Abstract
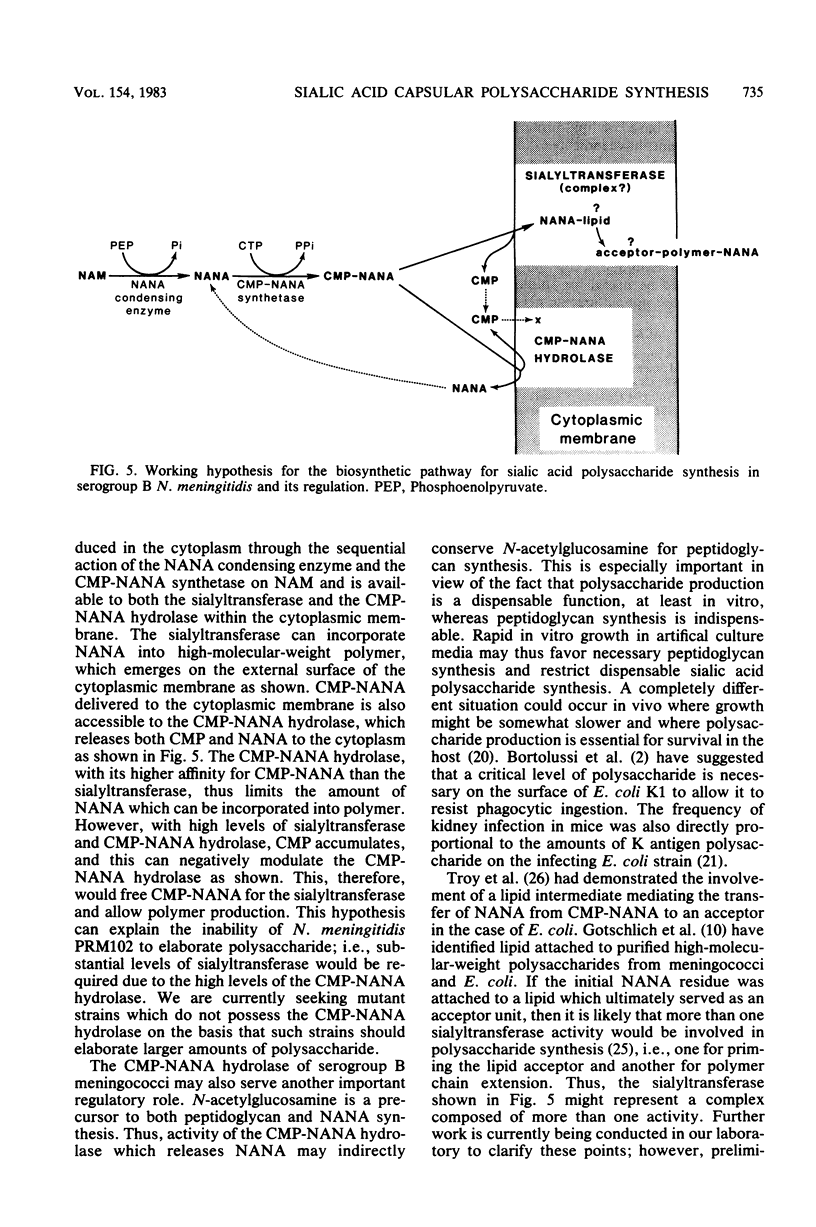
The pathway for biosynthesis of sialic acid capsular polysaccharide was examined in Neisseria meningitidis serogroup B strain M986 and in strain PRM102, an isogenic mutant defective in polysaccharide production. Strain PRM102 was found to possess only 25% of the level of sialyltransferase activity that was found in strain M986, but it had wild-type levels of both the N-acetylneuraminic acid (NANA) condensing enzyme and the CMP-NANA synthetase. A new meningococcal enzyme, a CMP-NANA hydrolase, was found in both meningococcal strains. This enzyme generated CMP and NANA from CMP-NANA, had a Km of 0.88 microM, had a Vmax of 10.75 nmol of NANA produced per h per mg of protein, and was completely inhibited by 45.3 microM CMP. The sialyltransferase, which also had CMP-NANA as substrate, was insensitive to CMP addition. Subcellular fractionation and purification of cytoplasmic and outer membranes on sucrose density gradients revealed that both the sialyltransferase and the CMP-NANA hydrolase were cytoplasmic membrane associated. The NANA condensing enzyme and the CMP-NANA synthetase were found to be cytosolic. A working hypothesis for the regulation of sialic acid polysaccharide synthesis was developed. The CMP-NANA hydrolase with its high affinity for CMP-NANA regulates polysaccharide formation by the sialyltransferase, whereas CMP, a product of both the sialyltransferase and the CMP-NANA hydrolase, modulates the activity of the hydrolase on the cytoplasmic membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKLOW R. S., WARREN L. Biosynthesis of sialic acids by Neisseria meningitidis. J Biol Chem. 1962 Nov;237:3520–3526. [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Devoe I. W., Golchrist J. E. Localization of tetramethylphenylenediamine-oxidase in the outer cell wall layer of Neisseria meningitidis. J Bacteriol. 1976 Oct;128(1):144–148. doi: 10.1128/jb.128.1.144-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Kean E. L., Bighouse K. J. Cytidine 5'-monophosphosialic acid hydrolase. Subcellular location and properties. J Biol Chem. 1974 Dec 25;249(24):7813–7823. [PubMed] [Google Scholar]
- Kundig J. D., Aminoff D., Roseman S. The sialic acids. XII. Synthesis of colominic acid by a sialyltransferase from Escherichia coli K-235. J Biol Chem. 1971 Apr 25;246(8):2543–2550. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod M. N., DeVoe I. W. Localization of carbonic anhydrase in the cytoplasmic membrane of Neisseria sicca (strain 19). Can J Microbiol. 1981 Jan;27(1):87–92. doi: 10.1139/m81-014. [DOI] [PubMed] [Google Scholar]
- Nicholson A. M., Glynn A. A. Investigation of the effect of K antigen in Escherichia coli urinary tract infections by use of a mouse model. Br J Exp Pathol. 1975 Dec;56(6):549–553. [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy F. A., 2nd The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol. 1979;33:519–560. doi: 10.1146/annurev.mi.33.100179.002511. [DOI] [PubMed] [Google Scholar]
- Troy F. A., McCloskey M. A. Role of a membranous sialyltransferase complex in the synthesis of surface polymers containing polysialic acid in Escherichia coli. Temperature-induced alteration in the assembly process. J Biol Chem. 1979 Aug 10;254(15):7377–7387. [PubMed] [Google Scholar]
- Troy F. A., Vijay I. K., Tesche N. Role of undecaprenyl phosphate in synthesis of polymers containing sialic acid in Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):156–163. [PubMed] [Google Scholar]
- WARREN L., BLACKLOW R. S. Biosynthesis of N-acetyl-neuraminic acid and cytidine-5'-monophospho-N-acetyl-neuraminic acid in Neisseria meningitidis. Biochem Biophys Res Commun. 1962 Jun 4;7:433–438. doi: 10.1016/0006-291x(62)90330-3. [DOI] [PubMed] [Google Scholar]
- WARREN L., BLACKLOW R. S. The biosynthesis of cytidine 5'-monophospho-n-acetylneuraminic acid by an enzyme from Neisseria meningitidis. J Biol Chem. 1962 Nov;237:3527–3534. [PubMed] [Google Scholar]