We have generated several stable cell lines expressing GFP-labeled centrin. This fusion protein becomes concentrated in the lumen of both centrioles, making them clearly visible in the living cell. Time-lapse fluorescence microscopy reveals that the centriole pair inherited after mitosis splits during or just after telophase. At this time the mother centriole remains near the cell center while the daughter migrates extensively throughout the cytoplasm. This differential behavior is not related to the presence of a nucleus because it is also observed in enucleated cells. The characteristic motions of the daughter centriole persist in the absence of microtubules (Mts), or actin, but are arrested when both Mts and actin filaments are disrupted. As the centrioles replicate at the G1/S transition the movements exhibited by the original daughter become progressively attenuated, and by the onset of mitosis its behavior is indistinguishable from that of the mother centriole. While both centrioles possess associated γ-tubulin, and nucleate similar number of Mts in Mt repolymerization experiments, during G1 and S only the mother centriole is located at the focus of the Mt array. A model, based on differences in Mt anchoring and release by the mother and daughter centrioles, is proposed to explain these results.
Introduction
During mitosis, animal cells inherit a single centrosome that contains a pair of centrioles, each of which is associated with a cloud of pericentriolar material (reviewed in Andersen 1999; Mogensen 1999; Schnackenberg and Palazzo 1999; Tassin and Bornens 1999). These two centrioles differ both structurally (Paintrand et al. 1992) and biochemically (Lange and Gull 1995): the older “mother” centriole, which was formed at least 1.5 generations earlier, carries two sets of appendages (distal and sub-distal). In contrast, the younger “daughter” centriole, which was formed during the previous S phase, lacks these structures. This structural asymmetry appears to be due partly to the semiconservative nature of centrosome replication (Kochanski and Borisy 1990).
Centrioles replicate during S phase concurrent with DNA replication. During this time a small “procentriole” bud forms adjacent to the proximal wall of each parenting centriole, which then gradually elongates (Kuriyama and Borisy 1981). By G2, when the cell possesses a 4N DNA content, it also possesses four centrioles arranged into two pairs referred to as diplosomes. Within each diplosome the mother and daughter centrioles are orthogonally oriented so that a line through the long axis of the daughter points to the wall of the mother. This relationship is then maintained through the ensuing mitosis. During the next cell cycle, a slow process of maturation of the daughter centriole takes place which transforms it into a fully differentiated centriole apparently similar in all respects to the mother centriole. In parallel, the centrosome matrix changes in size or structure, displaying a varying number of satellite structures, the significance of which is not clear (Rieder and Borisy 1982; Vorobjev and Chentsov 1982).
The function(s) of the centrioles within the centrosome remain unclear, although recent results demonstrate that they are required for organizing the centrosomal components into a single stable structure (Bobinnec et al. 1998). Since the reproductive capacity of a centrosome depends on its centriole content (Sluder and Rieder 1985), and since centrosomes lacking centrioles do not reproduce (Sluder et al. 1989), centrioles also likely play an important role in centrosome reproduction, which must be tightly controlled to maintain genetic stability.
The centrosomal components involved in microtubule (Mt) nucleation (e.g., γ-tubulin, HsSpc98p) are localized within the pericentriolar material (PCM) associated with each centriole (Moudjou et al. 1996; Tassin et al. 1998). At the EM level Mts are often seen in interphase cells to terminate on the sub-distal appendages surrounding the mother centriole (Gorgidze and Vorobjev 1995). It is unclear, however, if these sites nucleate Mt assembly (Chretien et al. 1997) or serve simply to anchor minus ends of Mts nucleated elsewhere in the PCM.
Centrin is a small (20 kD) protein that concentrates within the centriole distal lumen (Paoletti et al. 1996; Middendorp et al. 1997). This protein appears in the centriole as soon as centrioles begin to form, and then remains associated with this organelle as it matures (Paoletti et al. 1996). Its exact function(s) remain to be determined, but recent evidence suggests that at least one isoform (centrin 3) is involved in centrosome reproduction (Middendorp et al. 2000). Because of its small size and striking concentration within the centriole, we reasoned that centrin would be useful for defining the behavior of centrioles throughout the cell cycle, in living cells, if it was expressed as a fusion with the green fluorescent protein (GFP).
In this study, we used time-lapse fluorescence microscopy and serial section EM to define, for the first time, the in vivo behavior of centrioles during the vertebrate somatic cell cycle. The data reveal that the mother and daughter centrioles differ in their behavior and in their respective contributions to forming the interphase Mt array.
Materials and Methods
Cell Culture, Cloning, and Synchronization
L929, NIH 3T3, and HeLa-B cells were grown in DME medium (GIBCO) supplemented with 10% fetal calf serum.
Centrin cDNA was sub-cloned in the pEGFP-N1 vector (Clontech) and cells were transformed by electroporation. Stable clones expressing the centrin/GFP fusion protein were then isolated from each of the three parental cell lines, by using the limited dilution method in the presence of 500 μg/ml G418. Multiple independent clones, expressing ∼20 times the endogenous level of centrin, were kept for each of the three parental cell lines. The centrioles in all of the clones exhibited a similar behavior to that described here.
Synchronization in early G1 was accomplished by a single thymidine block of 20 h (5 mM thymidine for L929 and 2 mM for HeLa). Mitotic cells were then collected by shakeoff 8 or 20 h after releasing the block (for L929 and HeLa, respectively). Cells were then replated on coverslips and used 2 h later. Synchronization at the G1/S border was accomplished using the double-thymidine block technique. To determine the duration of S and G2 we incubated cells at various times after thymidine washout with 30 μM BrdU for 15 min and then analyzed them by FACS®. The maximum content of S cells (by BrdU incorporation) was reached 1–4 h after release. The maximum number of G2 cells (double DNA content and no BrdU incorporation) was observed ∼5 h after release in L929 cells and 9–10 h in HeLa cells.
Cell Enucleation
Enucleation was performed as described by Prescott et al. 1972. In brief, coverslip cultures were incubated at 37°C for 30 min in the presence of 1.5 μg/ml cytochalasin D (CD; Sigma) and then centrifuged at 15,000 g for 40 min at 37°C in the presence of CD. The cytoplasts were then rinsed with fresh drug-free medium and incubated for 4 h at 37°C before use. To obtain cytoplasts with more than two centrioles cells were treated with 0.25 μg/ml CD for 24–48 h to induce the formation of multinucleated cells (Carter 1967), and then enucleated as described above. To obtain centriole-free cytoplasts, or cytoplasts containing only one centriole, cells were enucleated in the presence of both 1.5 μg/ml CD and 5 μg/ml nocodazole (ND; Sigma; Karsenti et al. 1984).
Drug Treatments
We used a combination of ND (5 μM) and cold (40 min on ice) to depolymerize Mts. This treatment depolymerizes even the most stable Mts in L929 cells, and these do not reassemble when the cells are subsequently incubated in warm media containing 5 μM ND. To disrupt actin filaments cells were treated with 3 μg/ml of CD for 30 min. Latrunculin A (Molecular Probes) was used at 1 μM and added just before initiating observations. Butanedione Monoxime (BDM; Sigma) was used at 20 μM and cells were observed 30 min after treatment.
Microinjection of Rhodamine-Tubulin and Incorporation of the Shiga Toxin B Fragment
Rhodamine-tubulin (catalog number T331M; TEBU, Inc.) was microinjected using an automatic microinjector (Eppendorf). The B fragment of Shiga toxin was incorporated into cells using the method described by Mallard et al. 1998.
Microscopy and Data Processing
For time-lapse imaging cells were plated on #1 1/2 coverslips (L929 cells were plated on coverslips coated with collagen and fibronectin to induce cell flattening). For brief (<1 h) experiments cells were maintained at 37°C in sealed chambers containing complete phenol red-free culture medium supplemented with 20 mM Hepes. Open chambers equilibrated in 5% CO2 and maintained at 37°C were used for longer experiments. Rhodamine-labeled cells were mounted in hermetically sealed chambers containing Oxyrase and lactic acid (Vorobjev et al. 1997).
Time-lapse Z-sequences were collected on a Leica DMIRBE microscope controlled by Metamorph software (Universal Imaging). This microscope was equipped with a piezoelectric device for rapid and reproducible focal changes, a 100× 1.4 NA Plan Apo lens, and a cooled CCD camera (MicroMax 5 MHz; Roper Scientific). The final magnification on the camera chip was 84 nm/pixel. Using a DG4 illumination device (DeMey, J., and J.B. Sibarita, manuscript in preparation) we could collect a Z-sequence through an entire cell, of two different wavelengths, in under 2 s.
As a rule 6–10 sequential Z-axis images were collected in 0.5-μm steps every 2–30 s. However, as the cell rounded during late G2 and mitosis it was often necessary to collect as many as 30 Z-axis images. Centriole tracking was performed automatically by Metamorph in maximal-intensity projections computed from the original three-dimensional data sets.
Same Cell Correlative Video, Immunofluorescence, and/or Electron Microscopy
To identify cells followed in vivo thought subsequent preparative procedures we cultured them on Cellocate coverslips (Eppendorf). For indirect immunofluorescence studies they were then rapidly extracted with 0.2% NP-40 in BRB80 (80 mM KPIPES, pH 6.8, 1 mM MgCl2; 1 mM EGTA) for 30 s, followed by fixation in a mixture of 2% paraformaldehyde and 0.25% glutaraldehyde in PBS for 3 min. After reducing free aldehydes with 0.1% NaBH4 in PBS, the coverslips were incubated in primary antibodies followed by the appropriate secondary antibody coupled to either cyanine 3 (red channel; Jackson ImmunoResearch) or AMKA (blue channel; Jackson ImmunoResearch). The green channel was used to record the GFP signal which was preserved by our fixation protocol.
After immunostaining, cells that had been followed in vivo were relocated and imaged on a Leica DMRXA microscope. Image stacks (200-nm steps) were recorded using a piezoelectric objective positioning device and a MicroMAx CCD camera (Princeton Instruments). With a 100× 1.4 NA objective the final magnification on the chip was 67 nm/pixel. All centrin, ninein and γ-tubulin images shown in this paper are maximal intensity projections, while Mts are presented as self-luminous reconstructions.
Serial section EM of cells previously followed in vivo was performed as detailed by Rieder and Cassels 1999.
Supplemental Material
Video supplements for Fig. 2, Fig. 4, Fig. 5, and Fig. 9 are at http://www.jcb.org/cgi/content/full/149/2/317/DC1. To ensure a good resolution of the movies, please check that the monitor of your computer is set on millions of colors or true colors (32 bits). All of the videos correspond to cells or cytoplasts displayed on figures or from which data were extracted, except for Fig. 3 for which a movie showing a G2 cytoplast is added. Refer to the respective figure legend for further explanation.
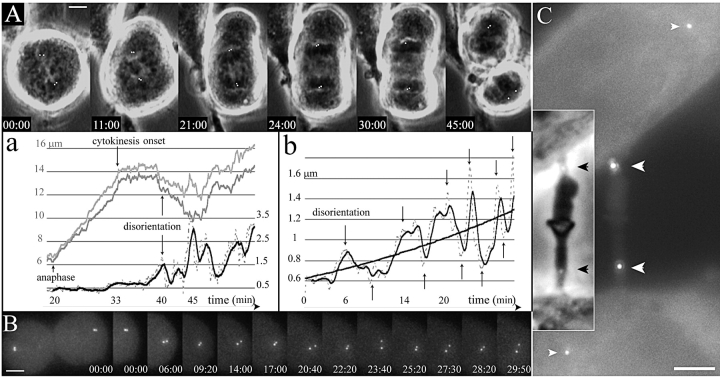
Figure 2.
Centriole disorientation occurs early after the onset of cytokinesis. (A) Selected frames from a time-lapse recording of mitosis in HeLa cell progressing from metaphase to telophase. The top row shows selected phase contrast pictures with the GFP signal in white (centrioles signals were manually enhanced), whereas the behavior of the bottom centrosome during the complete recording is described on the corresponding graphics (a). The two upper curves (grey) are the distance of each centriole of the bottom centrosome to a fixed point at the center of the metaphase plate in the first frame (scale on the left). It mainly shows the increasing distance during anaphase and the differential movements of each centriole after cytokinesis onset. The lower curves are the distance between the two centrioles of the bottom centrosome (dotted line), and a mobile mean fit (black line; scale in μm on the right). It shows an almost constant distance (the little increase at 33 min is due to a rotation of the diplosome) until time 40 min after metaphase shown on the top left (20 min after anaphase) when it reaches a distance incompatible with a close association of the two centrioles. (B) One sister cell spreading after furrowing onset. Z-series were acquired every 10 s. The bottom row shows the GFP signal of the right sister cell at times corresponding to the arrows on the graph (b), showing the alternative splitting and joining of the centrioles. The curves in b are the distance between the two centrioles of the right centrosome (dotted line), and a mobile mean fit (black line). A logarithmic fit shows the globally increasing distance between both centrioles. (C) Two daughter cells still linked by a midbody in early G1. Two centrioles are located in the midbody (large white arrowheads) while the other two are far inside the cells (small white arrowheads). (Inset) The two black arrowheads show the location of the centrioles on the phase contrast picture of the midbody. Supplemental video is available at http://www.jcb.org/cgi/content/full/149/2/317/DC1. Bars, 5 μm.
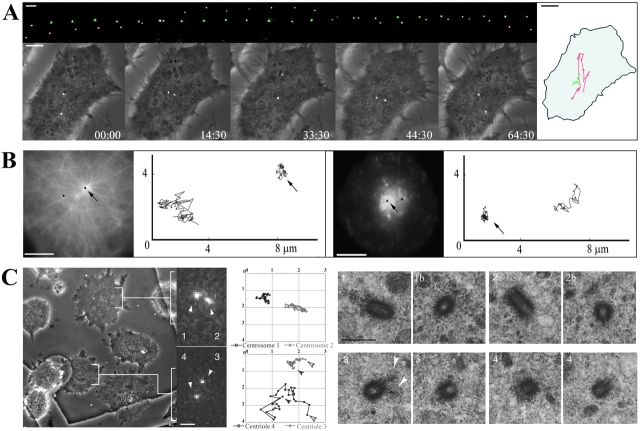
Figure 4.
(A) Centriole behavior in G1 cytoplasts. The top row is of GFP images (at 6-min intervals) in which the nonmotile centriole was colored green while the motile one red. In the bottom row the GFP images are superimposed on phase-contrast images of the same cell (time = min/s). The diagram on the right represents the centrioles trajectories in relation to the cell boundaries. (B) The immotile centriole acts as the centrosome. G1 cytoplasts injected with rhodamine-tubulin (left panel) or having incorporated the B fragment of Shiga toxin coupled with Cyanin 3 (right panel) were video-recorded in two channels (GFP and rhodamine) during 10 min with a 4-s time-lapse. The pictures shown correspond to the first frame. The trajectories of the centrioles (in black on the pictures) are shown on the right. Arrows point to the immotile centriole. (C) The immotile centriole is the mother centriole. Movements of centrioles were recorded during 20 min, with 30-s time-lapse, in cytoplasts seeded on a gridded coverslip which were then flat embedded and processed for EM (see Materials and Methods). (Left) Phase contrast and GFP signals of the last frame. (Middle) Trajectories. The GFP signal appears pixelized because pictures were acquired at low resolution (63× objective and binning mode) in order to have at least 10 cells in a field. In these conditions, it was not possible to resolve the buds in duplicating centrioles at the optical level. (Right) High-voltage EM after semi-thick serial sectioning. The top row corresponds to the upper cytoplast and the bottom row to the lower cytoplast. The upper cytoplast contains two immotile GFP dots (1 and 2) which were revealed as two diplosomes by EM (1 and 1b, 2 and 2b). The lower cytoplast contains a motile (4) and an immotile (3) GFP dot, which corresponded to two centrioles. Two consecutive serial sections of each centriole are presented. The immotile centriole was identified as the mother centriole by the presence of appendages (white arrowheads). Supplemental video is available at http://www.jcb.org/cgi/content/full/149/2/317/DC1. Bars: (A and B) 5 μm; (C, GFP signal image) 2 μm; (C, phase contrast image) 8 μm.
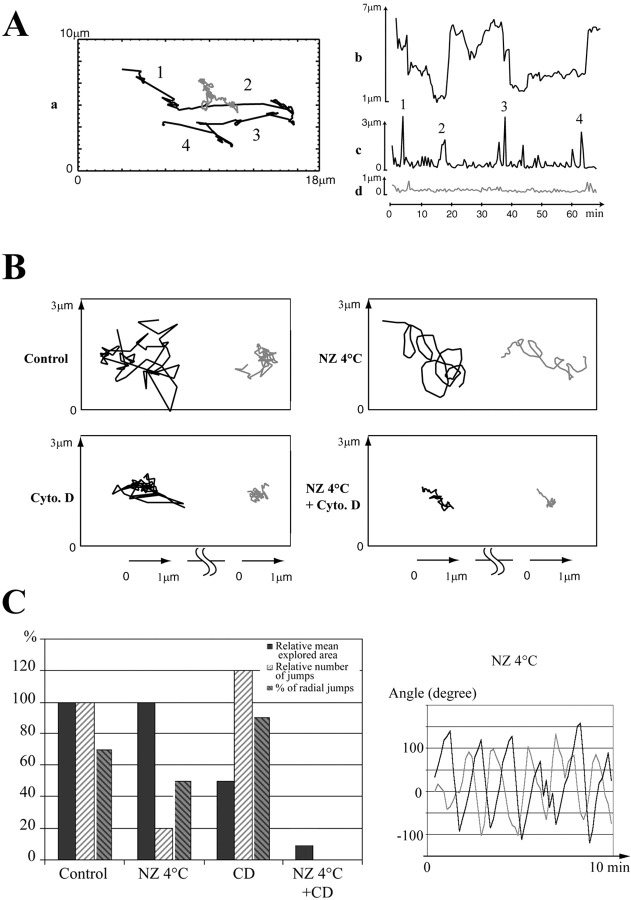
Figure 5.
Analysis of centriole movements in G1 cytoplasts. (A) Quantification of centrioles movements in the control G1 cytoplast shown in Fig. 4. (a) Corresponding trajectories; (b) distance between centrioles; (c and d) distances covered between two frames (30 s) by the daughter centriole (c) and the mother centriole (d). The numbers indicate periods of rapid movements of the daughter centriole. Between these periods the daughter centriole kept jolting while the mother centriole did not. (B) Drug effect on centrioles movements. The modifications of the trajectories induced by ND or CD are strikingly different, revealing that Mts and actin filaments drive two components of centrioles movements (see text). When both systems were impaired, centriole movements were minimal. (C, left) Quantification of the drug experiments on the movement of the daughter. Note that Mt disassembly reduced drastically the number of rapid movements (jumps) whereas impairing the actin system reduced the explored area. Note also that in CD-treated cytoplasts, movements of the daughter centriole are mostly radial with respect to the mother centriole. (Right) Correlation of mother and daughter centrioles movements in cytoplasts treated with ND. Each curve represents the angle variation over time between two consecutive segments of the trajectory of each centriole (dark line, motile centriole; grey line, immotile centriole). Note the phase shift between the two curves. Supplemental video is available at http://www. jcb.org/cgi/content/full/149/2/317/DC1.
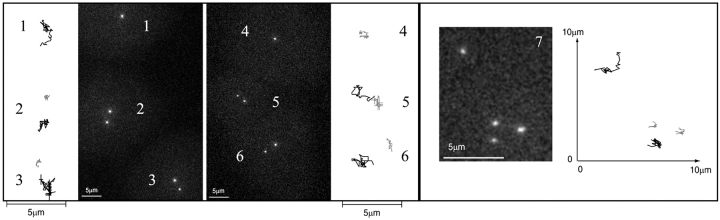
Figure 9.
G1 cytoplasts with variable numbers of centrioles. (Left) Two fields from two independent experiments are shown that both contain a cytoplast having only one centriole (1 and 4), and two cytoplasts having two centrioles (2, 3 and 5, 6). The trajectories video recorded during 10 min every 10 s are presented on each side. Cytoplast 1 contained a motile centriole while cytoplast 4 contained an immotile one. (Right) One G1 cytoplast (7) containing four centrioles (see Materials and Methods). The trajectories, recorded during 20 min every 30 s, show two motile and two immotile centrioles. The GFP pictures correspond to the first frame of each recording. Supplemental video is available at http://www.jcb.org/cgi/content/full/149/2/317/DC1.
Figure 3.
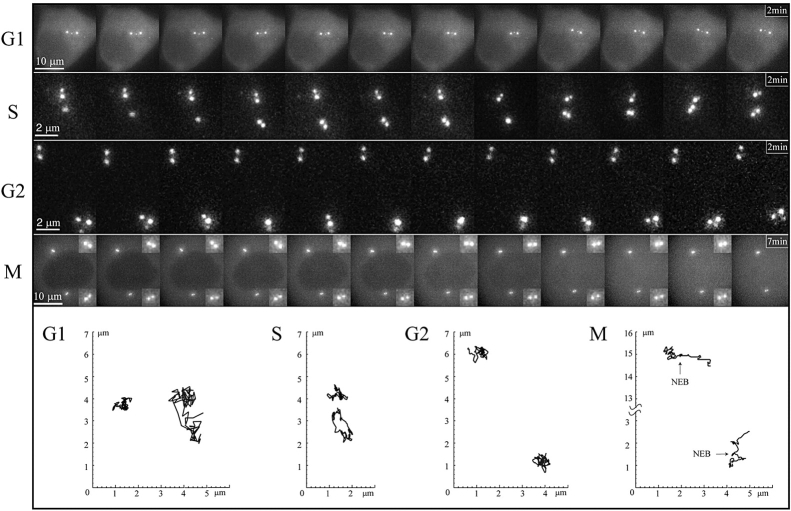
(Top) Selected frames from time-lapse sequences depicting centriole behavior during the cell cycle. The G1, S and G2 sequences were recorded at 1 frame/2 s, and every 5th frame is shown here. The M series was recorded at 1 frame/5 s, and every 7th frame is shown here (insets show each diplosome at threefold magnification). (Bottom) Position plots depicting the trajectories of the centrioles shown above. One centriole remains stationary during all phases of the cell cycle while the other moves extensively during G1 and then gradually becomes sessile. Note that one of the two diplosomes (the bottom one in these images) exhibits rocking movements during S and G2. Supplemental video is available at http://www.jcb.org/cgi/content/full/149/2/317/DC1.
Video 1 corresponds to the cell shown on Fig. 2 B.
Videos 2 and 3 correspond to the S and G2 cells shown on Fig. 3 and video 4 is a G2 cytoplast shown for comparison: the behavior of the diplosomes in G2 is the same in cells and in cytoplasts.
Video 5 corresponds to the G1 cytoplast shown on Fig. 4 A, rotated 90°. Videos 6 and 7 correspond to the G1 cytoplasts as shown in Fig. 4 injected with rhodamine-tubulin or having incorporated Shiga toxin B fragment coupled with rhodamine, respectively.
Videos 8–10 correspond to the G1 cytoplasts treated with nocodazole and cold, cytochalasin D, or both, respectively, and whose centrioles trajectories are shown on Fig. 5 B. Video 11 shows videos 8–10 one after the other in the same file, thus making easier the comparison between different treatments.
Videos 12 and 13 correspond to the fields containing three G1 cytoplasts shown respectively on the left and on the right in the left panel of Fig. 9. Video 14 corresponds to the G1 cytoplast containing four centrioles shown on the right panel of Fig. 9.
Results
Distribution of GFP-Centrin
We established several stable (>50 passages) cell lines that express centrin as a NH2-terminal fusion with GFP. Multiple clones that exhibit growth rates similar to the parental cell lines were isolated from HeLa, NIH-3T3, and L929 cells. In this report, we illustrate our finding using data obtained from L929 clones because the mother and daughter centrioles in these cells are sufficiently separated during G1 so that their individual behavior can be easily observed. In G1 HeLa cells, centrioles remain relatively close to each other while in NIH 3T3 they are usually separated by distances greater than those seen in L929 cells. Despite these differences, the centrioles behave the same in all three cell lines. Thus, the phenomena described here are exhibited by a number of different vertebrate somatic cell lines.
In all clones the distribution of GFP-centrin was very similar to that previously described by indirect immunofluorescence (Baron et al. 1992; Paoletti et al. 1996). The most obvious feature was a set of small strongly fluorescent dots that were usually located near the nucleus. In fixed cells these dots always stained with antibodies against typical centrosomal markers (e.g., poly-glutamylated tubulin, γ-tubulin, not shown).
In an asynchronous population the number of centrin-GFP dots varied from cell to cell. Most cells contained two individual dots, positioned at variable distances from one another, but some contained two pairs of dots. In the latter cells the two dots comprising each pair often differed in their intensity. Correlative LM/EM studies confirmed that individual dots seen at the LM level were single centrioles, while the paired dots corresponded to orthogonally oriented mother/daughter centriole pairs (i.e., a diplosome; not shown).
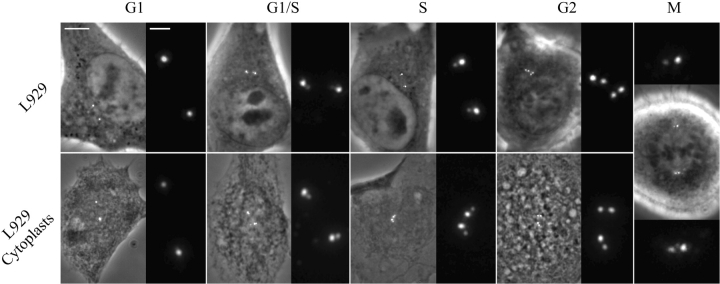
To determine how centrin/GFP labeling changes with respect to the centrosome cycle we investigated the centrin/GFP distribution in synchronized cell populations. When cells were synchronized by mitotic shakeoff and replated for 2 h, 94% of cells (n >200) contained two individual dots. By contrast when cells were synchronized by a double-thymidine block and then allowed to progress into S-phase, 87% cells contained two pairs of dots with one member of each pair significantly brighter than the other. Finally, when cells were released from a double-thymidine block and allowed to progress into G2, 69% of cells contained two pairs of dots each of which was approximately equal in intensity, 25% still exhibiting the S-phase pattern. It is noteworthy that the distance separating the two dots comprising each diplosome increased as the cells progressed through S-G2, likely reflecting the elongation of the pro-centriole. From these results we conclude that centrin/GFP can be used as a live cell marker for the formation and maturation of individual centrioles, and is thus a very good marker of cell cycle progression. The typical distribution of centrin/GFP during the cell cycle is summarized in Fig. 1 (top row).
Figure 1.
Cell cycle changes in the distribution of centrin/GFP. Top row, L929 cells; bottom row, cytoplasts obtained from the same clone. For each period, the left picture is a superimposition of the GFP fluorescence and phase-contrast images, and the right picture is a 4× magnification of the GFP channel. Note that the distance between the two GFP dots in each diplosome increases from G1/S to S and G2. Bar, 5 μm for phase contrast images and 1 μm for higher magnification.
To investigate the behavior of the two centrioles within the centrosome, we used both whole cells and enucleated cells (cytoplasts). Cytoplasts were used to determine if the behavior of centrioles is influenced by the presence of nucleus. Additionally, cytoplasts are very flat and immobile, facilitating observation and analysis of centriole movements. In our hands, cytoplasts were viable for >50 h. The typical distribution of centrin/GFP in cytoplasts during different periods of cell cycle is summarized in Fig. 1 (bottom row).
Centriole Behavior during the Cell Cycle
During mitosis each daughter cell inherits a single diplosome consisting of a closely associated mother and daughter centriole pair. We found that the mother and daughter centrioles comprising the diplosome separate into individual units during or just after telophase, well before the completion of cytokinesis (Fig. 2). Most of the time, that separation correlates with cell spreading. Once separated the mutual distance between the two centrioles fluctuates, but the average value increases steadily up to a few microns. From that moment on and up to the completion of cytokinesis (i.e., breaking of the midbody), the two centrioles exhibit dramatically different behaviors. One remains almost stationary and near the geometrical center of the forming daughter cell while the other is wandering around and eventually migrates around the nucleus and towards the forming midbody (Fig. 2 C). Although the movement of one centriole toward the midbody sometimes occurred only in one of the daughter cells, it was usually observed in both, just before the completion of cytokinesis.
We then asked if this differential behavior persists throughout interphase. We found that during G1 one centriole remained relatively stationary while the other wandered throughout the cytoplasm (Fig. 3). This exaggerated motion of one centriole was greatly attenuated during S and G2 when the centrioles were in the process of replicating (Fig. 3). However, the two forming diplosomes still exhibited different behaviors during S and G2; one remained stationary while the other exhibited rocking motions even when translational movements were suppressed in G2 (Fig. 3S and G2). This movement ceased during late G2/prophase before nuclear envelope breakdown.
Since the centrosome has been shown to be tightly associated with the nucleus in some cells (Bornens 1977; Fais et al. 1984), we asked if the relative immotility of one centriole during G1 was due to its attachment to the nucleus. To answer this question we followed the behavior of centrioles in cytoplasts obtained from L929 cells at various stages of the cell cycle. We found that the two centrioles in G1 cytoplasts behaved, in all respects, as those in nucleated cells (Fig. 4 A). It was noteworthy that the most stable centriole was always located near the geometrical center of the cell while the motile centriole could wander across the entire cell (Fig. 4 A). Centriole behavior in S or G2 cytoplasts was also indistinguishable from that observed in whole S or G2 cells. The ratio between the mean velocity of motile and nonmotile centrioles decreased from about 1.8 ± 0.4 during G1 to 1.3 ± 0.2 during S and further to 1 ± 0.1 during G2 (n ∼10 cells in each case).
We next asked how Mts were distributed around the motile and nonmotile centrioles. To answer this question we followed living cytoplasts after having either microinjected them with rhodamine-labeled tubulin or incubated them with a living marker for the Golgi apparatus (the internalized B fragment of the Shiga toxin whom internalization leads, at the steady-state to an accumulation in the Golgi; Mallard et al. 1998). We found that the centrally located nonmotile centriole was always positioned at the center of a radial array of rhodamine-labeled Mts, whereas the motile one was off-center and not associated with a significant Mt array (Fig. 4 B). The Shiga toxin B fragment stained a large central area that usually contained both centrioles. However, as a rule the nonmotile centriole was positioned in the center of this region.
Next we conducted a serial section EM analysis of cells previously followed in vivo to determine which of the centrioles in our cells was the mother (Fig. 4 C). In all seven cytoplasts containing one motile and one stationary centriole, the stationary centriole was always found to be the mother based on the presence of sub-distal appendages. By contrast, the centrioles in adjacent cytoplasts, in which all GFP-centrin dots remained relatively stationary, were always found to be replicating (n = 5).
Effects of Nocodazole and Cytochalasin D on Centriole Behavior during G1
To determine if the differential behavior of mother and daughter centrioles during G1 depends on the presence of Mts and/or actin filaments we analyzed centriole movements in G1 cytoplasts treated with either ND (5 μM), CD (3 μg/ml), or both. For these analyses we recorded time-lapse sequences at high temporal resolution (1 frame every 2 s). Even at this high framing rate we could follow centriole movement for ∼10 min before the GFP signal photobleached significantly. For these studies we chose to analyze two parameters of centriole behavior: the number of fast movement periods per unit of time, and the area covered by centriole motions (i.e., the root mean square of the excursion: [< (X − < X >)2 > . < (Y − < Y >)2 >]1/2). The latter parameter is independent of the framing rate and allows one to determine if the centriole exhibited persistent motion.
The results of these studies are summarized in Fig. 5. In most untreated cytoplasts the daughter centriole exhibited brief periods of rapid movement (numbers in Fig. 5 A) interrupted by longer periods of slower motion. The typical trajectory of the two centrioles is illustrated in Fig. 5 A. When compared with untreated cytoplasts, the trajectories of both centrioles became much smoother when Mts were completely disassembled by ND (Fig. 5 B). Under these conditions the centrioles no longer exhibited sudden direction changes, but the daughter centriole still remained more motile than the mother centriole. Even after a 4-h incubation in ND most mother centrioles remained positioned near the geometrical center of the cell. Remarkably, the mother and the daughter centrioles had very correlated movements (Fig. 5 C).
The extensive motion of daughter centrioles persisted in CD-treated cytoplasts, but the movements were more abrupt compared with untreated cytoplasts (Fig. 5 B). The same effect was observed when cytoplasts were treated with Latrunculin A (actin inhibitor) or BDM (a myosin inhibitor). In BDM-treated cytoplasts this effect was even more prominent, suggesting that the dependency of centriole movement on microfilaments is mediated by myosin (data not shown).
When Mts and microfilaments were both disrupted by the combined action of ND and CD all centrioles ceased moving and exhibited only brief tumbling motions (Fig. 5 B).
The Respective Contribution of the Mother and Daughter Centrioles in Forming the Interphase Mt Array
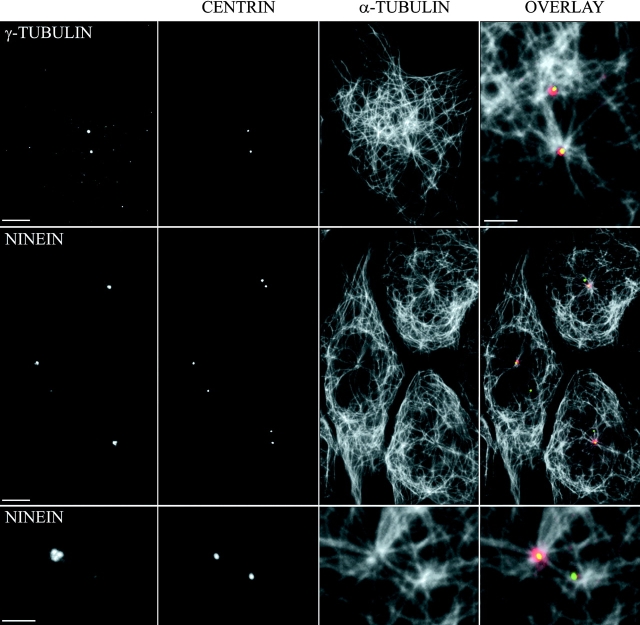
As noted above, we found that the nonmotile mother centriole was associated with a typical radial array of Mts whereas the motile daughter centriole had either no obvious direct interactions with the Mt array, or was associated with only a few Mts (Fig. 4 B). The different density of Mts associated with the mother and daughter centrioles in our cells could be due to differences in their nucleating potential and/or their ability to anchor Mts. In an attempt to differentiate between these possibilities, we determined the relative content between the mother and daughter centrioles of the Mt-nucleating protein γ-tubulin and the Mt-anchoring protein ninein (Mogensen et al. 1999). As illustrated in Fig. 6, the amount of γ-tubulin associated with each centriole (or diplosome during S-G2) was very similar. However, ninein was associated primarily if not exclusively with the mother centriole during G1, but with both diplosomes during S-G2 (Fig. 6).
Figure 6.
γ-Tubulin associates with both centrioles, whereas ninein associates with the mother centriole only. G1 cytoplasts from cells expressing GFP-centrin were fixed and stained with anti–α-tubulin antibody or with either an anti–γ-tubulin or an anti-ninein antibody. Note that the ninein staining on the daughter centriole is very weak, whereas it is conspicuous and organized in several blobs, most often three, on the mother centriole. Note also on the bottom row the converging bundles of Mts abutting in the ninein blobs. On the top row, one can see that nonastral Mts are numerous in the vicinity of the daughter centriole. Bars: 5 μm or 2 μm for the bottom row and the top-right picture.
As shown in Fig. 7 A, only the ninein-containing centriole maintained an aster of Mts after treatment with low doses of ND. In agreement with γ-tubulin immunolocalization, both centrioles re-nucleated a comparable number of Mts after complete disassembly (Fig. 7 B). Similar numbers of short Mts were found to be arranged radially around both centrioles 2 min after washing ND-treated cytoplasts. By contrast, Mt arrays were associated only with the ninein-containing mother centrioles 15 min after ND washout. After 15 min regrowth, a common and conspicuous radial array of Mts was observed if the two centrioles were close to each other (Fig. 7 B, top), but some “free” cortical Mts were also observed when the two centrioles were split apart (Fig. 7 B, bottom). Moreover, many free short Mts were observed at 5 min regrowth when centrioles were split.
Figure 7.
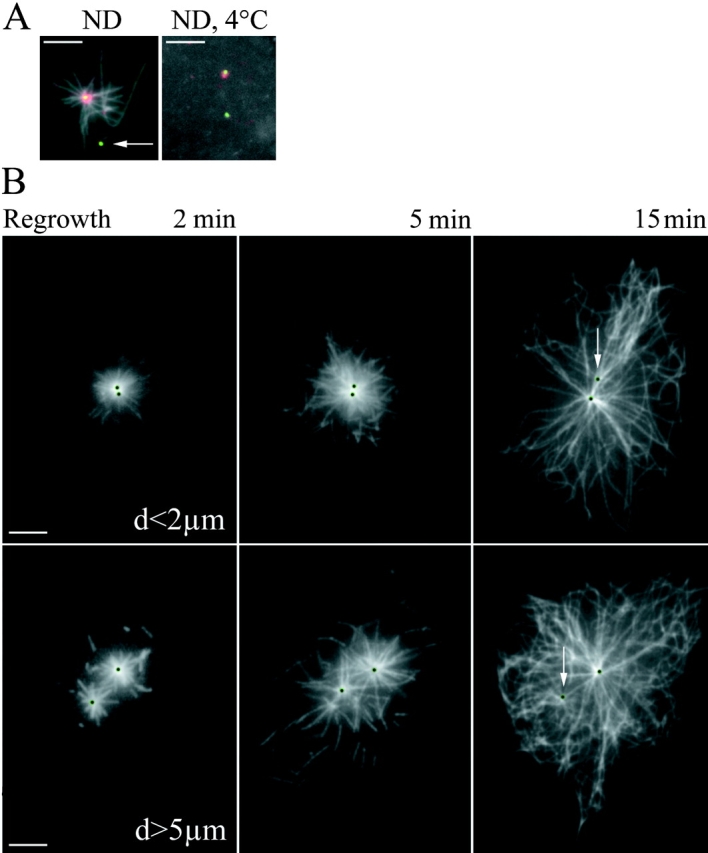
Stable Mts are anchored at the mother centriole and Mts are nucleated by both centrioles. (A, left) Cytoplasts treated during 10 min with 1 μM ND were fixed and stained with an anti–α-tubulin antibody (acquired in the blue channel and shown in grey), and an anti-ninein antibody (red). GFP-centrin signal is shown in green. Arrow points to the daughter centriole that does not anchor stable Mts. (Right) Cytoplasts treated during 40 min with 5 μM ND at 4°C do not contain Mts. Note that ninein is still associated with one centriole only. (B) Mt regrowth pattern depends upon the distance between both centrioles. After complete Mt depolymerization, the drug was washed and cytoplasts were fixed after 2, 5, or 15 min incubation at 37°C and stained with an anti–α-tubulin antibody. GFP signals are localized by black dots. White arrows indicate the daughter centriole, to which few Mts are associated at 15 min. (Top row) A typical Mt pattern observed in cytoplasts with two close centrioles. (Bottom row) Cytoplasts with distant centrioles. Note the released Mts at 5mn in the bottom row and the absence of peripheral Mts in the top row at 15 min. Bars, 5 μm.
Mts Organization and Centriole Behavior in Cytoplasts Containing One or More than Two Centrioles
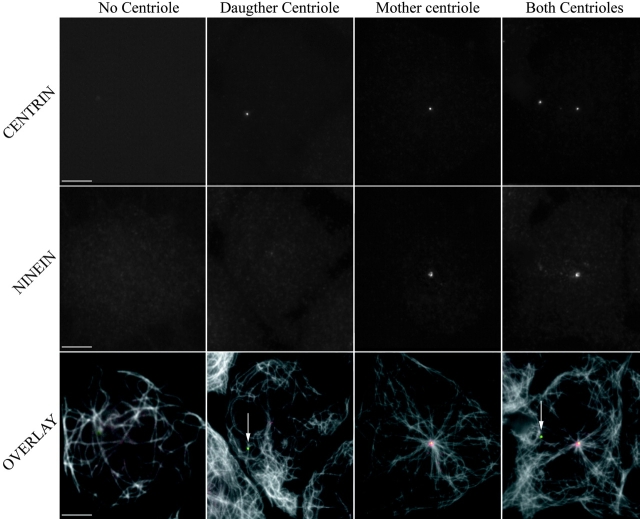
To further investigate the specific contribution of the daughter and the mother centrioles to the formation of the interphase Mt array, we enucleated synchronized G1 cells in the presence of both ND and CD which produced many cytoplasts that contained only one centriole (either mother or daughter) or no centrioles at all (Karsenti et al. 1984). When Mts were disassembled in the cytoplasts, and then allowed to repolymerize, the ninein-containing centrioles were always centrally located and associated with Mt arrays 15 min after ND washout, whereas those centrioles lacking ninein were in a peripheral location and not associated with radial Mt arrays (Fig. 8). In the latter cases, the Mts were curly, located primarily at the periphery of the cytoplast, and were more numerous than in cytoplasts lacking both centrioles. Peripheral Mts were also more abundant in cytoplasts with mother and daughter centrioles than in cytoplasts containing the mother centriole only. Thus, the presence of peripheral Mts correlates with the presence of the daughter centriole, suggesting that they were nucleated by the latter.
Figure 8.
Cytoplasts containing different centrosomes show different Mt organization. Cells were enucleated in the presence of ND in order to obtain cytoplasts with either no centriole, one centriole (daughter centriole or mother centriole), or two centrioles (see Materials and Methods). Mts were totally depolymerized. Cytoplasts were fixed after 15 min in regrowth conditions and stained for ninein (second row or red in the third row) and α-tubulin. GFP-centrin is shown on the first row. White arrows indicate the daughter centrioles. Bars, 5 μm.
This experiment also demonstrates that the mother centriole does not simply out-compete the daughter for those components (e.g., ninein) required for anchoring Mts. Rather, the younger daughter is not yet competent to recruit these components from the cytoplasmic pool and cannot mimic the mother in her absence.
In cytoplasts containing a single centriole, the centriole either remained relatively stationary or wandered throughout the cell (Fig. 9). An IMF analysis of these cells revealed that the stationary centriole was always associated with ninein and a Mt array, whereas the motile centriole lacked both (data not shown). Nonmotile centrioles were always positioned near the geometric center of cytoplasts containing only one centriole, whereas motile ones were often located near the periphery. These latter centrioles exhibited motions roughly parallel to the cell edge, seldom directed towards the cell center. This behavior differs from that found in cytoplasts containing a full complement of centrioles (i.e., one mother and one daughter) in which the daughter centriole often exhibits motions towards and away from the centrally located mother. Finally, in cytoplasts containing two mother and two daughter centrioles, two remained relatively motionless while two were highly motile (Fig. 9, right panel). As in our previous studies, the only centrioles associated with radial arrays of Mts were the immotile (mother) centrioles (not shown).
Discussion
Many observations of the centrosome in the past, either in situ or after isolation, have suggested a great complexity of this organelle. But most often obtained on fixed cells, structural features have been difficult to integrate into a coherent and reliable model of the centrosome organization. Here, by observing the centrosome in living cells, and in spite of a much lower resolution than that of EM, one could get at a more integrated view of the centrosome. Our work reveals novel features of the centrosome dynamics. First, splitting of centrioles in each postmitotic centrosome, which corresponds to the moment when orthogonal orientation is lost, occurs soon after anaphase, long before cytokinesis is completed. Second, the two centrioles of postmitotic cells demonstrate differential movements, one maintaining a central and stable location, whereas the other has a wide and eccentric trajectory. Third, the motile centriole progressively slows down from the onset of centriole duplication at the G1/S border, up to late G2; although maintaining a stable location within the cell once duplicated (in G2), the former motile centriole and its associated pro-centriole conserve more independence with respect to the surrounding cytoplasm until the onset of mitosis where it stops rocking completely. We also demonstrate a specific contribution of each centriole to the activity of the centrosome: both centrioles nucleate Mts but only the mother centriole anchors them. A general conclusion consistent with all the observations reported in this work is that the behavior of individual centrioles is maturation-dependent, correlated with the generation process of these organelles. In other words, one centriole cannot replace the other one in a centrosome.
The Two Centrioles-Centrosome: A Constitutive Generational Asymmetry of the Centriole Pair Necessary for Centrosome Function
Centriole Movements and the Interaction of the Centrosome with the Surrounding Cytoplasm.
The analysis of the centrosome dynamics in vivo suggests the existence of an intercentriole link. Even when Mts were totally depolymerized, there was still a strong correlation between the smooth movements of the two centrioles, even when they were microns apart (see Fig. 5 C). This is in agreement with the observation that, once isolated from cultured cells, centrosomes are always composed of the two centrioles associated with a complex filament network which seems to link them at the proximal ends (Bornens et al. 1987; Paintrand et al. 1992). The biochemical nature of this material, which corresponds broadly to the centrosome matrix, and the way in which the distance between centrioles is controlled (Ca2+, ATP, etc.) will deserve further study.
Wide excursions of the daughter centriole are mainly due to acto-myosin activity (see quantification in Fig. 5 B). This suggests that the motile centriole could be driven either by global cytoplasmic actin-dependent movements, or by a direct interaction with the acto-myosin system. Recent reports favor the last interpretation, as an accumulation of the myosin V isoform at the centrosome has been demonstrated (Espreafico et al. 1998; Tsakraklides et al. 1999). In addition, interactions of the microtubules with the actin cytoskeleton, through dynactin for example (Koonce et al. 1999), could also drive centriole movements (Euteneuer and Schliwa 1985).
A maturation-dependent anchorage of the centrosome within the cytoplasm, distinct from the Mt-dependent centering of the mother centriole, might involve the centrosomal matrix: when Mts were depolymerized in S or G2, the daughter centriole did not recover the motility observed in G1 (not shown). Interactions of the centrosomal matrix with other cytoskeletal components such as intermediate filaments or membranes will deserve further characterization. One may recall here that cells possessing a primary cilium provide an example of how the mother centriole could be anchored independently of the Mts. The distal end of the mother centriole interacts directly with the plasma membrane through its distal appendages. These structures which are observed in centrosomes isolated from cells which never grow a primary cilium (Paintrand et al. 1992) are thus candidates for anchoring the immotile centriole in the cytoplasm.
The centering of the mother centriole depends, however, on its Mt-anchoring activity. When Mts were completely depolymerized over several hours, the immotile centriole could be eventually found away from the cytoplast center. The centering capacity of an aster of Mts nucleated either from a bead bearing Mt seeds, or from isolated centrosomes, has been demonstrated in vitro in an artificial cell (Dogterom et al. 1995; Holy et al. 1997). We show here that in vivo the centering ability of the centrosome-Mts system relies on the mother centriole only and on its Mt-anchoring activity. In agreement with this conclusion, we observed that the motile centriole, even when alone in a cytoplast, was never located at the cell center.
The characteristic ninein staining of the mother centriole (see Fig. 6) might correspond to sub-distal appendages of the mother centriole (see Bouckson-Castaing et al. 1996, and unpublished observations). Independent arguments for a role of the sub-distal appendages in anchoring Mts was previously proposed (De Brabander 1982; Gorgidze and Vorobjev 1995). A role of ninein in mediating the anchoring of Mts to the apical plasma membrane in cochlear epithelial cells has been recently demonstrated during the postnatal differentiation of the mouse inner ear (Mogensen et al. 1999). Whether or not ninein is directly interacting with Mts as yet to be established.
The Release and Capture Model and the Regulation of Release Versus Capture by Centriole Splitting.
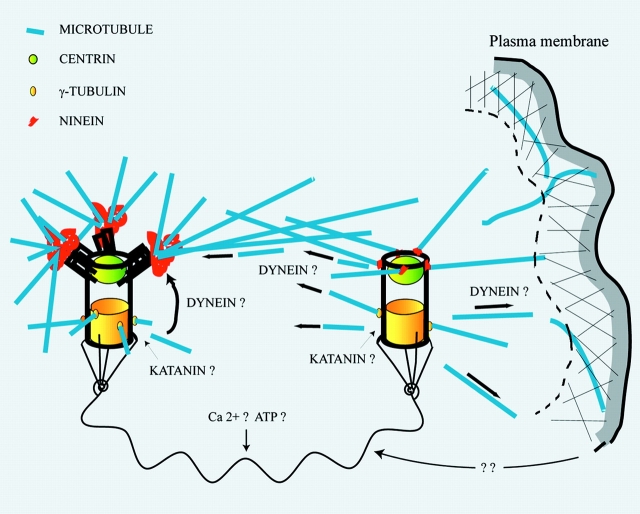
All the data presented in this work, particularly the strikingly different Mt arrays observed in cytoplasts containing either the mother or the daughter centriole (see Fig. 8), can be accounted for by the working model depicted in Fig. 10. It proposes that Mts are nucleated near centrioles (Fig. 7 B), then released and transported either to the ninein-containing complexes associated with the mother centriole or to other anchoring sites, mainly near or at the plasma membrane. Released Mts would be transported by dynein motors for example, as it was shown that perturbing dynactin activity greatly disturbed the organization of the Mt array. It has also been proposed that the dynein/dynactin complex could have a role in anchoring the Mts at the centrosome (Clark and Meyer 1999; Quintyne et al. 1999) and in the centrosome interaction with the cell cortex (Koonce et al. 1999). An important feature of this model is that the intercentriolar distance, which might itself be dependent on peripheral motility (see below), would regulate the release versus centrosomal capture balance of Mts within the cell.
Figure 10.
A model for the role of each centriole in the centrosome. The model proposes that Mts are nucleated near centriole walls, then released and transported either to the ninein-containing complexes associated with the mother centriole or to other anchoring sites, mainly near or at the plasma membrane. An important feature of this model is that the intercentriolar distance, which might itself be dependent on peripheral acto-myosin activity, would regulate the release of Mts to the cell periphery. These Mts being then involved in peripheral processes, the control of the intercentriolar distance would provide a feed-back loop for the regulation of the cortical acto-myosin system. The link between both centrioles is probably composed of many different proteins usually found in the PCM, and its overall length could be calcium dependent. The maturation-dependent anchoring of the centrosome organelle in the cytoplasm is not represented.
The release and capture model, first proposed by De Brabander 1982, was recently revived as a possible mechanism for understanding the redistribution of Mts away from the centrosome in highly polarized epithelial cells of the cochlea (Mogensen et al. 1997). The release of Mts in the cytoplasm, first proposed by Vorobjev and Nadezhdina 1987, has been a matter of debate (for a review, see Keating and Borisy 1999). The Mt release from the centrosome seems to be an active process, mediated by the severing protein katanin (McNally et al. 1996; Ahmad et al. 1999). As katanin seems to be active throughout cytoplasm, the major way to control the Mt array would be to control the anchoring and stabilizing activities.
Calcium-dependent regulation of the centrosome matrix (Baron et al. 1994; Paintrand et al. 1992) could be a way for the cell to reorganize its Mt array. When the matrix would be condensed by an appropriate mechanism, most of the nucleated Mts would be able to anchor on the mother centriole (Fig. 7 B, top), whereas when the matrix would be extended, released Mts could be transiently captured by capping elements at the cell periphery (Fig. 7 B, bottom). We must stress that the time course of daughter centriole excursion at the cell periphery (up to 15 min; see Fig. 4) is sufficient to release a number of Mts.
From the extensive data on fixed cells from various cell lines in the literature, one can observe that there is often a correlation between the extent of centriole splitting and the amount of Mts involved in the aster. All cell lines in which most of the Mts are participating in the aster, such as PtK1, CV1, COS, have two centrioles near one another at the center of the aster. Another way to control Mt organization in differentiated cells could be to redistribute anchoring proteins from the centrosome to other sites (Mogensen 1999; Mogensen et al. 1999).
Perspectives
The implication of the centrosome in cell motility is controversial as it has been shown that pieces of different kinds of cells can polarize and migrate (Malawista et al. 1983; Euteneuer and Schliwa 1984; Verkhovsky et al. 1999). However, if an acto-myosin cortical system is able to polarize and drive a piece of cell, it is not clear whether it is capable of integrating conflicting gradients. A cell often grows several lamellipodia suggesting that the peripheral acto-myosin system is not able to impose a unified polarity which is essential for cell integrity (the whole cell must go in the same direction). This problem of course would be expected to become more crucial as cell size increases. One may note, for example, that in small amoeba cells, like Dyctyostelium, the centrosome has a unique core structure rather than two, the repositioning of which seems to stabilize a pseudopodial extension and thus the direction of cell movement (Ueda et al. 1997). The two centrioles-centrosome might thus be necessary for coordinating the Mt array in large cells with the activity of the cortical actin. A centrally organized aster of stable Mts might be necessary for stabilizing the cell compartments and the nucleus with respect to the newly acquired position, whereas a more peripheral subset of released and transient Mts might be necessary for exploring a new step in locomotion. It is known, for example, that actin-dependent pseudopodial activity must be either stabilized by, or at least that it is somehow dependent on, peripheral Mts (Nathke et al. 1996; Schliwa et al. 1999).
Another situation in which the specific activity of each centriole might be required is cytokinesis during which a peculiar behavior of the centrosome organelle has been observed (Mack and Rattner 1993). The data presented in this work strongly suggests a specific function of centriole splitting for completion of the division process (see Fig. 2). At the end of telophase, a rapid (∼10 μm/min) and persistent movement of one of the two centrioles towards the mid-body takes place in one sister cell or in both, although at different moments. This phenomenon is currently under study.
In conclusion, our work reveals an unexpected complexity in the behavior of the centrosome. The analysis of the centriole movements and of the effect of cytoskeletal drugs has implications for the integration of the centrosome organelle within the cell. A specific role for each centriole in the Mt organizing activity of the centrosome is also demonstrated, which strongly suggests that centriole splitting could be a way to control the cellular array of Mts. This could have important implications for understanding how the centrosome activity and the plasma membrane activity could be coupled with each other during cell locomotion or cell division.
Acknowledgments
We thank Jan de Mey and Jean-Baptiste Sibarita for the setting of the video recording device which was very helpful; Olivier Cardoso and Virginie Emselem for helpful discussions on the analysis of the centriole movements; Andrew Matus for well-advised commentaries on this work; Yann Abraham for the GFP constructs and Claude Celati for her help in the cloning of stable cell lines; Bruno Goud and Ludger Johannes for the Shiga toxin B fragment; and Anne-Marie Tassin, Guy Keryer, Bruno Goud, Andrew Matus, and Don Cleveland for critical reading of the manuscript.
This work is supported by Centre National de la Recherche Scientifique, Institut Curie, Direction des Recherches, Etudes et Techniques and Association pour la Recherche sur le Cancer. It was also supported, in part, by the National Institute of Health grants GMS R01 59363 to A. Khodjakov and GMS R01 40198 to C.L. Rieder.
Footnotes
The online version of this article contains supplemental material.
Dr. Michel Bornens, Institut Curie, Section Recherche, UMR 144 du CNRS, 26, rue d'ULM, 75248 Paris Cedex 05, France. Tel.: 01 42 34 64 20. Fax: 01 42 34 64 21. E-mail: mbornens@curie.fr
Abbreviations used in this paper: CD, cytochalasin D; EM, electron microscopy; GFP, green fluorescent protein; LM, light microscopy; Mts, microtubules; ND, nocodazole; PCM, pericentriolar material.
References
- Ahmad F.J., Yu W., McNally F.J., Baas P.W. An essential role for katanin in severing Mts in the neuron. J. Cell Biol. 1999;145:305–315. doi: 10.1083/jcb.145.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.S.L. Molecular characteristics of the centrosome. Int. Rev. Cytol. 1999;187:51–109. doi: 10.1016/s0074-7696(08)62416-x. [DOI] [PubMed] [Google Scholar]
- Baron A.T., Greenwood T.M., Bazinet C.W., Salisbury J.L. Centrin is a component of the pericentriolar lattice. Biol. Cell. 1992;76:383–388. doi: 10.1016/0248-4900(92)90442-4. [DOI] [PubMed] [Google Scholar]
- Baron A.T., Suman V.J., Nemeth E., Salisbury J.L. The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J. Cell Sci. 1994;107:2993–3003. doi: 10.1242/jcs.107.11.2993. [DOI] [PubMed] [Google Scholar]
- Bobinnec Y., Khodjakov A., Mir L.M., Rieder C.L., Edde B., Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M. Is the centriole bound to the nuclear membrane? Nature. 1977;270:80–82. doi: 10.1038/270080a0. [DOI] [PubMed] [Google Scholar]
- Bornens M., Paintrand M., Berges J., Marty M.C., Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskelet. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Bouckson-Castaing V., Moudjou M., Ferguson D.J.P., Mucklow S., Belkaid Y., Milon G., Crocker P.R. Molecular characterisation of ninein, a new coiled-coil protein of the centrosome. J. Cell Sci. 1996;109:179–190. doi: 10.1242/jcs.109.1.179. [DOI] [PubMed] [Google Scholar]
- Carter S.B. Effects of cytochalasins on mammalian cells. Nature. 1967;213:261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Chretien D., Buendia B., Fuller S.D., Karsenti E. Reconstruction of the centrosome cycle from cryoelectron micrographs. J. Struct. Biol. 1997;120:117–133. doi: 10.1006/jsbi.1997.3928. [DOI] [PubMed] [Google Scholar]
- Clark I.B., Meyer D.I. Overexpression of normal and mutant Arp1(alpha)(centractin) differently affects Mt organisation during mitosis and interphase. J. Cell Sci. 1999;112:3507–3518. doi: 10.1242/jcs.112.20.3507. [DOI] [PubMed] [Google Scholar]
- De Brabander M. A model for the Mt organising activity of the centrosomes and kinetochores in mammalian cells. Biol. Intl. Reports. 1982;6:901–915. doi: 10.1016/0309-1651(82)90001-7. [DOI] [PubMed] [Google Scholar]
- Dogterom M., Maggs A.C., Leibler S. Diffusion and formation of Mt astersphysical processes versus biochemical regulation. Proc. Natl. Acad. Sci. USA. 1995;92:6683–6688. doi: 10.1073/pnas.92.15.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espreafico E.M., Coling D.E., Tsakraklides V., Krogh K., Wolenski J.S., Kalinec G., Kachar B. Localization of myosin-V in the centrosome. Proc. Natl. Acad. Sci. USA. 1998;95:8636–8641. doi: 10.1073/pnas.95.15.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U., Schliwa M. Persistant and directional motility of cells and cytoplasmic fragments in the absence of Mts. Nature. 1984;310:58–61. doi: 10.1038/310058a0. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., Schliwa M. Evidence for an involvement of actin in the positioning and motility of centrosomes. J. Cell Biol. 1985;101:96–103. doi: 10.1083/jcb.101.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais D., Nadezhdina E.S., Chentsov Iu.S. Evidence for the nucleus-centriole association in living cells obtained by ultracentrifugation. Eur. J. Cell Biol. 1984;33:190–196. [PubMed] [Google Scholar]
- Gorgidze L.A., Vorobjev I.A. Centrosome and Mts behavior in the cytoplasts. J. Submicrosc. Cytol. Pathol. 1995;27:381–389. [PubMed] [Google Scholar]
- Holy T.E., Dogterom M., Yurke B., Leibler S. Assembly and positioning of Mt asters in microfabricated chambers. Proc. Natl. Acad. Sci. USA. 1997;94:6228–6231. doi: 10.1073/pnas.94.12.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Kobayashi S., Mitchison T., Kirschner M. Role of the centrosome in organizing the interphase Mt arrayproperties of cytoplasts containing or lacking centrosomes. J. Cell Biol. 1984;98:1763–1776. doi: 10.1083/jcb.98.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating T.J., Borisy G.G. Centrosomal and non-centrosomal Mts. Biol. Cell. 1999;91:321–329. [PubMed] [Google Scholar]
- Kochanski R.S., Borisy G.G. Mode of centriole duplication and distribution. J. Cell Biol. 1990;110:1599–1605. doi: 10.1083/jcb.110.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce M.P., Köhler J.K., Schwartz J., Tikhonenko I., Gerish G. Dynein motor regulation stabilizes interphase Mt arrays and determines centrosome position. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:6786–6792. doi: 10.1093/emboj/18.23.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G.G. Centriole cycle in chinese hamster ovary cells as determined by whole-mount electron microscopy. J. Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange B.M., Gull K. Molecular marker for centriole maturation in the mammalian cell cycle. J. Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack G., Rattner J.B. Centrosome repositioning immediately following karyokinesis and prior cytokinesis. Cell Motil. Cytoskelet. 1993;26:239–247. doi: 10.1002/cm.970260307. [DOI] [PubMed] [Google Scholar]
- Malawista S.E., de Boisfleury-Chevance A., Maunoury R., Bessis M. Heat as a probe of centrosomal functiona phase-contrast and immunofluorescent study of human blood monocytes. Blood Cells. 1983;9:443–453. [PubMed] [Google Scholar]
- Mallard F., Anthony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally F.J., Okawa K., Iwamatsu A., Vale R.D. Katanin, the Mt-severing ATPase, is concentrated at centrosomes. J. Cell Sci. 1996;109:561–567. doi: 10.1242/jcs.109.3.561. [DOI] [PubMed] [Google Scholar]
- Middendorp S., Paoletti A., Schiebel E., Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc. Natl. Acad. Sci. USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorp S., Küntziger T., Abraham Y., Holmes S., Bordes N., Paintrand M., Paoletti A., Bornens M. A role for centrin 3 in centrosome reproduction. J. Cell Biol. 2000;148:405–416. doi: 10.1083/jcb.148.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M.M., Malik A., Bouckson-Castaing V., Bornens M. Microtubule release and capture and the role of the centrosomal protein ninein Mol. Biol. Cell. 10Suppl.1999. 257a [Google Scholar]
- Mogensen M.M. Mt release and capture in epithelial cells. Biol. Cell. 1999;91:331–341. [PubMed] [Google Scholar]
- Mogensen M.M., Mackie J.B., Doxsey S.J., Stearns T., Tucker J.B. Centrosomal deployment of gamma-tubulin and pericentrinevidence for a Mt-nucleating domain and a minus-end docking domain in certain mouse epithelial cells. Cell Motil. Cytoskelet. 1997;36:276–290. doi: 10.1002/(SICI)1097-0169(1997)36:3<276::AID-CM8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Moudjou M., Bordes N., Paintrand M., Bornens M. Gamma-tubulin in mammalian cellsthe centrosomal and the cytosolic forms. J. Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Nathke I.S., Adams C.L., Polakis P., Sellin J.H., Nelson W.J. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintrand M., Moudjou M., Delacroix H., Bornens M. Centrosome organization and centriole architecturetheir sensibility to divalent cations. J. Struct. Biol. 1992;108:107–128. doi: 10.1016/1047-8477(92)90011-x. [DOI] [PubMed] [Google Scholar]
- Paoletti A., Moudjou M., Paintrand M., Salisbury J.L., Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Prescott D.M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp. Cell Res. 1972;71:480. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Quintyne N.J., Gill S.R., Eckley D.M., Crego C.L., Compton D.A., Schroer T.A. Dynactin is required for Mt anchoring at centrosomes. J. Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Borisy G.G. The centrosome cycle in PtK2 cellsasymmetric distribution and structural change in the pericentriolar material. Biol. Cell. 1982;44:117–132. [Google Scholar]
- Rieder C.L., Cassels G. Correlative light and electron microscopy of mitotic cells in monolayer cultures. Methods Cell Biol. 1999;61:297–315. doi: 10.1016/s0091-679x(08)61987-1. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Euteneuer U., Graf R., Ueda M. Centrosomes, Mts and cell migration. Biochem. Soc. Symp. 1999;65:223–231. [PubMed] [Google Scholar]
- Schnackenberg B.J., Palazzo R.E. Identification and function of the centrosome centromatrix. Biol. Cell. 1999;91:429–438. [PubMed] [Google Scholar]
- Sluder G., Rieder C.L. Centriole number and the reproductive capacity of spindle poles. J. Cell Biol. 1985;100:887–896. doi: 10.1083/jcb.100.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Miller F.J., Rieder C.L. Reproductive capacity of sea urchin centrosomes without centrioles. Cell. Motil. Cytoskelet. 1989;13:264–273. doi: 10.1002/cm.970130405. [DOI] [PubMed] [Google Scholar]
- Tassin A.M., Bornens M. Centrosome structure and Mt nucleation in animal cells. Biol. Cell. 1999;91:343–354. [PubMed] [Google Scholar]
- Tassin A.M., Celati C., Moudjou M., Bornens M. Characterization of the human homologue of the yeast Spc98p and its association with gamma-tubulin. J. Cell Biol. 1998;141:689–701. doi: 10.1083/jcb.141.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakraklides V., Krogh K., Wang L., Bizario J.C.S., Larson R.E., Espreafico E.M., Wolenski J.S. Subcellular localization of GFP-myosin-V in live mouse melanocytes. J. Cell Sci. 1999;112:2853–2865. doi: 10.1242/jcs.112.17.2853. [DOI] [PubMed] [Google Scholar]
- Ueda M., Graf R., MacWilliams H.K., Schliwa M., Euteneuer U. Centrosome positioning and directionality of cell movements. Proc. Natl. Acad. Sci. USA. 1997;94:9674–9678. doi: 10.1073/pnas.94.18.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky A.B., Svitkina T.M., Borisy G.G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- Vorobjev I.A., Chentsov Y.S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982;98:938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev I.A., Nadezhdina E.S. The centrosome and its role in the organization of Mts. Int. Rev. Cytol. 1987;106:227–293. doi: 10.1016/s0074-7696(08)61714-3. [DOI] [PubMed] [Google Scholar]
- Vorobjev I.A., Svitkina T.M., Borisy G.G. Cytoplasmic assembly of Mts in cultured cells. J. Cell Sci. 1997;110:2635–2645. doi: 10.1242/jcs.110.21.2635. [DOI] [PubMed] [Google Scholar]