Abstract
The adenoviral early region 4 open reading frame 4 (E4orf4) death factor induces p53-independent apoptosis in many cell types and appears to kill selectively transformed cells. Here we show that expression of E4orf4 in transformed epithelial cells results in early caspase-independent membrane blebbing, associated with changes in the organization of focal adhesions and actin cytoskeleton. Evidence that E4orf4 can associate with and modulate Src family kinase activity, inhibiting Src-dependent phosphorylation of focal adhesion kinase (FAK) and paxillin while increasing phosphorylation of cortactin and some other cellular proteins, is presented. Furthermore, E4orf4 dramatically inhibited the ability of FAK and c-src to cooperate in induction of tyrosine phosphorylation of cellular substrates, suggesting that E4orf4 can interfere with the formation of a signaling complex at focal adhesion sites. Consistent with a functional role for E4orf4–Src interaction, overexpression of activated c-src dramatically potentiated E4orf4-induced membrane blebbing and apoptosis, whereas kinase dead c-src constructs inhibited E4orf4 effects on cell morphology and death. Moreover treatment of E4orf4-expressing cells with PP2, a selective Src kinase inhibitor, led to inhibition of E4orf4-dependent membrane blebbing and later to a marked decrease in E4orf4-induced nuclear condensation. Taken together, these observations indicate that expression of adenovirus 2 E4orf4 can initiate caspase-independent extranuclear manifestations of apoptosis through a modulation of Src family kinases and that these are involved in signaling E4orf4-dependent apoptosis. This study also suggests that Src family kinases are likely to play a role in the cytoplasmic execution of apoptotic programs.
Keywords: actin, blebbing, Src family kinase, focal adhesion kinase, cortactin
Introduction
During the last several years, a tremendous amount of work has led to new insights into the mechanisms underlying the central executioner core of the apoptotic machinery, which plays a crucial role in the maintenance of cellular integrity (Thompson 1995). Two major execution programs downstream of the death signal have emerged, one of which is the caspase pathway by which limited proteolysis of specific substrates lead to activation of enzyme activities, or inactivation of essential cellular components (Martin and Green 1995; Nicholson and Thornberry 1997). The second major execution program, the mitochondrial pathway, leads to release of proapoptotic molecules, some of which are involved in the activation of the so-called apoptosome (for review see Green and Reed 1998 and Thornberry and Lazebnick 1998; Kroemer et al. 1995). Despite the well-established importance of caspases in apoptotic processes, the existence of a caspase-independent pathway for execution of cell death is evidenced by molecules that can induce apoptosis, without the need for caspase activation; these include the proapoptotic Bcl-2 family members BAX and BAK (Xiang et al. 1996; McCarthy et al. 1997), AIF (which is released from mitochondria during apoptosis; Susin, et al. 1999), and expression of the adenovirus (Ad) early region 4 open reading frame 4 (E4orf4) death factor (Lavoie et al. 1998).
Apoptosis is also associated with profound modifications in the cytoplasmic architecture of cells, which are manifested by changes in adhesive mechanisms and localization of cytoskeletal proteins, induction of a rounded morphology, membrane blebbing, and shrinkage of the cells (for review see Mills et al. 1999). Recently, a model was proposed to recapitulate the mechanisms underlying the cytoplasmic execution phase of apoptosis, or extranuclear apoptosis, into three sequential phases: the release stage, blebbing, and the final condensation stage (Mills et al. 1999). The first stage is characterized by a reorganization of focal adhesions and actin changes to allow a rounder morphology (Bannerman et al. 1998; Huot et al. 1998; Levkau et al. 1998). Based on this model, the physiological apoptotic process induced by loss of extracellular matrix (ECM) attachments (anoikis) would represent a direct entry of cells into the release stage of the cytoplasmic execution of death. Cell–ECM interactions via integrin receptors promote the assembly of cytoskeletal and signaling molecules at sites called focal adhesion. These signaling complexes include Src family kinase members, focal adhesion kinase (FAK), adaptor molecules, phosphatidylinositol-3′-kinase, and phospholipase C (Miyamoto et al. 1995a,Miyamoto et al. 1995b; Plopper et al. 1995), and are involved in the propagation of survival signals via activation of downstream signaling pathways (for review see Giancotti 1997). The importance of FAK in regulating adhesion-dependent cell survival was demonstrated by the development of a constitutively activated form of FAK that resulted in a substantial protection of MDCK cells from anoikis (Frisch et al. 1996). Furthermore, inactivation of FAK by microinjection of specific peptides was shown to induce apoptosis (Hungerford et al. 1996). However, it is not understood how integrins, cell shape, and apoptotic signaling are integrated, and study of cytoplasmic events has lagged.
Recently, we and others have shown that expression of the Ad E4orf4 protein in several mammalian cell lines induces a p53-independent apoptotic pathway (Shtrichman and Kleinberger 1998;Lavoie et al. 1998; Marcellus et al. 1998). E4orf4 appears to kill transformed cells more selectively, as expression of oncogene in primary cell cultures was reported to sensitize these cells to E4orf4-induced apoptosis (Shtrichman et al. 1999). E4orf4-mediated apoptosis does not require activation of the zVAD-fmk–inhibitable caspases, and is associated with mitochondrial dysfunctions in CHO cells (Lavoie et al. 1998). Other cellular processes such as downregulation of virally induced signal transduction (Bondesson et al. 1996), regulation of gene expression (Muller et al. 1992), and differential splicing of adenovirus late mRNA (Kanopka et al. 1998) have been shown to be affected by Ad E4orf4. Additionally, E4orf4 was found to associate with the B55 subunit of protein phosphatase 2A (PP2A; Kleinberger and Shenk 1993), and very recently a close correlation between E4orf4 binding to PP2A and induction of apoptosis was reported in mutagenesis experiments (Shtrichman et al. 1999; Marcellus et al. 2000); however, no molecular mechanism was proposed to explain how E4orf4/PP2A interaction could transduce E4orf4 apoptotic signal. The mechanisms underlying initiation and execution of E4orf4 death pathway are still not understood, and the observations that this pathway appears to be p53-caspase–independent and specific to transformed cells is of great interest.
In this study, we report that E4orf4 initiates early morphological changes leading to membrane blebbing and provide evidence that these manifestations are involved in the propagation of E4orf4 death signal. We found that E4orf4 can associate with Src family kinases and dysregulate Src-dependent signaling. Moreover, the data obtained strongly suggest that Src family kinase activity plays a role in signaling caspase-independent E4orf4-induced membrane blebbing and nuclear condensation.
Materials and Methods
Expression Vectors and Mutagenesis
NH2-terminal epitope-tagged forms of Ad2 E4orf4 were generated by standard PCR method and subcloned into mammalian expression vectors. The Flag–E4orf4 construct was generated using a hemagglutinin (HA)-orf4/PUHD10-3 as the template (Lavoie, et al. 1998), and the sense and antisense oligos 5′-GGGGTACCG ATG GTT CTT CCA GCT CTT CC-3′ and 5′-CTA CTG TAC GGA GTG CGCC-3′, respectively. The resulting DNA fragment was subcloned into the KpnI–EcoRV sites of pcDNA3 FLAG type A (Invitrogen). All subsequent PCRs were performed using the Flag–E4orf4 construct as the DNA template. The myc–E4orf4 construct was obtained by using the sense and antisense oligos 5′-GGGGTACCG ATG GTT CTT CCA GCT CTT CC-3′ and 5′-ATTTAGGTGACACTATAG-3′ (SP6 primer), respectively, and the resulting DNA fragment was subcloned in frame into the EcoRI restriction site of the pCineo-myc vector (Theriault et al. 1999). The Flag–green fluorescent protein (GFP) expression vector has been described by Lee et al. 1999. The Flag–E4orf4–GFP construct was obtained by PCR using the sense and antisense oligos 5′-AGAGGGCC C ATG GTT CTT CCA GCT CTT CCA-3′ and 5′-GCA GAA TTC CTC GAG CTG TAC GGA GTG CGC-3′, respectively. The resulting DNA fragment was digested with ApaI and XhoI and inserted into the ApaI–XhoI sites of the Flag-GFP vector. Expression vector containing the mouse c-src cDNA was obtained from Dr. André Veillette (McGill University, Montréal, Québec, Canada). Activated c-src was generated by replacing the COOH-terminal regulatory Tyr 527 by Phe residue as previously described (Courtneige 1985; Reynolds et al. 1987), using the QuickChange Site-Directed Mutagenesis Kit (Stratagene). Two oligos, 5′-ACT GAG CCA CAG TTC CAG CCC GGG GAG-3′ and 5′-CTC CCC GGG CTG GAA CTG TGG CTC AGT-3′, were used to generate the mutation on mouse c-src. Kinase-deficient c-src was obtained by inactivating the ATP binding site upon replacing the Lys at position 295 by a Met residue as previously reported (Snyder et al. 1985; Roche et al. 1995), using the QuickChange Site-Directed Mutagenesis Kit (Stratagene). The following sense and antisense oligos were used to generate the mutation on mouse c-src: 5′-AGG GTT GCC ATC ATG ACT CTG AAG CCA GGC ACC-3′ and 5′-GCC TGG CTT CAG AGT CAT GAT GGC AAC CCT CGT-3′. In vitro kinase activities of the mutant Srcs were analyzed after isolation of the Src proteins expressed into 293T cells, using Raytide peptide as substrate, according to the manufacturer's protocol (Oncogene Research Products). The v-src construct was obtained by subcloning the avian v-src sequence into the BamHI site of pCDNA3 vector (Invitrogen) and was a gift from Dr. Jerry Pelletier (McGill University). Chicken c-srcY527F and c-srcK295R in pLNCX vector were provided by Dr. J.S. Brugge (Harvard Medical School, Boston, MA) and have been described previously (Thomas et al. 1991). The myc-FAK expression vector was provided by Dr. J. Thomas Parsons (University of Virginia School of Medicine, Charlottesville, Virginia) and has been described elsewhere (Xiong and Parsons 1997). Authenticity of all DNA fragments generated by PCR was confirmed by DNA sequence analysis.
Glutathione S-Transferase Fusion Proteins and Rabbit 2418 E4orf4 Antibody
The glutathione S-transferase (GST)–E4orf4 fusion was generated by PCR using the Flag–E4orf4 as template and the sense and antisense oligos 5′-GGGGTACC ATG GTT CTT CCA GCT CTT CC-3′ and 5′-ATTTAGGTGACACTATAG-3′ (SP6 primer), respectively. The DNA fragment was processed for subcloning into the BamHI–XhoI sites of pGEX4T-3 vector (Amersham Pharmacia Biotech). The GST-c-src was produced by PCR using mouse c-src as template and the sense and antisense oligos 5′-CAGGATCCAGGAATTCG ATG GGC AGC AAC AAG AGC-3′ and 5′-CGCTCGAG CTA TAG GTT CTC CCC GGG-3′, respectively. The resulting DNA fragment was subcloned into the EcoRI–XhoI sites of pGEX4T-3 vector. The recombinant plasmids were introduced into Escherichia coli BL21 DE3, and the fusion proteins were produced by growing 50-ml bacterial cultures to an OD between 0.9 and 1.1, and then treating the cultures with 0.5 mM IPTG for 1.5–3 h. Cells were recovered and resuspended in 1.5 ml of SB buffer (16 mM sodium phosphate, pH 7.4, 150 mM NaCl, 15% glycerol, 0.02% Triton X-100, 1 mM DTT, 15 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A) and sonicated using a Vibra Cell probe sonicator operating at setting 7.5 for 20 s at 4°C. Triton X-100 was added to the lysates to a final concentration of 1%, and the bacterial lysates were incubated on ice for 15 min, then centrifuged at 12,000 rpm in a Beckman SS34 rotor for 15 min. The supernatants were recovered, mixed with 55 μl of a 1:1 suspension of glutathione/Sepharose 4B beads, and the mixture was rotated at 4°C for 1 h. The beads were then washed extensively in SB buffer and used for in vitro binding assays, or the GST fusions were eluted from the beads with 100 mM Tris-HCl, pH 8.0, 120 mM NaCl, 20 mM reduced glutathione. Purified GST–E4orf4 fusion protein was injected into rabbits, then the immune serums were absorbed on immobilized GST and the purified IgG fraction was used in Western blot and immunofluorescence analyses. The specificity of the resulting 2418 anti-E4orf4 was tested by Western blot analysis of total cellular proteins from lysates of control cells versus lysates from cell transfected with E4orf4 and myc–E4orf4, and by immunofluorescence analysis of control cells versus cells transfected with E4orf4, showing specific staining only for cells transfected with E4orf4. The antibody also reacted specifically with immune complexes obtained upon immunoprecipitation of transfected Flag–E4orf4 into 293T cells using anti-Flag.
Cell Culture, Transfections, and Apoptosis Assays
293T were derived from human embryonic kidney cells and express Ad5 E1A and E1B proteins and large T antigen (Graham et al. 1977). 293T were maintained in DMEM and the human non–small-cell lung carcinoma H1299 cells (Mitsudomi et al. 1992) were maintained in αMEM. All culture media were supplemented with 10% FBS and streptomycin sulfate/penicillin (100 U/ml). Cells were grown in a humidified 5% CO2 atmosphere at 37°C. Transfections of 293T and H1299 were performed by the calcium phosphate method when cells reached 50–60% confluency. Cells were either seeded on regular culture dishes (H1299) or polylysine (293T; 10 μg/ml), or fibronectin-coated culture dishes when indicated (FALCON Biocoat; Becton Dickinson). 293T cells were transfected with 15 μg total DNA in 10-cm culture dishes, or with the corresponding ratio DNA/cells in other culture dishes, and the calcium-phosphate-DNA precipitates were left on cells for 7 h in the presence of chloroquine (25 μM). H1299 cells were transfected with 40 μg total DNA in 10-cm culture dishes or with 15 μg total DNA in 6-well plates. Viability was assessed at 16–20 h by Trypan blue exclusion assays, as previously described (Lavoie et al. 1998). Morphological/spreading analysis of 293T cells transfected with Flag–GFP, Flag–E4orf4–GFP, or Flag–GFP together with E4orf4 and/or Src constructs were realized 20–24 h after transfection, using a Nikon Diaphot-TMD equipped with a Hoffman modulation system and a thermoregulated chamber at 37°C. Cell adhesion assays were performed by plating cells (30,000 in serum-free media containing 0.5% BSA) on fibronectin-coated 96-well tissue culture plates 20–24 h after transfections. Cells were incubated at 37°C for 1 h, and adherent cells were quantified as described by Masson-Gadais et al. 1997. In brief, attached cells were gently washed with PBS and fixed in 1% glutaraldehyde/PBS for 30 min. Fixed cells were stained for 15 min and extracted in 1% SDS, after which absorbance was measured at 550 nm. Specific attachment to fibronectin was controlled by incubating cell suspensions in the presence of integrin β1–blocking antibody (2 mg/ml, mouse anti–human integrin β1 clone P4C10, GIBCO-BRL) before plating cells on coated dishes. Treatments with various drugs were performed by adding DMSO (0.2%), cytochalasin D (1 μM; Sigma-Aldrich), PP2 or PP3 (50 μM; Calbiochem-Novabiochem), or genistein (100 μM; Sigma-Aldrich) directly to the culture medium. After quantitative measurement of the various phenotypes, representative fields were captured by confocal microscopy using a Bio-Rad MRC-1024 imaging system mounted on a Nikon Diaphot-TMD equipped with an ×20 objective lens as previously described (Lavoie et al. 1995). Cells were kept in culture for an additional 24 h and the nuclear morphology of GFP-positive cells was analyzed. Nonadherent and adherent cells were washed in PBS, half of each cell suspension was lysed in SDS sample buffer, and equal amounts of total proteins were run on SDS-PAGE for Western blot analysis of expression levels of the exogenous proteins. The other half of the cell suspensions were resuspended in 100% methanol, fixed at −20°C for 5 min, and air-dried on glass coverslips. Staining of DNA was performed using 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes) as previously described (Lavoie et al. 1998), and specimens were analyzed by fluorescence microscopy using a Nikon Eclipse E600 equipped with a Micromax CCD camera. All quantitative measures were obtained by counting at least 300 cells and are representative of three independent experiments.
In Vitro Binding Assays
Control 293T cells or transfected cells with myc–E4orf4 from a 10-cm plate were washed in PBS and homogenized in 1.0 ml lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 10% glycerol, 1 mM Na3VO4, 15 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 1 mM β-glycerophosphate). After centrifugation at 11,000 g, supernatants were precleared with 50 μl of a 1:1 slurry of glutathione/Sepharose 4B for 1 h at 4°C. The Sepharose was then removed and the supernatants were incubated in the presence of 4 μg of immobilized GST fusions, freshly prepared as described above, for 4 h at 4°C (slurry of glutathione/Sepharose 4B was added up to 10 μl volume of packed beads/sample). The beads were recovered by centrifugation, washed three times in buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% NP-40, and 10% glycerol, and were then boiled in SDS sample buffer. The amount of E4orf4 absorbed was analyzed by Western blot using mouse 9E10 anti–c-myc antibody (Sigma-Aldrich). Binding of Src-kinases, PP2A, and NCK to GST–orf4 fusion was assessed using rabbit SRC2 anti-src-kinases antibody (Santa Cruz Biotechnology, Inc.), mouse anti-PP2A catalytic α (Transduction Laboratories), or rabbit anti-NCK (PharMingen) antibodies, respectively, for Western blot analysis of the absorbed material.
Immunoprecipitations, Cell Fractionation, and Blotting
For coimmunoprecipitations of E4orf4-Src kinases, 293T cells were harvested 24 h after transfection, washed in PBS, and resuspended in 1.5 ml hypotonic buffer (5 mM Tris-HCl, pH 7.4, 5 mM KCl, 1.5 mM MgCl2, 5 mM EDTA, 1 mM Na3VO4, 15 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin) per 10-cm culture plate. Cells were allowed to swell on ice for 15 min, and were disrupted by five cycles of freeze–thaw interspersed by five strokes with a Wheaton glass homogenizer fitted with a B pestle. Homogenates were centrifuged at 900 g for 15 min at 4°C, yielding the first pellets (P1) containing mainly nuclei and unbroken cells. Supernatants were then centrifuged at 25,000 g for 30 min at 4°C in a Beckman Ti75 rotor. The second pellets (P2) contained mainly the nuclear membranes, plasma membrane sheets, mitochondria, and Golgi sheets. The resulting supernatant was referred as S. The two pellets were resuspended in 1.5 ml modified-RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.05% SDS, 1 mM Na3VO4, 15 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 1 mM PMSF), and S fractions were supplied with 45 mM Tris-HCl, pH 7.4, 125 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.05% SDS. Lysates were precleared with protein G or A Sepharose (50 μl of 1:1 slurry) for 1 h at 4°C, and then Sepharose was removed and lysates were incubated with mouse 9E10 anti–c-myc antibody (Sigma-Aldrich), or rabbit SRC2 anti-Src antibody (Santa Cruz Biotechnology, Inc.) for 4–8 h at 4°C. Protein G or A Sepharose was then added to the lysates (30 μl of a 1:1 slurry), and incubation at 4°C was pursued for another 1 h, after which the beads were recovered, washed three times, and boiled in SDS sample buffer. Samples were resolved on 10 or 12.5% SDS-PAGE, transferred to nitrocellulose, and processed for immunoblotting using either mouse 9E10 anti–c-myc, mouse Ab-1 anti–v-src antibody (Calbiochem-Novabiochem), or rabbit SRC2 anti-Src antibody (Santa Cruz Biotechnology, Inc.). In vitro kinase assays to measure autophosphorylation of FAK or Src kinase activity were performed 20–24 h after the transfection. Cells were washed in PBS and lysed in 1 ml modified RIPA buffer per 10-cm culture plate. After centrifugation at 11,000 g, supernatants (equal amounts of total proteins) were processed for immunoprecipitation as described above, using rabbit C-20 anti-FAK (Santa Cruz Biotechnology, Inc.) or Ab-1 anti-src (Calbiochem-Novabiochem). The immune complexes were washed and resuspended in 20 μl 2× kinase buffer (100 mM Tris-HCl, pH 7.0, 0.2% β-mercaptoethanol, 20 mM MgCl2, 0.2 mM Na3VO4). Reactions were started by addition of 10 μCi γ-[32P]ATP, allowed to proceed for 15 min at 30°C, and then stopped by addition of 20 μl of 3× SDS sample buffer. Samples were boiled, resolved on 10% SDS-PAGE, and visualized by autoradiography. To assess the tyrosine phosphorylation level of FAK, paxillin, and cortactin, cells were lysed in 250 μl boiling SDS buffer (10 mM Tris-HCl, pH 7.4, 1% SDS, 1 mM Na3VO4) per 10-cm culture plate, boiled for 5 min, and passed through a 26-gauge needle five times. 750 μl water and 1 ml 2× modified-RIPA buffer were added to the denatured lysates, and the lysates (equal amounts of proteins) were processed as above for immunoprecipitation using rabbit C-20 anti-FAK (Santa Cruz Biotechnology, Inc.), mouse 349 antipaxillin (Transduction Laboratories), mouse 4F11 anti-cortactin antibody (Upstate Biotechnology), or mouse PY20 antiphosphotyrosine (Transduction Laboratories). Tyrosine phosphorylation was analyzed by immunoblotting using mouse RC20–HRP antiphosphotyrosine antibody (Transduction Laboratories), and levels of phosphorylated FAK, paxillin, and cortactin recovered in the anti-PY20 immune complexes were detected by immunoblotting with C20 anti-FAK, mouse 349 antipaxillin, or mouse 4F11 anticortactin, respectively. Protein concentrations were determined using the Bio-Rad assay. For cell fractionation analysis, transfected 293T cells from 6-cm culture dishes were lysed in Triton buffer (50 mM Tris-Hcl, pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 10% glycerol, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4, 15 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 1 mM PMSF, and 50 mM NaF), vortexed, and incubated for 30 min on ice. The lysates were then centrifuged at 12,000 g for 15 min at 4°C, the pellets were resuspended in 1 vol of Triton buffer, and 1 vol of 2× SDS sample buffer was added to each fraction. Equal volumes of the supernatant (S) and pellet (I) fractions were analyzed by SDS-PAGE to determine the relative amount of myc–E4orf4, Src kinases, and mitogen-activated protein (MAP) kinase extracellular-regulated kinase (ERK) in each fraction by immunoblotting with mouse 9E10 anti–c-myc (Sigma-Aldrich), mouse Ab-1 anti-src (Calbiochem-Novabiochem), and rabbit anti-ERK, respectively. The anti-ERK is a rabbit polyclonal antibody raised against a synthetic peptide corresponding to the 14 COOH-terminal amino acids of rat ERK2 (Huot et al. 1995). Proteins were detected immunologically after electrotransfer onto nitrocellulose membranes as described previously (Lavoie et al. 1995). Horseradish peroxidase–linked goat anti–rabbit, or anti–mouse IgG (Jackson ImmunoResearch Laboratories), revealed by the enhanced chemiluminescence detection system (Renaissance; NEN Life Science Products) was used to detect the antigen–antibody complexes.
Immunofluorescence and Confocal Fluorescence Microscopy
Immunofluorescence analyses were performed in H1299 cells or 293T cells plated on fibronectin-coated culture slides (FALCON Biocoat; Becton Dickinson Co.) 10–24 h after transfection. Cells were washed in PBS containing 1 mM MgCl2, fixed in 3.7% formaldehyde/PBS for 20 min, and then permeabilized in 0.2% Triton X-100/PBS for 5 min. Immunodetection of E4orf4 proteins was performed using either mouse 9E10 anti–c-myc antibody (Sigma-Aldrich), mouse HA.11 anti-HA antibody (BabCO), or purified rabbit polyclonal 2418 anti-E4orf4, as indicated in the figure legends, using previously described methods (Lavoie et al. 1995). Mouse anticortactin antibody (Transduction Laboratories) was used to analyze cortactin distribution, rabbit SRC2 anti-src antibody was used to detect c-src, and mouse PY20 antiphosphotyrosine antibody was used to visualize tyrosine phosphorylation at focal adhesion, followed by BODIPY-labeled goat anti–mouse, or Texas Red–conjugated anti–rabbit IgG (Molecular Probes). Staining of actin filaments was performed as previously reported (Lavoie et al. 1993), using FITC-labeled phalloidin. The cells were examined by confocal microscopy as previously reported, using a Bio-Rad MRC-1024 imaging system mounted on a Nikon Diaphot-TDM equipped with an ×60 objective lens with a 1.4 numerical aperture (Lavoie et al. 1995).
Results
E4orf4 Expression Induces Membrane Blebbing and Inhibition of Fibronectin-induced Cell Spreading
The first manifestations of E4orf4 expression in many cell types is cell rounding and membrane blebbing. This early effect of E4orf4 was striking in transformed cells and Fig. 1 shows examples of the early extranuclear manifestations of apoptosis in 293T and H1299 cells. 10–20 h after transfection of E4orf4, a large fraction of the 293T cell population retracted from the culture plate adopting a rounded morphology, and started to undergo dramatic membrane blebbing (Fig. 1 A). E4orf4-transfected cells showed a large number of prominent membrane blebs that covered the entire cytoplasmic area (Fig. 1 A, arrows in b and c), and this effect persisted until the onset of cell death. This phenotype was dependent on E4orf4 expression because transfection of a E4orf4–GFP fusion in either H1299 or 293T cells induced prominent membrane blebbing very early after transfection (onset at 10 h after transfection), whereas expression of GFP alone had no effect on cell morphology (Fig. 1 B). Early changes in morphology induced by E4orf4 expression were independent of caspase activation, as they were still observed in the presence of the pan-caspase inhibitor zVAD-fmk (Fig. 1A and Fig. B) when used at a concentration that inhibited cell fragmentation induced by puromycin in the same cell lines (Fig. 1 B, bottom). E4orf4-induced membrane blebbing was observed very early and clearly preceded cell death in 293T cells, as judged by Trypan blue exclusion assays (data not shown). This suggested that the blebbing-inducing activity of E4orf4 could lie upstream in the death pathway.
Figure 1.
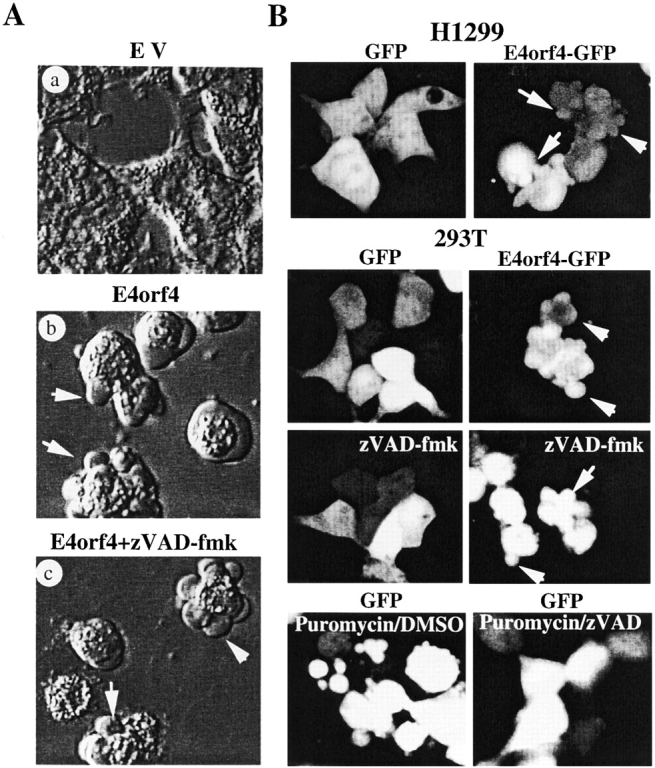
Expression of E4orf4 is associated with early membrane blebbing and inhibition of fibronectin-induced cell spreading. (A) 293T cells were transfected with either vector alone (a) or myc–E4orf4 (b and c), and 16–20 h after transfection cells were observed by phase contrast, using a Nikon Diaphot-TMD equipped with a Hoffman modulation system. The effect of zVAD-fmk was measured by adding the pan-caspase inhibitor (50 μM) at the time of transfection (c). Arrows in b and c show typical membrane blebs induced by E4orf4 expression. Each panel shows a representative field within the population of transfected cells, selected to show typical effects observed after we performed the same experiment at least four times. (B) 293T and H1299 cells were transfected with either FLAG–GFP (left) or FLAG–E4orf4–GFP (right), and 16–20 h after transfection positive cells were examined by fluorescence confocal microscopy. Arrows show E4orf4–GFP-positive cells undergoing membrane blebbing. The pan-caspase inhibitor (50 μM) was added at the time of transfection and did not affect E4orf4–GFP–induced membrane blebbing, whereas it strongly inhibited puromycin-induced cell fragmentation in GFP-transfected cells treated with 10 μM puromycin for 16 h (bottom, compare puromycin/DMSO versus puromycin/zVAD-fmk). (C) Fibronectin-induced spreading is inhibited in cells expressing E4orf4. 293T cells were transfected with either vector alone (a) or myc–E4orf4 (b). 20 h after transfection, cells were lifted up, resuspended in serum-free medium, transferred to fibronectin-coated culture dishes, and allowed to spread for 60 min at 37°C. Spreading of cells was observed by phase contrast, and panels show the representative phenotypic effects observed. Percentages of spread cells were obtained by counting the cells presenting a flat morphology with cellular extensions relative the total number of cells. At least 300 cells were counted for each condition and the data are representative of three independent experiments (means ± SE). Cell viability was assessed by Trypan blue exclusion on an aliquot of cells taken up before plating on fibronectin (data not shown), and no significant effect on survival was measured at that time. (D) Fibronectin adhesion is not inhibited by expression of E4orf4. 293T cells transfected with vector alone (EV) or myc–E4orf4 (E4orf4) were processed as in C and after a 1-h incubation period at 37°C cell adhesion to fibronectin was quantified as described in Materials and Methods. Attached cells were fixed and stained, and absorbance was measured at 550 nm after extraction. The right side shows image of a 96-well plate in which vector-only (EV) or E4orf4-transfected cells were allowed to attach, after extraction of the dye. Incubations with integrin β1 blocking antibody before cell plating strongly inhibited cell adhesion showing specific attachment on fibronectin. Data are the means ± SE of two independent experiments performed in duplicate (graphical analysis).
To further study the effects of E4orf4 expression on cell shape, transfections were performed into 293T cells and 20 h later the kinetics of cell spreading on fibronectin was analyzed in vector only (EV)–transfected cells as compared with cells transfected with E4orf4. 1 h after transferring cells to fibronectin-coated dishes in serum-free media, most of the empty vector-transfected cells had reattached to the culture dish and displayed a spread morphology (Fig. 1 C). In contrast, a majority of the cells transfected with E4orf4 remained round as spreading was inhibited by more than threefold over a 1-h period as compared with cells transfected with EV. This was not caused by cell death since at that time no significant effect on cell survival was observed (<10% cell death in EV- and E4orf4-transfected cells as measured by Trypan blue exclusion). Inhibition of cell spreading could be caused by E4orf4 interference with signals induced by cell adhesion, or by inhibition of cell adhesion itself. To discriminate between these two possibilities, we measured the ability of E4orf4-expressing cells to adhere to ECM proteins. Transfected 293T cells were processed as above and cell adhesion to fibronectin was quantitated as described in Materials and Methods after the 1-h incubation period. No significant difference was observed between control cells as compared with E4orf4-expressing cells that adhered as well to fibronectin (Fig. 1 D). When adhesion was measured after incubating cells with integrin β1–blocking antibody, adhesion to fibronectin was severely impaired in control and E4orf4-expressing cells, indicating that cell attachment to fibronectin was specific. As E4orf4-expressing cells were able to adhere to ECM but unable to spread, this suggests that E4orf4 may interfere with the propagation of adhesion signals.
E4orf4 Associates with Src Family Kinases
To determine whether E4orf4 could directly associate with cellular proteins involved in the formation of focal adhesion signaling complexes, pull-down assays were performed using a GST–E4orf4 fusion to absorb naive 293T cell lysates, and the absorbed material was analyzed by Western blot using different antibodies. When a pan-Src antibody recognizing multiple members of the src gene family was used, we detected a significant amount of Src family kinases bound to the GST–E4orf4 fusion as compared with GST alone (Fig. 2 A, left). The GST–E4orf4 fusion also pulled down the catalytic subunit of PP2A, as previously reported (Kleinberger and Shenk 1993). In contrast, E4orf4 binding to the adaptor protein NCK was barely detectable. The ability of E4orf4 to associate with Src was further analyzed using a GST fusion of mouse c-src to absorb lysates from 293T cells transfected with a myc-tagged E4orf4. Addition of epitope-tag at the NH2 terminus of E4orf4 has been shown previously to have no effect on its apoptotic activity (Lavoie et al. 1998), nor did it interfere with the localization of the protein (see Fig. 3 A and 7). The results show that the c-src fusion pulled down the expressed myc–E4orf4, further indicating that E4orf4 can associate with c-src in vitro (Fig. 2 A, right).
Figure 2.
(A) E4orf4 associates with Src kinases in vitro. Naive lysates of 293T cells were prepared as described in Materials and Methods and incubated with 4 μg of GST alone or with purified GST–E4orf4 bound to glutathione Sepharose. The beads were washed and resuspended in SDS sample buffer and the bound material was transferred onto nitrocellulose after SDS-PAGE. Immunological detections were performed using antibodies recognizing the PP2A catalytic subunit (positive control), Src family kinases, or NCK, and detection was performed by chemiluminescence. For myc–E4orf4 binding to recombinant GST–c-src, the assay was performed as described above, except that 293T lysates of cells transfected with myc–E4orf4 were incubated with 4 μg of either GST or GST–c-src bound to glutathione Sepharose. Total lysates (TL) represent 5% of the input lysates. (B) Coimmunoprecipitation of v-src with myc–E4orf4 in fractions of cellular membranes. Transfections of v-src with EV, or v-src together with myc–E4orf4 (E4orf4) were performed in 293T cells. 24 h after transfection, cells were homogenized and fractionated as described in Materials and Methods. Immunoprecipitations of myc–E4orf4 were performed in each cellular fraction using anti–c-myc antibody. The E4orf4 IPs were analyzed by SDS-PAGE followed by electrotransfer onto nitrocellulose, and immunological detections were performed using Ab-1 anti-src antibody, and anti–c-myc antibody. Immunoblots of total lysates (TL) show the levels of v-src expression and represent 5% of the input lysates. (C) Coimmunoprecipitation of myc–E4orf4 with endogenous Src family kinases. Myc–E4orf4 was transfected in 293T cells, and 24 h after transfection cells were processed for fractionation as in B. Immunoprecipitations of endogenous Src family kinases were performed in P1 and P2 fractions using a pan-src antibody (SRC2) which was replaced by unrelated rabbit serum in parallel experiments (IgG). The immune complexes were analyzed by Western blot analysis after SDS-PAGE and electrotransfer onto nitrocellulose. Anti–c-myc antibody revealed the presence of the myc–E4orf4 in Src family kinase IPs of the P2 fractions, whereas myc–E4orf4 was barely detected in IPs obtained using the unrelated rabbit serum. Immunoblots of total lysates (TL) show the levels of endogenous Src family kinases and transfected myc–E4orf4 and represent 2% of the input lysates.
Figure 3.
(A) Immunolocalization of c-src and E4orf4. 293T cells were transfected with c-src alone (a and b), HA–E4orf4 alone (c and d), c-src together with HA-orf4 (e and f), or activated c-src (Y527F) together with HA-orf4 (g and h) . Similar transfections were performed in H1299 cells except that a myc–E4orf4 construct was used (a′–h′). Double immunostaining using anti-SRC2 and anti-HA (a–h), or anti-SRC2 and anti-myc (a′–h′) was performed 24 h after transfection as described in Materials and Methods and specimens were analyzed by fluorescence confocal microscopy. Typical staining of exogenous c-src in 293T (b, d, f, and h) and H1299 cells (b′, d′, f′, and h′) are shown and E4orf4 staining in the same cells is presented in panels a, c, e, and g and a′, c′, e′, and g′. Arrows show c-src and E4orf4 colocalization in or surrounding membrane blebs. (B) Src kinase activity can modulate E4orf4 subcellular distribution. 293T cells were transfected with myc–E4orf4 alone, or together with c-src, kinase-dead c-src (K295M), activated c-src (Y527F), or v-src. Cell extractions were performed in 0.5% Triton buffer and total proteins from equal portions of the soluble (S) and insoluble (I) fractions were run on SDS-PAGE. The proportion of E4orf4, Src, and ERK in each fraction was evaluated on immunoblots using anti–c-myc, Ab-1 anti-src, and rabbit anti-ERK, respectively, and E4orf4 distributions were quantitated from scanned enhanced chemiluminescence-derived images by densitometric analyses with the software program NIH Image.
We then asked whether E4orf4–Src association could be detected in vivo. Myc–E4orf4 was cotransfected into 293T cells with a plasmid encoding v-src, and coimmunoprecipitations were performed on cellular fractions. Using this fractionation protocol, E4orf4 expression was enriched in the P2 fraction containing mainly cellular membranes. More importantly, up to 10% of the v-src expressed in the membrane fraction (P2) was recovered in myc–E4orf4 immune complexes (IPs), indicating that a subpopulation of E4orf4 present in cellular membranes was associated with v-src in vivo (Fig. 2 B, left). Moreover, E4orf4–Src association also was detected upon immunoprecipitation of endogenous Src family kinases in 293T cells transfected with myc–E4orf4 using a pan-src antibody (Fig. 2 C; SCRC2 IP). Parallel control experiments using unrelated rabbit serum did not precipitate myc–E4orf4 protein in similar conditions (Fig. 2 C, IgG IP). The association between E4orf4 and Src family kinases was quite robust, since complexes were recovered under relatively stringent conditions (in modified RIPA containing SDS). Coimmunoprecipitation of E4orf4 with endogenous Src family kinases was successfully performed in CHO fibroblasts as well, indicating that E4orf4 association with Src kinases is not restricted to 293T cells (data not shown).
E4orf4 and c-src Colocalize in Membrane Blebs and E4orf4 Association with the Cytoskeleton is Promoted by Overexpression of Activated Src Kinases
To study the physiological relevance of E4orf4 association with Src family kinases, the subcellular distribution of the two proteins was analyzed by immunofluorescence and confocal microscopy. Cellular staining of endogenous Src family kinases using rabbit SRC2 antibody was very faint and close to background (Fig. 3 A, d and d′). Therefore, c-src and activated c-src were overexpressed in either 293T or H1299 cells to allow a better detection of its subcellular localization. As previously reported (Reynolds et al. 1989; Kaplan et al. 1994; Schaller et al. 1999), exogenous c-src exhibited a diffuse staining pattern with prominent perinuclear and membrane staining in the two cell lines (Fig. 3 A, b and b′). Immunostaining of NH2-terminal epitope-tagged versions of E4orf4 (HA–orf4, myc–E4orf4; Fig. 3a and Fig. b), or non-tagged E4orf4 (Fig. 7), using anti-HA, anti-myc, or purified rabbit anti-E4orf4 in either 293T or H1299 cells gave similar staining patterns (Lavoie et al. 1998). E4orf4 protein was diffusely distributed throughout whole cells but in a majority of cells a stronger staining was detected at the nuclear level (Fig. 3 A, c and c′), although blebbing cells demonstrated a stronger cytoplasmic staining (see also Fig. 7 B). When E4orf4 and c-src were coexpressed, the cytoplasmic staining of E4orf4 appeared more intense (Fig. 3 A, e and e′,). More interestingly, E4orf4 and c-src were colocalized in or surrounding membrane blebs. E4orf4 and c-src colocalization in membrane area was even more striking in cells expressing an activated c-src (Fig. 3 A, g, h, g′ and h′). This suggests that subpopulations of E4orf4 and c-src proteins are localized in close proximity within membrane regions that are involved in bleb formation.
Figure 7.

E4orf4-induced focal adhesion misassembly, actin changes, and translocation of cortactin to the cell periphery. (A) Redistribution of tyrosine phosphorylated proteins at the cell periphery, and increased size of focal contacts in E4orf4-expressing cells. H1299 were plated on fibronectin-coated culture slides and transfected with vector alone (a and b) or E4orf4 (c–f). 16 h after transfection, cells were fixed and double stained for antiphosphotyrosine (a, c, and e) and E4orf4 (b, d, and f), using PY20 antiphosphotyrosine and rabbit 2418 anti-E4orf4, respectively. Specimens were analyzed by fluorescence confocal microscopy. Panel a shows the ventral face of representative control cells to visualize the organization of tyrosine phosphorylated proteins at focal contacts. Arrows in b and e show increased focal contacts formed by tyrosine phosphorylated proteins that relocated at the cell periphery and are more apical in E4orf4-expressing cells, as they started to adopt a rounder morphology before the onset of membrane blebbing. (B) E4orf4-induced reorganization of the actin cytoskeleton. 293T cells were transfected with vector only (a and b) or with HA–E4orf4 (c–j), and 24 h after transfection cells were fixed and double stained for actin filaments and E4orf4 using FITC-labeled phalloidin and anti-HA followed by Texas Red–labeled anti–mouse, respectively. Specimens were analyzed by fluorescence confocal microscopy and representative control cells (a and b) or E4orf4-expressing cells in various stages of blebbing (c–j) are shown. Panels c–h show examples of E4orf4-expressing cells in the early stages of membrane blebbing when actin reorganized into very dense fibers at the cell periphery (arrows), whereas i and j show a typical E4orf4-expressing cell in the active-late stage of membrane blebbing, when actin accumulated around membrane blebs that contain high amounts of E4orf4 protein. (C) Expression of E4orf4 induces early redistribution of cortactin at the cell periphery and in membrane blebs. H1299 seeded in fibronectin-coated culture slides were transfected with vector only (a and b) or with E4orf4 (c–f). 24 h after transfection, cells were fixed and double stained for endogenous cortactin (a, c, and e) and transfected E4orf4 (b, d, and f) using mouse anticortactin (Transduction Laboratories) and rabbit 2418 anti-E4orf4, respectively. Specimens were analyzed by fluorescence confocal microscopy and representative cells are shown. In typical control cells (EV), cortactin was present diffusely in cytoplasmic and perinuclear regions (a and b). In E4orf4-expressing cells that started to round up, cortactin was enriched at the cortical membrane, and colocalized with E4orf4 in membrane protrusions (arrows in c and d). Panels e and f show E4orf4-positive cells undergoing active blebbing, in which cortactin and E4orf4 staining were enriched in membrane blebs.
Previous studies have shown that activated c-src partitions to Triton-insoluble cytoskeletal complexes (Clark and Brugge 1993; Kaplan et al. 1995; Schlaepfer et al. 1998). To look biochemically at the effect of Src kinases on the subcellular distribution of E4orf4, 293T cells were transfected with myc–E4orf4 alone, or with myc–E4orf4 together with c-src, kinase-dead c-src (c-srcK295M), activated c-src (c-src Y527F), or v-src. Cell lysis was performed in 0.5% Triton buffer to analyze E4orf4 distribution in the soluble (S) and insoluble (I) fractions. 24 h after transfection, ∼30% of E4orf4 protein was recovered in the insoluble fraction, indicating that a subpopulation of E4orf4 is associated with cytoskeletal components (Fig. 3 B). In cells cotransfected with wild-type c-src or kinase-deficient c-src, no major change in the distribution of E4orf4 was observed, although a modest increase in the amount of insoluble E4orf4 could be detected when wild-type c-src was overexpressed. In contrast, when activated c-src or v-src was coexpressed together with E4orf4, >60–90% of E4orf4 protein was associated with the cytoskeletal fraction, together with increased levels of src species. However, the cellular distribution of endogenous MAP kinase ERK in the same lysates remained unaffected (Fig. 3 B, bottom). Interestingly enough, we observed an increase in E4orf4 distribution to cytoskeletal fractions in absence of activated Src 48 h after transfection (>55%; data not shown). This suggests that translocation of E4orf4 to a cytoskeletal compartment may be associated with cell progression through the death pathway. Taken together, the results indicate that a subpopulation of E4orf4 is associated with the cytoskeleton and that overexpression of activated Src kinases leads to increased levels of E4orf4 in cytoskeletal complexes.
Expression of E4orf4 Leads to a Specific Modulation of Src-mediated Tyrosine Phosphorylation of Cellular Substrates and Dysregulates Src-dependent Signaling at Focal Adhesion
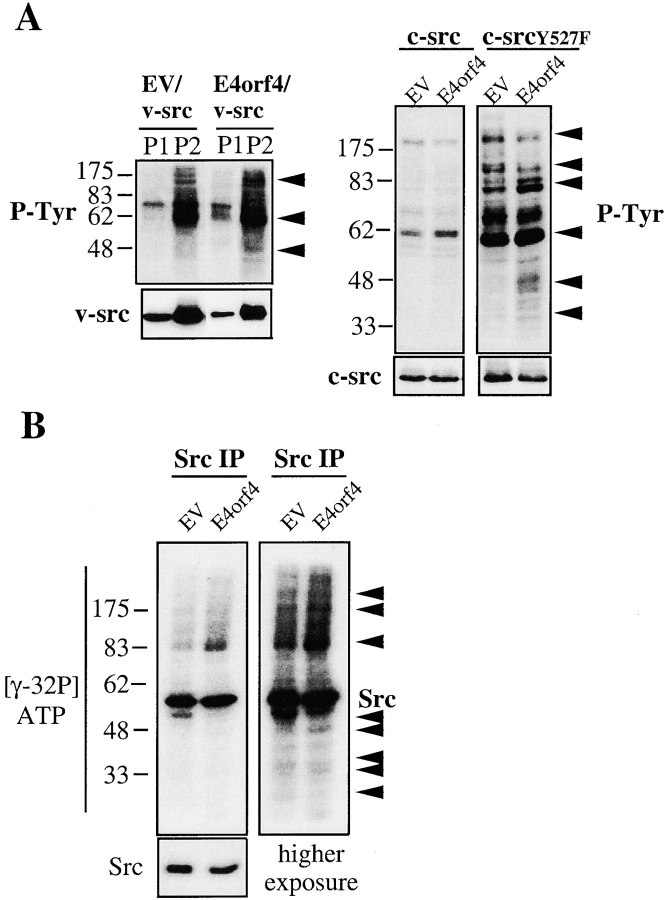
To determine if E4orf4–Src association could have some functional relevance, we looked at the tyrosine phosphorylation of cellular proteins induced by overexpression of Src kinases, in the presence or absence of E4orf4 expression. Expression of E4orf4 did not induce a general up- or downregulation of v-src, c-src, or activated c-src–dependent tyrosine phosphorylation of cellular proteins (Fig. 4A and Fig. B). Rather, there was a decrease in the tyrosine phosphorylation of specific proteins (200-, 125-kD ranges), whereas some other proteins presented higher levels of tyrosine phosphorylation (80–85-, 62-, 45–48-kD ranges) in E4orf4/Src–expressing cells, as compared with cells expressing comparable amounts of Src proteins alone (Fig. 4 A, arrows). Furthermore, in vitro–labeled IPs of endogenous Src isolated from E4orf4-only expressing cells presented differences in the intensity of Src-associated proteins as compared with control cells (EV). This indicates that E4orf4 expression caused a modulation of endogenous Src association with and phosphorylation of cellular proteins (Fig. 4 B). However, no major difference was observed between the in vitro kinase activity of c-src isolated from E4orf4-expressing cells and from that of cells expressing c-src alone, as measured on an exogenous peptide substrate (data not shown). This suggests that E4orf4 does not act primarily on Src intrinsic tyrosine kinase activity but may rather lead to a modulation of Src-dependent tyrosine phosphorylation of specific substrates.
Figure 4.
Effect of E4orf4 expression on Src kinase-induced tyrosine phosphorylation of cellular proteins. (A) 293T cells were transfected with v-src alone (EV/v-src) or together with E4orf4 (E4orf4/v-src); with c-src alone (c-src/EV) or together with E4orf4 (c-src/E4orf4); or with activated c-src alone (c-srcY527F/EV) or together with E4orf4 (c-srcY527F/E4orf4). 24 h after transfection, cells were lysed either in hypotonic buffer and fractionated in nuclear-enriched (P1) and membrane-enriched (P2) fractions, or in SDS sample buffer. Equal amounts of total proteins were run on SDS-PAGE and tyrosine phosphorylation of cellular proteins was analyzed by immunoblotting using RC20-HRPO antiphosphotyrosine. Immunoblots with Ab-1 anti-src show the level of transfected v-src, and c-src species were detected using SRC2 anti-src. Arrows indicate proteins presenting a decreased or increased level of tyrosine phosphorylation in cells expressing E4orf4. (B) 293T cells were transfected with EV or with myc–E4orf4 (E4orf4). 24 h after transfection, cells were lysed in modified RIPA, endogenous c-src was immunoprecipitated from an equal amount of total proteins (800 μg), and Src IPs were labeled by the addition of γ-[32P]ATP. Labeled Src IPs were resolved by SDS-PAGE and visualized by autoradiography (short and long exposures are shown). Arrows indicate Src-associated proteins differentially labeled in the IPs isolated from E4orf4-expressing cells.
Strong biochemical and genetic evidence has implicated Src family kinases in integrin-mediated signaling at focal adhesions and this event is concomitant with rapid tyrosine phosphorylation of focal adhesion proteins (Parsons 1996; Burridge and Chrzanowska-Wodnicka 1996; Hanks and Polte 1997; Klinghoffer et al. 1999). Recruitment of Src family kinases to integrin-based signaling complexes is thought to occur primarily via interaction with another tyrosine kinase, FAK (Schlaepfer and Hunter 1996; Cobb et al. 1994; Schlaepfer et al. 1994; Schaller et al. 1999). The subsequent phosphorylation of FAK by Src family kinase at multiple tyrosine residues is believed to increase its catalytic activity and allow further interactions with other signaling proteins such as GRB2 (Schlaepfer and Hunter 1996; Calalb et al. 1995; Schlaepfer et al. 1998). To characterize the effect of E4orf4 on Src-dependent signaling and to gain insight into the molecular mechanisms involved in E4orf4-dependent inhibition of fibronectin-induced cell spreading, we studied whether early expression of E4orf4 could affect the ability of Src to send downstream signals to focal adhesion proteins. Overexpression of v-src in H1299 or 293T cells led to an increase in the tyrosine phosphorylation level of endogenous FAK, which was associated with a dramatic increase in its autophosphorylation (Fig. 5 A). This was consistent with a previous report showing that Src-dependent phosphorylation of FAK in the kinase domain at Tyr-576–Tyr-577 enhances FAK catalytic activity (Calalb et al. 1995). In marked contrast, the tyrosine phosphorylation level of endogenous FAK was not increased by v-src when E4orf4 was coexpressed, nor was the autophosphorylation stimulated in these cells, indicating that E4orf4 expression led to a strong inhibition of v-src–induced FAK phosphorylation and activation. This was not due to inhibition of v-src expression by E4orf4, as equivalent expression levels were detected in E4orf4-expressing cells (Fig. 5 A, TL). Furthermore, a 50% decrease in the basal level of FAK phosphorylation was observed in cells expressing E4orf4 only, suggesting that E4orf4 interfered with FAK phosphorylation by endogenous Src as well.
Figure 5.
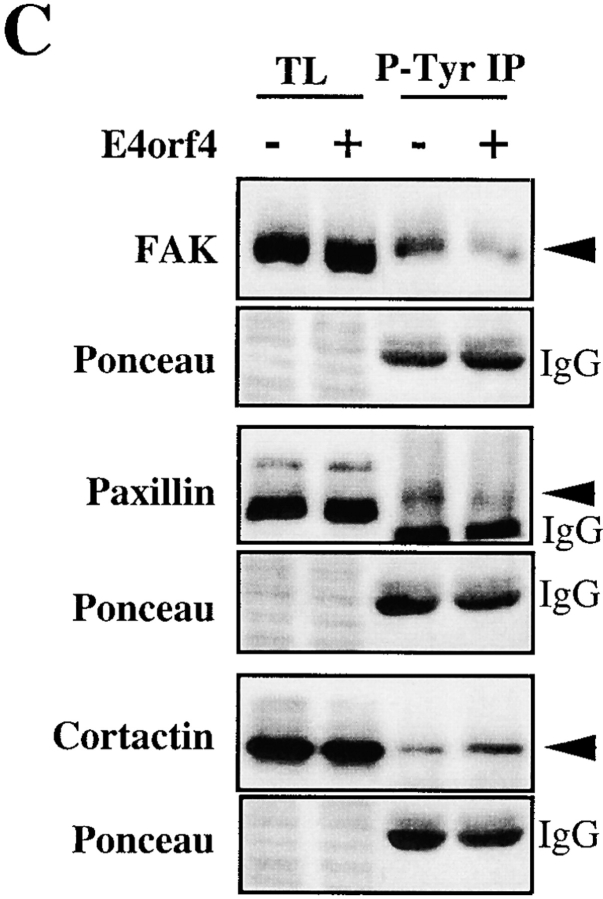
Expression of E4orf4 inhibits tyrosine phosphorylation of FAK and paxillin, whereas it increases tyrosine phosphorylation of cortactin. (A) H1299 cells were transfected with EV (EV−), Flag–E4orf4 alone (E4orf4−), or v-src alone (EV+) or together with Flag–E4orf4 (E4orf4+). 20 h after transfection, cells were lysed as described in Materials and Methods and endogenous FAK was immunoprecipitated. Tyrosine phosphorylation was analyzed by immunoblotting of equal amounts of FAK IPs using RC20-HRPO antiphosphotyrosine antibody (P-Tyr), after SDS-PAGE and electrotransfer onto nitrocellulose. Western blot analysis of the IPs with anti-FAK is also shown, and the levels of E4orf4 and v-src in total lysates (TL) were analyzed by blotting with anti-Flag and Ab-1 anti-src, respectively. Autophosphorylation of endogenous FAK was also analyzed in H1299 and 293T cells transfected as above, by incubating the FAK immune complexes with γ-[32P]ATP. Labeled FAK IPs were resolved by SDS-PAGE and visualized by autoradiography. (B) 293T cells were transfected as in A and cells were lysed as described in Materials and Methods 24 h after transfection. Half of the lysates were immunoprecipitated with antipaxillin antibody, and the other half with anticortactin. Paxillin, cortactin, and tyrosine phosphorylation levels of IPs were analyzed by Western blot using appropriate antibodies as indicated. The levels of expression in total lysates for transfected E4orf4 and v-src, and for endogenous paxillin and cortactin are shown (TL). Note the decrease in v-src–induced tyrosine phosphorylation of paxillin and the increased level of cortactin phosphorylation by v-src in lysate from E4orf4-expressing cells. (C) 293T cells were transfected with vector alone (−) or with myc–E4orf4 (+) and 24 h after transfection cells were lysed and equal amounts of lysates (1.5 mg) were processed for immunoprecipitation using PY20 antiphosphotyrosine antibody. Equal amounts of IPs were resolved by SDS-PAGE and analyzed by immunoblotting for FAK, paxillin, and cortactin using appropriate antibodies. The results show that in the same lysate isolated from E4orf4-expressing cells, there was less phosphorylated FAK and paxillin (74 and 50% inhibition, respectively), whereas phosphorylated cortactin was more abondant (3.5-fold increase). The levels of FAK, paxillin, and cortactin in IPs from control cells as compared with E4orf4-expressing cells were quantitated from scanned enhanced chemiluminescence–derived images by densitometric analyses with the software program NIH Image, and the results were corrected for transfection efficiency (50% in 293T cells). Ponceau staining of total lysates and IgGs show equivalent amounts for the various analyses. Ponceau staining of total proteins on SDS-PAGE is shown as loading control.
We next examined the v-src phosphorylation of two other proteins: paxillin, a cytoskeletal protein that is tyrosine phosphorylated in response to cell adhesion and is a likely substrate for FAK (Schaller and Parsons 1995; Burridge et al. 1992), and cortactin, an F-actin binding protein that is a major substrate for Src family kinases (Nada et al. 1994). As a consequence of E4orf4 expression, the basal level of paxillin tyrosine phosphorylation was severely decreased (by 60–70%) and in E4orf4/v-src–expressing cells, paxillin phosphorylation was also strongly inhibited as compared with cells expressing v-src only (Fig. 5 B). This was consistent with inhibition of v-src–dependent activation of FAK in E4orf4-expressing cells. In contrast, E4orf4 expression had no inhibitory effect on the tyrosine phosphorylation of cortactin induced by v-src expression, but rather increased the phosphorylation of this protein in the same lysates (Fig. 5 B). Interestingly, although the p80/85 anticortactin antibody most frequently recognized an 80-kD band on Western Blot analysis of 293T lysates (TL), it repeatedly immunoprecipitated doublet bands (70–80-kD ranges) that could result from degradation. However, the basal level of cortactin tyrosine phosphorylation was barely detectable in 293T in these conditions.
To study further the effect of E4orf4 on the tyrosine phosphorylation of Src substrates in the absence of coexpressed activated Src, 293T cells were transfected with EV or with E4orf4, and tyrosine phosphorylated proteins were immunoprecipitated from equal amounts of denatured cell lysates using antiphosphotyrosine antibody. The amounts of endogenous FAK, paxillin and cortactin recovered in IPs of tyrosine phosphorylated proteins were then determined by immunoblotting of equal amounts of IPs with anti-FAK, antipaxillin, and anticortactin, respectively (Fig. 5 C). Once again, a strong decrease was observed in the amount of FAK and paxillin proteins recovered in antiphosphotyrosine IPs of E4orf4-expressing cells as compared with control cells (74% and 50% inhibition, respectively), further indicating that E4orf4 expression inhibited the basal tyrosine phosphorylation of FAK and paxillin. In contrast, there was a 3.5-fold increase in the amount of cortactin recovered in antiphosphotyrosine IPs from E4orf4-expressing cells as compared with control cells, suggesting that tyrosine phosphorylation of cortactin was increased in the same lysate of cells expressing E4orf4. Because we never succeeded in showing directly increased cortactin phosphorylation in cortactin immunoprecipitations (phosphotyrosine levels were undetectable in our cell system), we can not completely rule out the possibility that the increased recovery of cortactin in phosphotyrosine immunoprecipitations may result from E4orf4-mediated changes in tyrosine phosphorylation of a cortactin-associated protein. However, it is unlikely considering that the lysates were denatured before immunoprecipitation. At any rate, these observations strongly suggest that expression of E4orf4 causes a specific inhibition of Src-dependent tyrosine phosphorylation of focal adhesion proteins, whereas it increases the tyrosine phosphorylation of other Src substrates and, presumably, of cortactin.
A recent study reported that the simultaneous overexpression of FAK and c-src results in the enhancement of the tyrosine phosphorylation of a limited number of cellular substrates, and that under these conditions paxillin phosphorylation is largely cell adhesion dependent (Schaller et al. 1999). To further study the effect of E4orf4 expression on Src-dependent signaling at focal adhesion, we examined whether early expression of E4orf4 could affect the tyrosine phosphorylation of cellular proteins induced by coexpression of FAK and c-src after transferring cells to fibronectin-coated dishes in serum-free media for a 1-h period. Overexpression of c-src alone induced an increase mainly in the tyrosine phosphorylation of 60–62-kD proteins in 293T cells (see Fig. 4 A for a higher exposure), whereas overexpression of FAK alone resulted in little change in the tyrosine phosphorylation pattern of cellular proteins, except for the exogenous FAK itself that was tyrosine phosphorylated (Fig. 6), as previously shown (Schaller and Parsons 1995; Schaller et al. 1999). However, when c-src and FAK were simultaneously expressed, a dramatic increase in the tyrosine phosphorylation of cellular proteins was detected (Fig. 6, c-src+FAK), indicating that FAK and c-src cooperated to enhance the tyrosine phosphorylation of cellular substrates. Coexpression of E4orf4 together with FAK and c-src induced a strong inhibition of the FAK/c-src–dependent stimulation of tyrosine phosphorylation. This was not due to inhibition of FAK and c-src expression by cotransfection of E4orf4, since equivalent expression levels of the tyrosine kinases were detected (compare myc-FAK, c-src, and myc–E4orf4 immunoblots in EV- versus E4orf4-transfected cells).
Figure 6.
E4orf4 inhibits the tyrosine phosphorylation of cellular substrates induced by coexpression of c-src and FAK. 293T cells were transfected with c-src alone, myc-FAK alone, c-src together with myc-FAK, or myc–E4orf4 alone, myc–E4orf4 and c-src, myc–E4orf4 and myc-FAK, or a combination of the three plasmids. 24 h after transfection, cells were lifted, resuspended in serum-free medium, transferred to fibronectin-coated dishes, and incubated at 37°C for a 1-h period before cell lysis in SDS sample buffer. Equal amounts of total proteins were run on SDS-PAGE followed by electrotransfer on nitrocellulose, and cell adhesion–dependent tyrosine phosphorylation induced by coexpression of c-src and FAK was evaluated in E4orf4-expressing cells (E4orf4), as compared with control cells transfected with the corresponding empty vector (EV). Immunoblotting was performed using RC20-HRPO antiphosphotyrosine to visualize tyrosine phosphorylation of cellular proteins, or with anti–c-myc and Ab-1 anti-src to detect the expression levels of E4orf4 and FAK, or c-src, respectively.
E4orf4 Expression Causes a Reorganization of Tyrosine Phosphorylated Proteins at Focal Adhesions, Actin Changes, and Relocation of Cortactin at the Cell Periphery
To correlate the early morphological changes observed in E4orf4-expressing cells with E4orf4-induced dysregulation of Src tyrosine phosphorylation, we examined the functional consequences of E4orf4 expression on the localization of tyrosine phosphorylated proteins at focal adhesions. Proteins phosphorylated on tyrosine are normally concentrated in focal adhesions, forming a typical pointed pattern along the ventral face of spread cells (Fig. 7 A, a) (Burridge et al. 1992). In E4orf4-expressing cells, the tyrosine phosphorylated proteins accumulated in patches, forming bigger focal contacts clustered at the periphery of cells adopting a rounder morphology (Fig. 7 A, c and e). This phenotype was similar to that reported in FAK-deficient cells (Ilic et al. 1995) and correlated with inhibition of FAK phosphorylation in E4orf4-expressing cells. This suggests that E4orf4 may trigger early changes in focal adhesions resulting in a more static and round cell shape typical of the release phase of extranuclear apoptosis (Mills et al. 1999). We then looked at the organization of actin fibers that anchor at focal adhesions. Actin staining using FITC-labeled phalloidin in 293T cells revealed a pattern of very thin fibers spanning the ventral face of cells (Fig. 7 B, a). Early expression of E4orf4 produced a dramatic rearrangement of actin into dense fibers that accumulated at the periphery of the cells (Fig. 7 B, c–h), and later around membrane blebs (Fig. 7 B, i and j). As actin is believed to provide the force generation needed for membrane blebbing (Mills et al. 1998), the redistribution of focal adhesions that correlated with formation of a dense actin network at the periphery of E4orf4-expressing cells may underlie the accumulation of F-actin required for membrane blebbing (Huot et al. 1998).
Cortactin is believed to be involved in the organization and remodeling of the cortical actin skeleton (Vuori and Ruoslahti 1995; Patel et al. 1998). The protein has recently been shown to translocate to the plasma membrane after growth factor stimulation (Weed et al. 1998) and hypertonicity-induced cell shrinkage (Kapus et al. 1999). Although the role of its tyrosine phosphorylation has not been clarified entirely, cytoskeletal changes have been associated with it (Wu et al. 1991; Nada et al. 1994; Huang et al. 1997). Because E4orf4 expression appeared to increase Src-mediated phosphorylation of cortactin, we examined the subcellular localization of cortactin in E4orf4-expressing cells as compared with cells transfected with EV. Control cells displayed cortactin staining within the cytoplasm and perinuclear area as previously shown in other cell types (Huang et al. 1998; Fig. 7 C, a). However, in cells expressing E4orf4, the distribution of cortactin was altered, being enriched at the cortical membrane in regions where membrane protrusions started to form (Fig. 7 C, c and d). Interestingly, E4orf4 also localized to these cortical regions, and in cells undergoing blebbing cortactin and E4orf4 were enriched in membrane blebs (Fig. 7 C, e and f). Thus the increased phosphorylation of cortactin correlated with its redistribution to cortical structures in E4orf4-expressing cells adopting a rounder morphology, although it is not yet clear whether the tyrosine phosphorylation of cortactin is required for its relocation to membrane blebs.
E4orf4-induced Membrane Blebbing and Nuclear Condensation Is Regulated by Src Kinase Activity
To assess the role of Src family kinases in E4orf4-mediated membrane blebbing and apoptosis, a cotransfection assay was developed in 293T cells, using GFP as a reporter to visualize the cellular morphology in live cells. Overexpression of activated mouse or chicken c-src alone did not induce membrane blebbing, but when expressed together with E4orf4, the number of GFP-positive blebbing cells was increased by more than twofold as compared with cells expressing E4orf4 alone (Fig. 8 A). Furthermore, the extent of blebbing within individual cells was markedly potentiated in terms of number and size of blebs per cell, which showed a “popcorn-like” morphology (Fig. 8 A, arrows). In contrast, coexpression of the kinase-deficient mouse and more significantly of the kinase-dead chicken c-src together with E4orf4 consistently inhibited the appearance of blebbing cells (50% inhibition with chicken kinase-dead c-src/ccsrcK295R). More strikingly, positive cells demonstrated a flat morphology and the size and number of blebs within individual cells was strongly decreased. In these conditions, the kinase-deficient chicken c-src construct was better expressed in 293T cells than was the mouse c-src, which may explain the stronger inhibition of E4orf4-induced membrane blebbing; when higher levels of expression were achieved with mouse c-srcK295M, E4orf4 expression was strongly increased and no clear interpretations could be made. A strong correlation was established between the extent of membrane blebbing observed 24 h after transfection and E4orf4-dependent DNA condensation 48 h after transfection. Again, coexpression of activated c-src constructs and E4orf4 produced more than twofold increase in the number of GFP-positive cells presenting an apoptotic nuclear morphology, and the extent of nuclear shrinkage and condensation was also higher. On the other hand, expression of kinase-deficient c-src constructs to a similar level inhibited the appearance of apoptotic nuclei by 30–50% as compared with cells expressing E4orf4 only (Fig. 8 B). Additionally, the extent of E4orf4-dependent DNA condensation was markedly decreased as revealed by a smaller degree of nuclear shrinkage and lower intensity in DAPI staining (Fig. 8 B, arrows). As the level of E4orf4 expression was similar in cells expressing the activated c-src and the kinase-deficient c-src, the difference measured in E4orf4-mediated membrane blebbing and apoptosis probably resulted from differences in Src kinase activity (Fig. 8 C).
Figure 8.
E4orf4-induced membrane blebbing and apoptosis is modulated by Src kinase activity. (A) 293T cells were transfected with 0.25 μg of Flag-GFP and 3 μg of empty vector (GFP+EV) alone, or together with 1 μg of activated mouse or chicken c-src (mcsrcY527F/ccsrcY527F) or 1 μg of mouse or chicken kinase-deficient c-src (mcsrcK295M/ccsrcK295R). In parallel experiments, similar transfections were performed except that the empty vector was replaced by myc–E4orf4 (2μg; GFP+E4orf4). 24 h after transfection, the cellular morphology of transfected cells was examined in live cells and typical blebbing phenotypes of E4orf4-expressing cells are presented on the right. Arrows show the “popcorn-like” morphology of GFP-positive cells cotransfected with E4orf4 and activated c-src plasmids that presented a higher number and size of blebs in individual cells, as compared with GFP-positive cells transfected with E4orf4+GFP only. At least 300 GFP-positive cells were counted for each transfection and the percentage of blebbing cells was determined from the number of GFP-positive cells that arbored more than one bleb per cell relative to the total number of GFP-positive cells (graphical representation on the right). The data are the means ± SE of at least three independent experiments. (B) The same cells were kept at 37°C for an additional 24 h (48 h after transfection), following witch non-adherent and adherent cells were washed in PBS and the nuclear morphology was analyzed by DAPI staining after cell fixation. Specimens were analyzed by fluorescence microscopy and representative GFP-positive cells (GFP staining) with the corresponding nuclear staining (DAPI) are presented for each transfection. Arrows show GFP-positive cells that presented typical E4orf4-dependent apoptotic nuclear morphology. Percentages of apoptotic nuclei were obtained by counting at least 300 GFP-positive cells for each condition and are expressed as the number of cells presenting nuclear condensation relative to the total number of GFP-positive cells. The data are representative of at least three independent experiments (means ± SE). (C) Before cell fixation, aliquots of each cell population were kept and lysed in SDS sample buffer. Expression levels of exogenous proteins were analyzed by immunoblotting with the corresponding antibodies as indicated and tyrosine phosphorylation of cellular proteins was visualized using RC20-HRPO antiphosphotyrosine for immunoblot analysis of equal amounts of total proteins run on SDS-PAGE followed by electrotransfer onto nitrocellulose (Ponceau staining of gels are shown as loading controls).
As another approach, we used chemical inhibitors in attempts to reverse the blebbing phenotype in 293T cells expressing high levels of E4orf4. 24 h after E4orf4/GFP transfection, cells were incubated for 1 h in the presence of either a selective inhibitor of Src family kinase activity, PP2 (Hanke et al. 1996), or genistein, a general tyrosine kinase inhibitor. Additionally, PP3, which has no inhibitory effect on Src family kinase activity but inhibits the activity of EGFR kinase (Traxler et al. 1997), was used as a negative control, and the actin polymerization inhibitor cytochalasin D was used as a positive control to inhibit membrane blebbing, as previously reported (Huot et al. 1998; Mills et al. 1998). After a 1-h incubation period in the presence of cytochalasin D, E4orf4-dependent membrane blebbing was strongly inhibited as compared with cells treated with vehicle only (Fig. 9A and Fig. B). This was consistent with the notion that the actin skeleton is required for membrane blebbing. More importantly, inhibition of membrane blebbing by cytochalasin D correlated with a marked decrease in the number of E4orf4-expressing cells presenting apoptotic nuclear morphology 48 h after transfection (24 h after cytochalasin D addition). This strongly suggests that the early morphological changes that precede cell death are involved in signaling execution of E4orf4-dependent death pathway. Significantly, E4orf4-induced membrane blebbing was similarly inhibited after a 1 h-treatment in the presence of PP2 (Fig. 9A and Fig. B), when used at a concentration that inhibited the tyrosine phosphorylation of cellular proteins induced by overexpression of activated c-src in parallel experiments (data not shown). However, under the same experimental conditions, PP3 did not interfere with E4orf4-induced membrane blebbing but rather consistently produced a modest increase, whereas genistein decreased E4orf4-dependent blebbing less efficiently than PP2. Consistent with a functional role for E4orf4-dependent modulation of Src activity, PP2 produced a twofold decrease in the number of apoptotic E4orf4-expressing cells 48 h after transfection (Fig. 9 B). Taken together, the results strongly suggest that modulation of Src kinase activity by E4orf4 is involved in triggering E4orf4-dependent membrane blebbing and apoptosis.
Figure 9.
Src family kinase activity is required for maximal E4orf4-induced membrane blebbing and apoptosis. (A) 293T cells were transfected with 3 μg of myc–E4orf4 together with 0.25 μg of Flag–E4orf4. 24 h after transfection, various drugs were added in individual cultures of E4orf4-expressing cells as follows: 0.2% DMSO (vehicle only), 1 μM cytochalasin D, 50 μM PP2, 100 μM genistein, or 50 μM PP3. 1 h after addition of the drugs, the cellular morphology of GFP-positive cells was examined. Arrows show cells undergoing E4orf4-dependent membrane blebbing, which was strongly inhibited in cell populations treated with cytochalasin D or with the Src family kinase inhibitor PP2. (B) Quantification of the effects of chemical drugs on E4orf4-dependent membrane blebbing and on E4orf4-dependent apoptosis 24 h after addition of the drugs (48 h after E4orf4 transfection). 1 h after addition of the drugs, at least 300 GFP-positive cells were counted for each treatment (described in A) and the percentage of blebbing cells was determined from the number of GFP-positive cells arboring more than one bleb per cell relative to the total number of GFP-positive cells. The data are representative of at least three independent experiments. Cells were then kept for an additional 24 h at 37°C, following which non-adherent and adherent cells were washed in PBS and the nuclear morphology was analyzed by DAPI staining after cell fixation. Specimens were analyzed as described in Fig. 8 B, and the percentages of apoptotic nuclei were obtained by counting at least 300 GFP-positive cells for each condition and are expressed relative to the number of cells presenting nuclear condensation relative to the total number of GFP-positive cells. The data are representative of at least three independent experiments. (C) Before cell fixation, aliquots of each cell population were kept and lysed in SDS sample buffer. Expression levels of exogenous Flag–GFP and myc–E4orf4 were analyzed by immunoblotting with anti-Flag and anti–c-myc antibodies, respectively, and show equivalent amounts of E4orf4 for each condition.
Discussion
We have shown previously that expression of Ad E4orf4 is sufficient to trigger a p53-independent apoptosis pathway that does not require activation of the zVAD-fmk–inhibitable caspases (Lavoie et al. 1998). Our present study provides two original and important findings. First, the results strongly suggest that E4orf4 action as a death factor may rely on its ability to associate with and dysregulate Src family kinase signaling at focal adhesions. Second, evidence is provided that E4orf4-induced early membrane blebbing is modulated by Src family kinase activity and is involved in triggering execution of the death pathway. Finally, the data obtained using E4orf4 as a model system suggest that modulation of Src family kinases could play a role in the cytoplasmic execution of apoptotic pathways, characterized by caspase-independent changes in cellular morphology and membrane blebbing.
Src Family Kinases and Downstream Signaling Pathways As Direct Targets of the Ad E4orf4 Death Factor
When looking at the early steps of E4orf4-induced cell death, we found that cells undergo a rapid change in morphology associated with cell rounding, membrane blebbing, and inhibition of fibronectin-induced cell spreading. This phenotype appeared as early as 10 to 16 h after transfection and before the onset of DNA condensation in 293T cells, indicating that the changes in cellular morphology probably preceded the ensuing death of the cells. We found that E4orf4 can associate with Src family kinases both in vitro and in vivo, and that expression of E4orf4 leads to a rapid and specific modulation of Src-dependent tyrosine phosphorylation of cellular proteins, rather than causing a general up- or downregulation of kinase activity. More specifically, early expression of E4orf4 inhibited Src-dependent tyrosine phosphorylation of focal adhesion–associated proteins such as FAK and paxillin, whereas other Src substrates, such as cortactin, presented higher levels of tyrosine phosphorylation. This was correlated with a redistribution of tyrosine phosphorylated proteins at the periphery of the cells, leading to a misassembly of focal adhesions that was accompanied by a dramatic reorganization of the actin skeleton. FAK is believed to play a major role in the dynamic turnover of focal adhesions essential for cell spreading and migration (Ilic et al. 1995). As Src family kinases are important regulators of tyrosine phosphorylation at focal adhesions (Klinghoffer et al. 1999), E4orf4 inhibition of Src/FAK-dependent signaling may be required to trigger the initial phase leading to E4orf4-dependent changes in cell shape allowing membrane blebbing.
E4orf4 effect on Src-dependent tyrosine phosphorylation was similar to that reported by inhibiting Src recruitment to integrins upon depletion of caveolin by antisense methodology in kidney 293 cells (Wei et al. 1999). Indeed, the possibility that E4orf4 association with Src kinases could regulate Src interactions with specific cellular proteins is supported by experiments showing that E4orf4 can interfere with the ability of FAK and c-src to cooperate in induction of tyrosine phosphorylation of cellular substrates. A recent study has provided evidence that FAK may function to direct phosphorylation of cellular substrates by recruitment of Src kinases (Schaller et al. 1999). FAK can associate with Src kinases via an SH2-dependent interaction involving the major site of FAK autophosphorylation, tyrosine 397 (Cobb et al. 1994; Schaller et al. 1994; Xing et al. 1994; Eide et al. 1995), and additionally, FAK contains a binding site for the SH3 domain of Src that may contribute to stabilization of the FAK–Src complex (Thomas et al. 1998). Although the molecular determinants of E4orf4/Src association has not been determined yet, preliminary results indicated that a highly conserved NH2-terminal, proline-rich motif, which contains a consensus sequence for Src family kinase SH3 binding (LPxLPxPP), can mediate E4orf4 binding to v-src SH3 domain in vitro (our unpublished data), raising the possibility that E4orf4 could change substrate binding via the SH3 domain. We have also observed that E4orf4 itself is tyrosine phosphorylated in cells coexpressing activated c-src or v-src, and that E4orf4 contains two potential consensus sequences for Src kinase SH2-binding (our unpublished data). However, it is not clear yet whether E4orf4 directly binds physically to Src family kinases in vivo, if E4orf4 association with Src kinases is facilitated by binding to another protein like PP2A as is the case for polyomavirus middle T antigen (Markland and Smith 1987; Glenn and Eckhart 1993; Glenn and Eckhart 1995; Campbell et al. 1995), or, alternatively, if E4orf4/Src association is indirect and mediated by adaptor molecules. Whatever the case, the results clearly show that E4orf4 expression interferes with Src signaling to focal adhesion and future work will help clarify the molecular mechanisms that are involved in E4orf4 modulation of Src kinase activity.
A Positive Role for Src Kinase Activity in E4orf4 Signaling of Caspase-independent Apoptosis
Functional analysis in cells coexpressing either activated c-src or kinase-deficient c-src together with E4orf4 revealed a close correlation between Src kinase activity, E4orf4-dependent membrane blebbing, and E4orf4-mediated DNA condensation. Furthermore, inhibition of Src family kinase activity using the selective inhibitor PP2 strongly inhibited membrane blebbing in E4orf4-expressing cells and as a consequence, E4orf4-mediated DNA condensation was markedly decreased. Although PP2 was shown recently to inhibit PDGFR and Abl tyrosine kinases, as well as some serine kinases (Liu et al. 1999), inhibition of E4orf4-induced apoptosis by this more selective inhibitor was consistent with a similar inhibition observed using Src interfering constructs (see level of inhibition Fig. 8A and Fig. B). The milder inhibition observed in the presence of genistein may be explained by the broader inhibitory action of this tyrosine kinase inhibitor, that may impact on signaling pathways important for survival, thus introducing unspecific toxic effects in these assays. Additionally, genistein was found to be less potent than PP2 in inhibiting the increased tyrosine phosphorylation induced by activated Src in our cell system (data not shown), although the reason for this is not clear. However, pharmacological analysis coupled with coexpression experiments strongly suggests that E4orf4 association with and modulation of Src kinase activity is likely to play an active role in mediating the early changes in cell shape, which may require the regulation of other signaling molecules by positive phosphorylation in addition to downregulation of FAK signaling at focal adhesions. It also implies that early membrane blebbing can be required as part of a discrete apoptotic program leading to caspase-independent nuclear condensation and cell death, supporting previous observations made in other apoptotic systems when caspase activity was inhibited (McCarthy et al. 1997). The observation that treatment with the actin polymerization inhibitor cytochalasin D resulted in a similar reversion of E4orf4-dependent membrane blebbing and later to inhibition of nuclear condensation further indicates that membrane blebbing is involved in signaling E4orf4 death pathway and stresses that the morphological changes occur before execution of the death pathway. These data also emphasize the importance of actin polymerization and remodeling in the release and blebbing phase of extranuclear apoptotic mechanisms.
The change in morphology associated with the release phase of extranuclear apoptosis was shown to correlate with a reorganization of actin into a peripheral cortical ring (Bannerman et al. 1998; Huot et al. 1998; Levkau et al. 1998). Soon after E4orf4 expression, we observed high amounts of polymerized actin forming dense fibers that accumulated at the cell periphery, indicating that E4orf4 may directly signal actin changes required for a rounder cell shape and force generation for membrane blebbing. Actin polymerization (Huot et al. 1998), actin-membrane linking proteins (Martin et al. 1995; Cryns et al. 1996; Nath et al. 1996; Wang et al. 1998), and small GTPases (Rudel and Bokoch 1997; Lee et al. 1997; Mills et al. 1998) have been implicated in apoptosis-related morphological changes and membrane blebbing. Immunolocalization of E4orf4 revealed that a subpopulation of the protein is localized in membrane blebs, together with exogenously expressed c-src. Furthermore, biochemical evidence was obtained that E4orf4 can associate with cytoskeletal components and, interestingly enough, higher amounts of E4orf4 were found associated with the cytoskeleton when activated Src were expressed. Although it is not clear yet if E4orf4 distribution to cytoskeletal fractions required E4orf4/Src association and/or modulation by endogenous Src kinases, the data clearly indicate that an increase in Src kinase activity can modulate E4orf4 distribution to the cytoskeleton, where E4orf4 could signal actin remodeling. In cells expressing E4orf4, cortactin together with E4orf4 relocalized in cortical membrane regions and later in membrane blebs. Moreover in v-src–expressing cells, cortactin phosphorylation was increased in the presence of E4orf4 and the results obtained upon phosphotyrosine immunoprecipitations from cells expressing E4orf4 only are also consistent with increased tyrosine phosphorylation of cortactin. In principle, the increased tyrosine phosphorylation of cortactin induced by E4orf4 expression, along with its redistribution to the cortical membrane, could play an active role in reorganizing the actin cytoskeleton during the release stage and/or the blebbing phase. In vitro phosphorylation of cortactin by Src was shown to downregulate its actin cross-linking activity (Huang et al. 1997), and hypertonicity-induced cell shrinkage was associated with phosphorylation of cortactin by the Src family kinase member fyn, suggesting a role in shrinkage-related reorganization of cytoskeleton (Kapus et al. 1999). However, still it is not clear whether phosphorylation of cortactin is required for actin remodeling and E4orf4 might provide a molecular tool to understand the regulation of cortactin function and its potential role in membrane blebbing. Nevertheless, although a clear modulation of cortactin localization by E4orf4 was observed, more direct evidence that E4orf4 can modulate cortactin phosphorylation by endogenous kinases in other transformed cell lines will be needed. The dramatic reorganization of the actin skeleton observed in E4orf4-expressing cells also raises the possibility that E4orf4 may modulate the activity of the major regulators of actin dynamic, the small Rho GTPases. This GTP-binding protein family for which the best characterized members are Cdc42, Rac1, and RhoA, was shown to control diverse biological processes such as cytoskeletal organization, gene transcription, adhesion, and apoptosis (for review see Aspenstrom 1999). Indeed, all three major Rho family members were recently involved in CTL- and Fas-induced killing, presumably through their effect on the actin cytoskeleton (Subauste et al. 2000), illustrating the complex multifunctional role of the Rho proteins in apoptosis induction. As Src family kinases are believed to modulate Rho activity through tyrosine phosphorylation of the p190 RhoGAP protein (Chang et al. 1995; Roof et al. 1998; Fincham et al. 1999), one possibility is that E4orf4 would cause actin changes via regulation of Rho function through modulation of Src family kinases. In future studies, we hope to elucidate the molecular mechanisms involved in E4orf4-dependent remodeling of the actin skeleton and thus to identify specific actin structures and dynamics involved in apoptosis induction.
In light of the results presented in this paper, we propose that E4orf4 may trigger the direct entry of cells into the release and blebbing phases of extranuclear apoptosis. E4orf4 would cause a misassembly of focal adhesions through inhibition of Src-dependent regulation of FAK signaling, coupled with a reorganization of the actin cytoskeleton through modulation of actin regulators such as cortactin. In a number of studies, integrin–FAK signaling complexes have been implicated in the regulation of anchorage-dependent cell survival, and upregulation of FAK signaling was shown to transform cells by providing survival signals allowing anchorage-independent growth (Frisch et al. 1996; Hungerford et al. 1996). As Src transformation was reported to protect MDCK cells and HUVECs from anoikis (Frisch and Francis 1994; Re et al. 1994), Src family kinase activation may mimic ECM survival signals required by transformed cells (irrespective of cell type) to survive in suspension. This suggests that malignant cells must somehow upregulate signaling intermediates in this adhesion pathway (like Src) in order to metastasize, and to survive and grow in inappropriate environments. The observation that E4orf4 can target and dysregulate Src-dependent signaling could provide part of an explanation for the apparent E4orf4 selectivity towards transformed cells (Shtrichman et al. 1999). Our data also support a model in which oncogenes such as Src may cotransduce survival and proapoptotic signals, and that the balance between those in a given tissue would be a critical determinant in the initiation and maintenance of a tumor (Pelengaris et al. 2000). Our work also provides the first evidence that adenoviruses express a protein capable of associating with and altering the function of Src-related kinases, as is the case for most DNA tumor viruses (Messerschmitt et al. 1997).
Acknowledgments
We thank Dr. Stephen Lee (University of Ottawa, Ottawa, Canada) for providing the FLAG–GFP expression vector, Dr. André Veillette (Department of Biochemistry, McGill University and IRCM, Montréal, Canada) for the mouse c-src expression vector, Dr. Jerry Pelletier (McGill University, Department of Biochemistry, Montréal, Canada) for the v-src plasmid, Dr. J.S. Brugge (Department of Cell Biology, Harvard Medical School, Boston) for the chicken c-src constructs, and Dr. J. Thomas Parsons (Department of Microbiology, University of Virginia School of Medicine, Charlottesville, Virginia) for the myc-FAK construct. We are also grateful to Dr. Gordon C. Shore for his support in starting this project and for critical reading of the manuscript, to Dr. Jacques Landry for helpful discussions, and to Drs. Jacques Landry, Jacques Huot, and Alan Anderson for critical reading of this manuscript. We also thank Dr. Benedicte Masson-Gadais for technical assistance in performing cell adhesion assays.
This work was supported by a Terry Fox Research Grant (No. 009058) from the National Cancer Institute of Canada (NCIC), and J.N. Lavoie is a Scholar from the Medical Research Council of Canada (MRC).
Footnotes
Abbreviations used in this paper: Ad, adenovirus; ECM, extracellular matrix; E4orf4, early region 4 open reading frame 4; ERK, extracellular-regulated kinase; EV, vector alone; FAK, focal adhesion kinase; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, a hemagglutinin; IP, immune complex; PP2A, protein phosphatase 2A.
References
- Aspenstrom P. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- Bannerman D.D., Sathyamoorthy M., Goldblum S.E. Bacterial lipopolysaccharide disrupts endothelial monolayer of adherens junction proteins. J. Biol. Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- Bondesson M., Ohman K., Manervik M., Fan S., Akusjarvi G. Adenovirus E4 open reading frame 4 protein autoregulates E4 transcription by inhibiting E1A transactivation of the E4 promoter. J. Virol. 1996;70:3844–3851. doi: 10.1128/jvi.70.6.3844-3851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C.E., Romer L.H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cells adhesion to extracellular matrix. J. Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb M.B., Polte T.R., Hanks S.K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activitya role for Src family kinases. Mol. Cell. Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.S., Auger K.R., Hemmings B.A., Roberts T.M., Pallas D.C. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J. Virol. 1995;39:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.H., Gill S., Settleman J., Parsons S.J. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 1995;130:355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E.A., Brugge J.S. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol. Cell. Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B.S., Schaller M.D., Leu T.H., Parsons J.T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol. Cell. Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneige S.A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO (Eur. Mol. Biol. Organ.) J. 1985;4:1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns V.L., Bergeron L., Zhu H., Li H., Yuan J. Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1beta-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J. Biol. Chem. 1996;271:31277–31282. doi: 10.1074/jbc.271.49.31277. [DOI] [PubMed] [Google Scholar]
- Eide B.L., Turck C.W., Escobedo J.A. Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Mol. Cell. Biol. 1995;15:2819–2827. doi: 10.1128/mcb.15.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham V.J., Chudleigh A., Frame M.C. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J. Cell Sci. 1999;112:947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- Frisch S.M., Francis H. Disruption of epithelial cell–matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S.M., Vuori K., Ruoslahti E., Chan-Hui P.Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F.G. Integrin signalingspecificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Glenn G.M., Eckhart W. Mutation of a cysteine residue in polyomavirus middle T antigen abolishes interactions with protein phosphatase 2A, pp60c-src, and phosphatidylinositol-3 kinase, activation of c-fos expression, and cellular transformation. J. Virol. 1993;67:1945–1952. doi: 10.1128/jvi.67.4.1945-1952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn G.M., Eckhart W. Amino-terminal regions of polyomavirus middle T antigen are required for interactions with protein phosphatase 2A. J. Virol. 1995;69:3729–3736. doi: 10.1128/jvi.69.6.3729-3736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green D., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hanke J.H., Gardner J.P., Dow R.L., Changelian P.S., Brissette W.H., Weringer E.J., Pollok B.A., Connelly P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hanks S.K., Polte T.R. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Huang C., Ni Y., Wang T., Gao Y., Haudenschild C.C., Zhan X. Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:13911–13915. doi: 10.1074/jbc.272.21.13911. [DOI] [PubMed] [Google Scholar]
- Huang C., Liu J., Haudenschild C.C., Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- Hungerford J.E., Compton M.T., Matter M.L., Hoffstrom B.G., Otey C.A. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J. Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot J., Lambert H., Lavoie J.N., Guimond A., Houle F., Landry J. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur. J. Biochem. 1995;227:416–427. doi: 10.1111/j.1432-1033.1995.tb20404.x. [DOI] [PubMed] [Google Scholar]
- Huot J., Houle F., Rousseau S., Deschesnes R.G., Shah G.M., Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J. Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Kanopka A., Muhlemann O., Petersen-Mahrt S., Estmer C., Ohrmalm C., Akusjarvi G. Regulation of adenovirus alternative RNA splicing by dephosphorylation of SR proteins. Nature. 1998;393:185–187. doi: 10.1038/30277. [DOI] [PubMed] [Google Scholar]
- Kaplan K.B., Bibbins K.B., Swedlow J.R., Arnaud M., Morgan D.O., Varmus H.E. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K.B., Swedlow J.R., Morgan D.O., Varmus H.E. c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- Kapus A., Szaszi K., Sun J., Rizoli S., Rotstein O.D. Cell shrinkage regulates Src kinases and induces tyrosine phosphorylation of cortactin, independent of the osmotic regulation of Na+/H+ exchangers. J. Biol. Chem. 1999;274:8093–8102. doi: 10.1074/jbc.274.12.8093. [DOI] [PubMed] [Google Scholar]
- Kleinberger T., Shenk T. Adenovirus E4orf4 protein binds to protein phosphatase 2A, and the complex down regulates E1A-enhanced junB transcription. J. Virol. 1993;67:7556–7560. doi: 10.1128/jvi.67.12.7556-7560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinghoffer R.A., Sashsenmaier C., Cooper J.A., Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Petit P., Zamzami N., Vayssiere J.L., Mignotte B. The biochemistry of programmed cell death. FASEB (Fed. Am. Soc. Exp. Biol.) J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Lavoie J.N., Hickey E., Weber L.A., Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J. Biol. Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie J.N., Lambert H., Hickey E., Weber L.A., Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell. Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J.N., Nguyen M., Marcellus R.C., Branton P.E., Shore G.C. E4orf4, a novel adenovirus death factor that induces p53-independent apoptosis by a pathway that is not inhibited by zVAD-fmk. J. Cell Biol. 1998;140:637–645. doi: 10.1083/jcb.140.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., MacDonald H., Reinhard C., Halenbeck R., Roulston A., Shi T., Williams L.T. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Neumann M., Stearman R., Stauber R., Pause A., Pavlakis G.N., Klausner R.D. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Mol. Cell. Biol. 1999;19:1486–1497. doi: 10.1128/mcb.19.2.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bishop A., Witucki L., Kraybill B., Shimizu E., Tsien J., Ubersax J., Blethrow J.O., Morgan D., Shokat K.M. Structural basis for selective inhibition of Src family kinases by PP1. Chem. Biol. 1999;6:671–678. doi: 10.1016/s1074-5521(99)80118-5. [DOI] [PubMed] [Google Scholar]
- Levkau B., Herren B., Koyama H., Ross R., Raines E.W. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellus R.C., Lavoie J.N., Boivin D., Shore G.C., Ketner G., Branton P.E. The early region 4 orf4 protein of human adenovirus type 5 induces p53-independent cell death by apoptosis. J. Virol. 1998;72:7144–7153. doi: 10.1128/jvi.72.9.7144-7153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellus R.C., Chan H., Paquette D., Thirlwell S., Boivin D., Branton P.E. Induction of p53-independent apoptosis by the adenovirus e4orf4 protein requires binding to the βα subunit of protein phosphatase 2A. J. Virol. 2000;74:7869–7877. doi: 10.1128/jvi.74.17.7869-7877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Smith A.E. Mutants of polyomavirus middle T antigen. Biochim. Biophys. Acta. 1987;907:299–321. doi: 10.1016/0304-419x(87)90011-4. [DOI] [PubMed] [Google Scholar]
- Martin S.J., Green D.R. Protease activation during apoptosisdeath by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Martin S.J., O'Brien G.A., Nishioka W.K., McGahon A.J., Mahboubi A., Saido T.C., Green D.R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J. Biol. Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- Masson-Gadais B., Salers P., Bongrand P., Lissitzky J.-C. PKC regulation of microfilament network organization in keratinocytes defined by a pharmacological study with PKC activators and inhibitors. Exp. Cell Res. 1997;236:238–247. doi: 10.1006/excr.1997.3721. [DOI] [PubMed] [Google Scholar]
- McCarthy N.J., Whyte M.K., Gilbert C.S., Evan G.I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J. Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmitt A.S., Dunant N., Ballmer-Hofer K. DNA tumor viruses and Src family tyrosine kinases, an intimate relationship. Virology. 1997;227:271–280. doi: 10.1006/viro.1996.8316. [DOI] [PubMed] [Google Scholar]
- Mills J.C., Stone N.L., Erhardt J., Pittman R.N. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J.C., Stone N.L., Pittman R.N. Extranuclear apoptosisthe role of the cytoplasm in the execution phase. J. Cell Biol. 1999;146:703–707. doi: 10.1083/jcb.146.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsudomi T., Steinberg S.M., Nau M.M., Carbone D., D'Amico D., Bodner S., Oie H.K., Linnoila R.I., Mulshine J.L., Minna J.D. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene. 1992;7:171–180. [PubMed] [Google Scholar]
- Miyamoto S., Akiyama S.K., Yamada K.M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function Science. 267 1995. 883 885a [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Coso O.A., Gutkind J.S., Burbelo P.D., Akiyama S.K., Yamada K.M. Integrin functionmolecular hierarchies of cytoskeletal and signaling molecules J. Cell Biol. 131 1995. 791 805b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U., Kleinberger T., Shenk T. Adenovirus E4orf4 protein reduces phosphorylation of c-Fos and E1A proteins while simultaneously reducing the level of AP-1. J. Virol. 1992;66:5867–5878. doi: 10.1128/jvi.66.10.5867-5878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada M., Aizawa S., Nakagawa H. Identification of major tyrosine-phosphorylated proteins in Csk-deficient cells. Oncogene. 1994;9:3571–3578. [PubMed] [Google Scholar]
- Nath R., Raser K.J., Stafford D., Hajimohammadreza I., Posner A., Allen H., Talanian R.V., Yuen P., Gilbertsen R.B., Wang K.K. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cellscontributory roles of both protease families in neuronal apoptosis. Biochem. J. 1996;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D.W., Thornberry N.A. Caspaseskiller proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Parsons J.T. Integrin-mediated signallingregulation by protein tyrosine kinases and small GTP-binding proteins. Curr. Opin. Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- Patel A.S., Schechter G.L., Wasilenko W.J., Somers K.D. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- Pelengaris S., Rudolph B., Littlewood T. Action of Myc in vivo — proliferation and apoptosis. Curr. Opin. Genet. Dev. 2000;10:100–105. doi: 10.1016/s0959-437x(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Plopper G.E., McNamee H.P., Dike L.E., Bojanowski K., Ingber D.E. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol. Biol. Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F., Zanetti A., Sironi M., Polentarutti N., Lanfrancone L., Dejana E., Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J. Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.B., Vila J., Lansing T.J., Potts W.M., Weber M.J., Parsons J.T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO (Eur. Mol. Biol. Organ.) J. 1987;6:2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.B., Roesel D.J., Kanner S.B., Parsons J.T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol. Cell. Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S., Koegl M., Barone M.V., Roussel M.F., Courtneidge S.A. DNA synthesis induced by some but not all growth factors requires Src family protein tyrosine kinases. Mol. Cell. Biol. 1995;15:1102–1109. doi: 10.1128/mcb.15.2.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof R.W., Haskell M.D., Dukes B.D., Sherman N., Kinter M., Parsons S.J. Phosphotyrosine (p-Tyr)-dependent and -independent mechanisms of p190 RhoGAP-p120 RasGAP interactionTyr 1105 of p190, a substrate for c-Src, is the sole p-Tyr mediator of complex formation. Mol. Cell. Biol. 1998;18:7052–7063. doi: 10.1128/mcb.18.12.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T., Bokoch G.M. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Schaller M., Parsons T. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high affinity binding site for crk. Mol. Cell. Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Hildebrand J.D., Shannon J.D., Fox J.W., Vines R.R., Parsons J.T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Hildebrand J.D., Parsons J.T. Complex formation with focal adhesion kinaseA mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell. 1999;10:3489–3505. doi: 10.1091/mbc.10.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases [published erratum appears in Mol. Cell. Biol. 1996. 16:7182–7184] Mol. Cell. Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hanks S.K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D., Jones K.C., Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinasesummation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol. Cell. Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtrichman R., Kleinberger T. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J. Virol. 1998;72:2975–2982. doi: 10.1128/jvi.72.4.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtrichman R., Sharf R., Barr H., Dobner T., Kleinberger T. Induction of apoptosis by adenovirus E4orf4 protein is specific to transformed cells and requires an interaction with protein phosphatase 2A. Proc. Natl. Acad. Sci. USA. 1999;96:10080–10085. doi: 10.1073/pnas.96.18.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M.A., Bishop J.M., McGrath J.P., Levinson A.D. A mutation at the ATP-binding site of pp60v-src abolishes kinase activity, transformation, and tumorigenicity. Mol. Cell. Biol. 1985;5:1772–1779. doi: 10.1128/mcb.5.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste M.C., Von Herrath M., Benard V., Chamberlain C.E., Chuang T.H., Chu K., Bokoch G.M., Hahn K.M. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J. Biol. Chem. 2000;275:9725–9733. doi: 10.1074/jbc.275.13.9725. [DOI] [PubMed] [Google Scholar]
- Susin S.A., Lorenzo H.K., Zamzami N., Marzo I., Snow B.E., Brothers G.M., Mangion J., Jacotot E., Costantini P., Loeffler M. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Theriault J.R., Charette S.J., Lambert H., Landry J. Cloning and characterization of hGMEB1, a novel glucocorticoid modulatory element binding protein. FEBS (Feb. Eur. Biochem. Soc.) Lett. 1999;452:170–176. doi: 10.1016/s0014-5793(99)00634-1. [DOI] [PubMed] [Google Scholar]
- Thomas J.E., Soriano P., Brugge J.S. Phosphorylation of c-src on tyrosine 527 by another protein tyrosine kinase. Science. 1991;254:568–571. doi: 10.1126/science.1719633. [DOI] [PubMed] [Google Scholar]
- Thomas J.W., Ellis B., Boerner R.J., Knight W.B., White G.C., II, Schaller M.D. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J. Biol. Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A., Lazebnick Y. Caspasesenemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Traxler P., Bold G., Frei J., Lang M., Lydon N., Mett H., Buchdunger E., Meyer T., Mueller M., Furet P. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J. Med. Chem. 1997;40:3601–3616. doi: 10.1021/jm970124v. [DOI] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Tyrosine phosphorylation of p130cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- Wang K.K., Posmantur R., Nath R., McGinnis K., Whitton M., Talanian R.V., Glantz S.B., Morrow J.S. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- Weed S.A., Du Y., Parsons J.T. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 1998;111:2433–2443. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- Wei Y., Yang X., Liu Q., Wilkins J.A., Chapman H.A. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 1999;144:1285–1294. doi: 10.1083/jcb.144.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Reynolds A.B., Kanner S.B., Vines R.R., Parsons J.T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Chao D.T., Korsmeyer S.J. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Chen H.C., Nowlen J.K., Taylor S.J., Shalloway D., Guan J.L. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol. Biol. Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Parsons J.T. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J. Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]