (Walch-Solimena). In this study, we establish a role for the COOH terminus of Sec2p in the efficient polarized delivery of Sec4p vesicles.
Previous work has shown that COOH-terminal truncation of Sec2p produces a defective protein causing a temperature-sensitive growth and secretion phenotype (Nair et al. 1990). However, the results presented here show that loss of the COOH terminus of Sec2p does not reduce its nucleotide exchange activity (GTP on-rate) to the extent that would cause temperature sensitivity. In support of this, we expressed levels of full-length Sec2p that were undetectable by Western blot, with no resulting growth defect. These cells would have a much greater decrease in Sec2p function than the ∼20% decrease in exchange activity observed for the truncated protein Sec2-59(1-374)-GFP. Thus, the COOH terminus must have another function.
Full-length Sec2-GFP localizes to sites of exocytosis, including the presumptive bud site, small buds and the mother/daughter neck (Fig. 3). A 58–amino acid domain, present in the COOH terminus, was shown to be necessary but not sufficient for this localization. Sec2 proteins, either lacking the 58–amino acid domain, or possessing a point mutation within this domain (sec2-78) are not concentrated at sites of exocytosis. In addition, this domain is required for the tight membrane association displayed by full-length and not truncated Sec2 proteins. The localization of Sec2p to sites of exocytosis correlates with its membrane association.
The localization of Sec2p to exocytic sites is reminiscent of Sec4p localization (Novick and Brennwald 1993; Walch-Solimena et al. 1997), and is similar to that observed for other late-acting secretory proteins functioning at the sites of polarized growth; namely, Sec5p (Mondesert et al. 1997), Sec3p (Finger et al. 1998), Sec8 (TerBush and Novick 1995), Sec1p (Carr et al. 1999), and Exo84p (Guo et al. 1999a). As in the case of Sec4p, the localization of Sec2-GFP to these sites requires the ongoing production of secretory vesicles as well as the function of the actin-based cytoskeleton and the Myo2p motor. We interpret this localization to reflect the association of Sec2-GFP with secretory vesicles that are concentrated at sites of exocytosis in an actin-dependent fashion. There are several situations in which the localization of Sec2-GFP differs from that of Sec4p. Sec4p localization is independent of all of the late acting Sec proteins except Sec2p, while Sec2-GFP requires, in addition to Sec4p function, the function of Sec1p, Sec6p, and Sec9p as well. We postulate that Sec2p normally recycles after activating Sec4p. Mutants defective in Sec1p, Sec6p, and Sec9p function are blocked after Sec2p has recycled, leading to an accumulation of vesicles carrying Sec4p but not Sec2-GFP. Other mutants are blocked before this event, leading to the accumulation of vesicles carrying both Sec4p and Sec2-GFP. In support of this hypothesis we have shown that membrane association of full-length Sec2-GFP is perturbed in both sec6-4 and sec9-4 mutant cells at the restrictive temperature, but not in sec3-2 or sec15-1 cells.
We propose that Sec2p associates with secretory vesicles and this allows its nucleotide exchange activity greater accessibility to Sec4p, whose activation is required for vesicle transport. When Sec2p membrane attachment is compromised and the activation of Sec4p occurs less efficiently (as in a sec2(1-450), sec2-59(1-374), or sec2-78 mutant cells), vesicles accumulate in a random fashion. Yeast overexpressing truncated sec2 alleles bypass the need for Sec2p membrane attachment and thereby overcome this growth defect. In our study this increase in expression occurs when sec2(1-450), sec2-59(1-374), or sec2-78 are fused to GFP and integrated at the LEU2 locus. Sec2 proteins defective for membrane attachment may still interact with Sec4p during transport, however, we propose that this interaction is partially compromised. This explains the detection of both Sec2-59(1-374)p and Sec2-78p in association with the vesicular pool in previous work from our lab (Walch-Solimena et al. 1997). It is also possible that the interaction of truncated Sec2 proteins with Sec4p is able to withstand sucrose, as was used in the earlier study (Walch-Solimena et al. 1997), but not iodixanol gradient centrifugation, as used here.
If Sec2p function is required for efficient vesicle targeting, Sec4p activation may be required for the transport event and/or upon arrival at sites of exocytosis. Analogous to Rab 5 function during endosome fusion (Rybin et al. 1996), Sec4p may also undergo continuous cycles of exchange during transport in order to ensure that it is in its activated GTP-bound form, ready to interact with its docking effector, Sec15p (Guo et al. 1999b), upon reaching the destination. To date, Sec15p is the only effector known for Sec4p and sec15-1 is not defective for the polarized delivery of vesicles to exocytic sites. We therefore anticipate the existence of an additional Sec4p effector that would serve to couple Sec4 activation to the vesicle transport machinery. This effector would be analogous to the Golgi-associated kinesin-like effector for Rab6 which plays a role in Golgi dynamics in mammalian cells (Echard et al. 1998).
Vectorial transport of post-Golgi vesicles in yeast is mediated by the actin cytoskeleton. Post-Golgi vesicles accumulate randomly in LAT-A–treated cells (Karpova et al. 2000), reminiscent of the accumulation of vesicles in an act1 or sec2 mutant background (Novick and Botstein 1985; Walch-Solimena et al. 1997). Indirect evidence has implicated the unconventional type V myosin, Myo2p, as the motor involved in the transport process in yeast since mutations in MYO2, or overexpression of the Myo2p-tail domain, lead to a random accumulation of vesicles (Govindan et al. 1995; Karpova et al. 2000; Reck-Peterson et al. 1999; Schott et al. 1999). Accordingly, Sec4p (Walch-Solimena et al. 1997) and Sec2p (Fig. 4 and text) are mislocalized in myo2-66. Sec2-GFP is partially mislocalized in myo2-2. This allele specifically affects vacuolar inheritance and has no growth phenotype and no defect in Sec4p localization. The myo2-2 mutation is in the tail domain and Myo2-2p is severely impaired for vacuole binding. This mutation might also affect the interaction between Myo2-2p and vesicles to some extent as well. In this case, vesicle transport may be mildly affected to the point where Sec2p is partially mislocalized but with no effect on growth rate.
In mammalian cells, filamentous actin, organized at the cell periphery, may play a role in short-range movement or capture of vesicles transported there via a long-range microtubule-based mechanism (Wu et al. 1998). In yeast microtubules are not required for post-Golgi vectorial transport. In dilute mice, a defective myosin Va leads to a perinuclear distribution of melanocytes causing a lighter colored coat. Normal melanocytes possess randomly distributed melanosomes which cause the normal, darker, coat color (Provance et al. 1996; Wei et al. 1997). These results and the visualization of melanosome dynamics in dilute melanocytes (Wu et al. 1998) imply that myosin Va may act to tether these vesicles to the cell periphery. In support of this, disruption of actin in melanoma cells leads to a phenotype similar to that observed in dilute melanocytes (Koyama and Takeuchi 1980). In humans, myosin Va may be the gene involved in Griscelli's syndrome, where patients have a diluted pigment as well as immunodeficiency and/or neurological phenotypes (Griscelli et al. 1978; Haraldsson et al. 1991; Hurvitz et al. 1993; Klein et al. 1994; Pastural et al. 1997). Studying the actin-mediated transport of vesicles in yeast may shed light on the interactions between Rab proteins and unconventional myosins in transport.
In conclusion, we have characterized a 58–amino acid domain in the COOH terminus of Sec2p that regulates the association of Sec2p with membranes. Sec2p membrane association is correlated with a polarized localization, reminiscent of Sec4p localization. The data presented here are consistent with a model where Sec2p is carried on post-Golgi vesicles, activating Sec4p either before or during transport. This implies the existence of an unidentified Sec4p transport effector. This effector may be part of a transport complex that links post-Golgi vesicles to the actin cytoskeleton via the associated motor Myo2p. Elucidating the molecular machinery linking vesicles to cytoskeletal systems will shed light on the mechanisms responsible for movement and localization of post-Golgi vesicles.
Acknowledgments
We would like to thank K. Kinzler for making the SAGE library available and P. Crews for providing us with LAT-A. Thanks to S. Reck-Peterson for critically reading of the manuscript and all members of the Novick lab, past and present, for stimulating discussions.
This work was supported by grants from the National Institutes of Health to P.J. Novick. N.B. Elkind was supported by a Human Frontiers Long Term Fellowship. C. Walch-Solimena was supported by Deutsche Forschungsgemeinschaft Fellowship.
Footnotes
Abbreviations used in this paper: GFP, green fluorescent protein; LAT, latrunculin; sec, secretory.
References
- Adams A.E.M., Pringle J.R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae . J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K.R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D.G. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keranen S., Bankaitis V., Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Carr C.M., Grote E., Munson M., Hughson F.M., Novick P.J. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett N.L., Weisman L.S. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc. Natl. Acad. Sci. USA. 1998;95:14799–14804. doi: 10.1073/pnas.95.25.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T., Gussow D., Jones P.T. General applications of PCR to gene cloning and manipulation. In: McPherson M.J., Quirke P., Taylor G.R., editors. PCR. Vol. 1. Oxford University Press; Oxford: 1996. pp. 207–209. [Google Scholar]
- Cormack B.P., Valdivia R.H., Falkow S. FACS optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Coue M., Brenner S.L., Spector I., Korn E.D. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Echard A., Jollivet F., Martinez O., Lacapere J.J., Rousselet A., Janoueix-Lerosey I., Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Finger F.P., Hughes T.E., Novick P.J. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Garrett M.D., Kabcenell A.K., Zahner J.E., Kaibuchi K., Sasaki T., Takai Y., Cheney C.M., Novick P.J. Interaction of Sec4 with GDI proteins from bovine brain, Drosophila melanogaster and Saccharomyces cerevisiae. Conservation of GDI membrane dissociation activity. FEBS Lett. 1993;331:233–238. doi: 10.1016/0014-5793(93)80343-s. [DOI] [PubMed] [Google Scholar]
- Garrett M.D., Novick P.J. Expression, purification, and assays of Gdi1p from recombinant Escherichia coli . Methods Enzymol. 1995;257:232–240. doi: 10.1016/s0076-6879(95)57028-4. [DOI] [PubMed] [Google Scholar]
- Garrett M.D., Zahner J.E., Cheney C.M., Novick P.J. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gournier H., Stenmark H., Rybin V., Lippe R., Zerial M. Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1930–1940. doi: 10.1093/emboj/17.7.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B., Bowser R., Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 1995;128:1055–1067. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscelli C., Durandy A., Guy-Grand D., Daguillard F., Herzog C., Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am. J. Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- Guo W., Grant A., Novick P. Exo84p is an exocyst protein essential for secretion J. Biol. Chem. 274 1999. 23558 23564a [DOI] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for sec4p, targeting secretory vesicles to sites of exocytosis EMBO (Eur. Mol. Biol. Organ.) J. 18 1999. 1071 1080b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., Fink G.R. Guide to yeast genetics and molecular biology. In: Abelson J.N., Simon M.I., editors. Methods in Enzymology. Vol. 194. Academic Press, Inc; San Diego, CA: 1991. [PubMed] [Google Scholar]
- Haraldsson A., Weemaes C.M., Bakkeren J.A., Happle R. Griscelli disease with cerebral involvement. Eur. J. Pediatr. 1991;150:419–422. doi: 10.1007/BF02093723. [DOI] [PubMed] [Google Scholar]
- Hurvitz H., Gillis R., Klaus S., Klar A., Gross-Kieselstein F., Okon E. A kindred with Griscelli diseasespectrum of neurological involvement. Eur. J. Pediatr. 1993;152:402–405. doi: 10.1007/BF01955897. [DOI] [PubMed] [Google Scholar]
- Johnston G.C., Prendergast J.A., Singer R.A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T.S., Reck-Peterson S.L., Elkind N.B., Mooseker M.S., Novick P.J., Cooper J.A. Role of the actin cytoskeleton and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae . Mol Biol. Cell. 2000;In press doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Philippe N., Le Deist F., Fraitag S., Prost C., Durandy A., Fischer A., Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J. Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- Koyama Y.I., Takeuchi T. Differential effect of cytochalasin B on the aggregation of melanosomes in cultured mouse melanoma cells. Anat. Rec. 1980;196:449–459. doi: 10.1002/ar.1091960410. [DOI] [PubMed] [Google Scholar]
- Lillie S.H., Brown S.S. Suppression of a myosin defect by a kinesin-related gene. Nature. 1992;356:358–361. doi: 10.1038/356358a0. [DOI] [PubMed] [Google Scholar]
- Lillie S.H., Brown S.S. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae . J. Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S.H., Brown S.S. Smy1p, a kinesin-related protein that does not require microtubules. J. Cell Biol. 1998;140:873–883. doi: 10.1083/jcb.140.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesert G., Clarke D.J., Reed S.I. Identification of genes controlling growth polarity in the budding yeast Saccharomyces cerevisiaea possible role of N-glycosylation and involvement of the exocyst complex. Genetics. 1997;147:421–434. doi: 10.1093/genetics/147.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J., Muller H., Peterson M., Novick P. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J. Cell Biol. 1990;110:1897–1909. doi: 10.1083/jcb.110.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature sensitive mutant of Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Novick P., Brennwald P. Friends and familythe role of the Rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Pastural E., Barrat F.J., Dufourcq-Lagelouse R., Certain S., Sanal O., Jabado N., Seger R., Griscelli C., Fischer A., de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat. Genet. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- Pfeffer S.R. Motivating endosome motility. Nat. Cell Biol. 1999;1:E145–E147. doi: 10.1038/14097. [DOI] [PubMed] [Google Scholar]
- Provance D.W., Jr., Wei M., Ipe V., Mercer J.A. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc. Natl. Acad. Sci. USA. 1996;93:14554–14558. doi: 10.1073/pnas.93.25.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D.W., Schott D.H., Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson S.L., Novick P.J., Mooseker M.S. The tail of a yeast class V myosin, Myo2p, functions as a localization domain. Mol. Biol. Cell. 1999;10:1001–1017. doi: 10.1091/mbc.10.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin V., Ullrich O., Rubino M., Alexandrov K., Simon I., Seabra M.C., Goody R., Zerial M. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P.J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. [Google Scholar]
- Schliwa M. Molecular motors join forces. Nature. 1999;397:204–205. doi: 10.1038/16577. [DOI] [PubMed] [Google Scholar]
- Schott D., Ho J., Pruyne D., Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Shochet N.R., Kashman Y., Groweiss A. Latrunculinsnovel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- TerBush D.R., Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae . J. Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Velculescu V.E., Zhang L., Zhou W., Vogelstein J., Basrai M.A., Bassett J., Bassett D.E., Hieter P., Vogelstein B., Kinzler K.W. Characterization of the yeast transcriptome. Cell. 1997;88:243–251. doi: 10.1016/s0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C., Collins R.N., Novick P.J. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N.C., Novick P.J. Purification and characterization of constitutive secretory vesicles from yeast. J. Cell Biol. 1987;105:163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Wu X., Hammer J.A., 3rd. The predominant defect in dilute melanocytes is in melanosome distribution and not cell shape, supporting a role for myosin V in melanosome transport. J Musc. Res. Cell Motil. 1997;18:517–527. doi: 10.1023/a:1018659117569. [DOI] [PubMed] [Google Scholar]
- Wu X., Bowers B., Rao K., Wei Q., Hammer J.A., 3rd. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]